Abstract
The salivary glands of adults concentrate nitrate from the plasma into saliva where it is converted to nitrite by bacterial nitrate reductases. Nitrite can play a beneficial role in adult gastrointestinal and cardiovascular physiology. When nitrite is swallowed, some of it is converted to nitric oxide (NO) in the stomach and may then exert protective effects in the gastrointestinal tract and throughout the body. It has yet to be determined either when newborn infants acquire oral nitrate reducing bacteria or what the effects of antimicrobial therapy or premature birth may be on the bacterial processing of nitrate to nitrite. We measured nitrate and nitrite levels in the saliva of adults and both preterm and term human infants in the early weeks of life. We also measured oral bacterial reductase activity in the saliva of both infants and adults, and characterized the species of nitrate reducing bacteria present. Oral bacterial conversion of nitrate to nitrite in infants was either undetectable or markedly lower than the conversion rates of adults. No measurable reductase activity was found in infants within the first two weeks of life, despite the presence of oral nitrate reducing bacteria such as Actinomyces odontolyticus, and Veillonella atypica, and Rothia mucilaginosa. We conclude that relatively little nitrite reaches the infant gastrointestinal tract due to the lack of oral bacterial nitrate reductase activity. Given the importance of the nitrate-nitrite-NO axis in adults, the lack of oral nitrate-reducing bacteria in infants may be relevant to the vulnerability of newborns to hypoxic stress and gastrointestinal tract pathologies.
Keywords: newborn, nitrate, nitrite, nitric oxide, nitrate reducing bacteria, gastrointestinal tract
Introduction
Until recently nitrite and nitrate were thought to be biologically inert at physiological concentrations. However, evidence now indicates that these anions play a significant role in cardiovascular homeostasis, and in responses to hypoxic and ischemic stress (See review by Lundberg et al [1]). Nitrite has been found to provide protective effects in various animal models of ischemia-reperfusion [2–5]. Clinical trials are currently evaluating the safety and efficacy of nitrite in pulmonary hypertension and myocardial infarction. While the mechanism underlying the protective effects of nitrite is not yet well understood, a common hypothesis posits that nitrite is reduced to nitric oxide (NO), a reaction that can either occur spontaneously under acidic conditions such as those found in the stomach [6], or which may be catalyzed by a number of metal-containing proteins [7]. Irrespective of the mechanism, there is growing consensus that nitrite is cytoprotective during ischemia-reperfusion insult which has lead to an interest in the factors that determine plasma nitrite concentrations.
Nitrate, which is inert in mammalian tissues, is converted to nitrite by bacteria normally found on the dorsal surface of the adult tongue [1, 8]. These microbes act upon nitrate that is concentrated in the saliva by the salivary glands or ingested in the diet. Dietary nitrate primarily derives from vegetables [9] in the adult diet and from breast milk and formula in the diet of newborns [10]. These microbes also act on nitrate that is actively transported from plasma into the saliva by the salivary glands [9]. The resulting salivary nitrite is swallowed, and can then be converted to NO by non-enzymatic disproportionation in the acidic environment of the stomach [11, 12]. It can also enter the circulation where it may serve as a long-term reservoir of NO-bioactivity [1]. Increasing dietary nitrate lowers blood pressure [13, 14], improves exercise performance [1, 15, 16], and increases plasma nitrite concentrations [14, 17]. Disruption of the salivary nitrate-nitrite-NO pathway in adults by not swallowing saliva prevents the fall in blood pressure associated with ingestion of nitrate [13]. Thus, there is strong evidence that nitrate-reducing oral bacteria play an important role in determining plasma nitrite concentrations and the cardiovascular homeostasis of adults.
The bacterial colonization of the mouth and gastrointestinal tract of newborn infants begins at birth and progresses over the first few weeks of life [18]. This progression is almost certainly altered in an intensive care setting where sanitary practices and the use of antibiotics may diminish oral bacterial quantity and nitrate-reducing capacity. The appearance of nitrate-reducing bacteria in the mouths of newborn infants, whether in outpatient or intensive care settings, has not been characterized.
Our previous work has shown that plasma nitrite concentrations are lower in newborn infants in the neonatal intensive care unit compared to adults [19]. We postulate that this is in part due to low oral nitrate reductase activity in newborn infants. The current studies were designed to measure the activity of nitrate reducing bacteria in saliva, to characterize the type of oral nitrate reducing bacteria in the mouth of newborn infants, and to compare newborn and adult salivary nitrite and nitrate concentrations. We also tested the hypothesis that elimination of oral nitrate-reducing bacteria leads to decreases in circulating nitrite in adults. Given the importance of the nitrate-nitrite-NO axis in adults, the appearance and activity of oral nitrate-reducing bacteria in infants may contribute to reduced plasma nitrite levels and may be a significant factor in the course of development.
Methods
All experimental protocols were approved by the Institutional Review Board of Loma Linda University. Newborn study subjects were recruited from the neonatal intensive care unit, well-baby nursery, and pediatric outpatient clinic of Loma Linda University Children’s Hospital. Infants were considered to be term if born after 36 weeks gestation, and to be preterm if born before 35 weeks gestation; they were excluded from the study if they were born at 35 or 36 weeks gestation. In addition, infants with congenital malformations were excluded from the study. The infants’ gestational age, birth weight, type of feeding, and antibiotic therapy were recorded. Adult subjects were healthy males and females who, ranged from 24 to 72 years of age, had not used antiseptic mouthwash within 24 hours, and had not received antibiotic treatment within two weeks prior to the study. Separate cohorts of adults were studied for the salivary nitrate and nitrite concentrations and nitrate reductase activity portions of the study.
Salivary nitrite and nitrate concentrations
To determine baseline salivary nitrite and nitrate concentrations, samples were collected from infants and adults by gently rotating a sterile cotton swab (Kendall Q-Tips®, Tyco Healthcare Group, Mansfield, MA) against the sublingual posterior aspect of the tongue for 90 seconds. Samples were collected at least one hour following meals. The cotton end of the swab was removed and immediately transferred to 500 μL of water. The weight of the cotton swab was recorded to the nearest milligram before and after placement in the mouth to determine the amount of saliva collected. The amount of nitrite and nitrate collected in the swab was determined by comparison with standard curves generated from known nitrite or nitrate concentrations in 200 μl of water soaked into swabs and prepared on each day samples were assayed. Both nitrite and nitrate standards resulted in a linear relationship between the nominal and measured concentrations (nitrite R2 = 0.98, nitrate R2 = 0.87). Nitrite levels were consistently found to be below the lower limit of assay detection (10 nM) in samples of dry swabs, demonstrating no background contamination. There was measurable background contamination of nitrate (60.32 ± 11.61 μM) in the dry swabs, which was subtracted from the raw nitrate levels by the use of the standard curve.
Salivary nitrate reductase activity
Salivary samples were collected by rotating a sterile cotton swab in a single 360-degree twisting motion against the dorsum of the tongue. The volume of saliva collected was determined via the weight gain of the swab. The swab tip was immediately transferred to 3.0 ml of sterile anaerobic BHI broth (BD Bacto™ Brain Heart Infusion; BD Bacto™ Yeast Extract, Becton Dickinson, Franklin Lakes, New Jersey; L-Cysteine Hydrochloride Monohydrate, Fisher Scientific, Pittsburg PA; Hemin; Menadione; Sigma, St Louis MO) and then placed in a water bath held at 37 C. Within ten minutes of placing the swab in broth, a baseline nitrite measurement was recorded and then 10 mM nitrate was added (final concentration 333 μM) to provide substrate for the bacterial conversion to nitrite. Nitrite concentrations were then measured at five-minute intervals beginning after the first collection at one minute, and continuing for 30 minutes. The nitrate reductase activity was calculated from the slope of a linear regression fit to the plot of increasing nitrite concentrations over time, with the result providing nanomoles of nitrite production per minute per milligram of saliva collected.
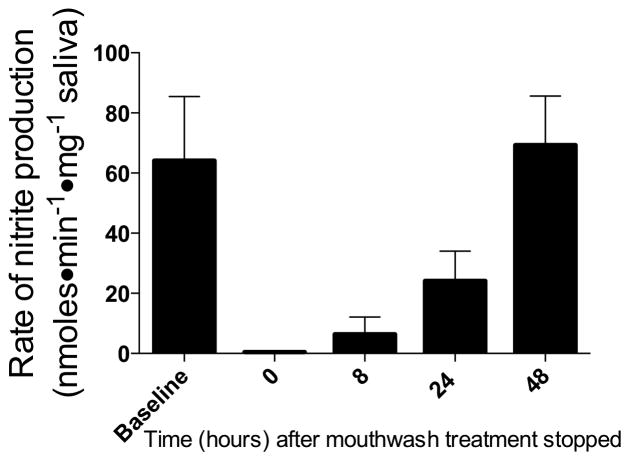
We verified this method of measuring oral nitrate reductase activity by treating four adult subjects with six antiseptic mouth rinse treatments consisting of 0.12% chlorhexidine solution (Peridex®, 3M ESPE Dental, St Paul, MN) over a period of three days (12-hour intervals). Baseline activities were recorded before the mouth rinse treatment, and immediately following the last mouth rinse treatment, demonstrating that the subjects no longer had any measurable activity (Figure 1). Subsequent measurements at 8, 24, and 48 hours after the last treatment indicated a return to baseline levels of nitrate reductase activity within 48 hours.
Figure 1.
Effect of antiseptic mouthwash on the assay for oral nitrate-reducing bacteria. Following six treatments with antimicrobial mouth rinse at twelve hour intervals, oral nitrate reductase activity in four adult subjects was decreased to unmeasurable levels compared to baseline values. Nitrate reductase activity returned to normal within 48 hours following the last antimicrobial mouth rinse treatment.
To verify that nitrate was being metabolized in the broth as nitrite concentrations were increasing, we also measured the rate of nitrate disappearance using swab samples from five adult subjects. Over the course of the 30-minute experiment, the mean rate of nitrate disappearance was 103 ± 20 nanomoles•min−1•mg−1 of saliva, while the mean rate of nitrite production was 47 ± 17 nanomoles•min−1•mg−1.
Effect of antimicrobial mouth rinse on blood and saliva nitrite and nitrate concentrations in adults
Twenty-four normal healthy adults received six mouth rinse treatments over a period of three days (at 12-hour intervals) with either 0.12% chlorhexidine solution (n=12) or saline solution (n=12) as placebo control. Saliva and venous blood samples were collected just prior to the first mouth rinse, and then again one to two hours following the final mouth rinse. Blood was immediately added to a nitrite preservation solution (4:1 v/v) [20], deproteinized by methanol precipitation (1:1 v/v), and stored at −70 C until assay. Approximately 5 ml of saliva was collected by expectoration of passively secreted saliva into a 50 ml tube, which was immediately frozen until assay at a later date. Subjects were asked to refrain from eating high-nitrate foods (e.g. beets, radishes, hotdogs, and leafy greens) from 24 hours prior to the first sample until after the collection of the final sample.
Salivary bacterial analyses
To assess for the presence of bacteria capable of nitrate reductase activity, PCR analysis and real-time PCR was performed on bacterial DNA isolated from saliva collected from healthy infants in the pediatric outpatient clinic, and from preterm infants in the NICU and from healthy adults after six chlorhexidine treatments. We chose to test for the presence of the four most prevalent nitrate reducing bacterial species in the adult mouth [21]: Veillonella atypica, Actinomyces odontolyticus, Rothia mucilaginosa, and Staphylococcus epidermidis.
Saliva was collected using a Vacutainer™ anaerobic specimen collection vial (Becton-Dickinson, Sparks, Maryland) by rotating a sterile anaerobic specimen collector swab in one 360-degree rotation against the dorsum of the tongue. Swabs were stored in an anaerobic specimen collector for up to 48 hours at room temperature after which they were placed in 5.0 ml of sterile anaerobic BHI broth. The swabs were then incubated overnight at 37 C in an anaerobic chamber (0% O2, 99.8% N2, 0.2% H2), until the broth had reached an optical density of 0.8 to 1.0 (600 nm). Bacterial DNA was extracted using a Wizard® Genomic DNA purification kit (Promega Corporation, Madison, WI) following the manufacture’s protocol for isolating genomic DNA from gram positive bacteria. For efficient lysis, 60 μl of 10mg/ml lysozyme and 60 μl of 10 mg/ml lysostaphin (Sigma-Aldrich®, St. Louis, MO) were added to each sample. The DNA was resuspended in 100 μl of rehydration solution, quantified using a NanoDrop 2000 (Thermo Scientific, Wilmington, DE) and then stored at −70 C.
PCR analysis
Qualitative PCR was performed on the DNA samples initially to confirm the accuracy of the primers used for the four bacterial species of interest, and to confirm the presence of target bacterial genes in the DNA samples. The primers for PCR analysis are listed in Table 1 and were designed using the NCBI primer select program. The reaction mixture (50 μl) contained 1 μg of template DNA in the high fidelity PCR master mix (Invitrogen, Carlsbad, CA). PCR cycling conditions were 1 cycle of 5 min at 94 C followed by 30 cycles of 30 sec at 94 C, 30 sec at 54 C and 1 min at 68 C with final extension of 5 min at 68 C.
Table 1.
Priming sequences used for bacterial PCR analyses
| Bacterial species | Target gene primer set |
|---|---|
| 1. Veillonella atypica | Forward primer-5′-GTGCTGCAGAGAGTTTGATCCTGGCTCAG -3′ |
| Reverse primer-5′-CACGGATCCTACGGGTACCTTGTTACGACTT -3′ | |
| 2. Rothia mucilaginosa | Forward primer-5′-AGCCTCAGGGATTGATGGGTTCTT-3′ |
| Reverse primer-5′-TTCTGGTGGTTGTACAGGGCGTTA-3′ | |
| 3. Actinomyces odontolyticus | Forward primer-5′-GCGGATTAATTCGATGCAACGCGA-3′ |
| Reverse primer-5′ CATTGTAGCATGCGTGAAGCCCAA-3′ | |
| 4. Staphylococcus epidermidis | Forward primer-5′-CTGCCTTTCAATGCGAGTTGGCTT-3′ |
| Reverse primer-5′-ACAGCTAAACTTGCAGCATGTGGG-3′ |
Real time PCR analysis
Real time PCR was carried out on 1 μg aliquots of purified DNA using a Smart cycler II (Cepheid, Sunnyvale CA). The primers for the real time analysis are listed in Table 1. The amplification efficiency of each primer set was determined empirically by using DNA template dilutions over four orders of magnitude. The amplification efficiency for each primer set varied between 95.1% and 102.5%, showing that the amplicons were generated with comparable efficiency. The specificity of the amplification was ascertained based on the melting peak generated during each run. The real time-PCR reaction contained 12.5 μl of QuantiTect SYBR Green qPCR master mix (Qiagen, Valencia, CA), 0.2 μM of each gene-specific primer and 1 μl of DNA template. The cycling conditions were 50 C for 2 min, 95 C for 2 min, then 40 cycles of 94 C for 15 s, 58 C for 30 s, and 72 C for 30 s. Distilled water was used as a negative control in each run. All reactions were carried out in triplicate. A standard curve was generated using dilution of the DNA from each species of bacteria plotted against the threshold value (Ct). The concentration of DNA was converted to copy number using the formula where n = the number of target sequence copies per microliter, NA = Avagadro’s constant (mol−1), m = mass of the amplicon per microliter, MW = mean molecular weight of 1 base pair, and L = amplicon length in base pairs. The Ct values obtained were plotted against DNA concentration to generate standard curves from which the cutoff Ct for each bacteria was determined. The cutoff Ct value, above which the absence of the bacteria is indicated, was 43 for Veillonella atypica, 49 for Rothia mucilaginosa, 39 for Actinomyces odontolyticus, and 47 for Staphylococcus epidermidis.
Nitrite and nitrate assays
Nitrite concentrations were measured by triiodide chemiluminescence as described by Pelletier et al [20], enabling quantification above 10 nM with a precision of ±5 nM. Nitrate concentrations were measured by incubation of 205 μl of sample with 5 μl nitrate reductase enzyme (10 U/ml solvent, Roche, Indianapolis IN) in 10 μl of 1M HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer solution (Fisher Scientific, Pittsburgh PA), 10 μl of 0.1 mM flavin adenine dinucleotide disodium salt hydrate (Sigma Aldrich, St Louis MO), and 20 μl of 1 mM nicotinamide adenine dinucleotide phosphate-oxidase tetrasodium salt (Roche, Indianapolis IN) at 37 C for 45 min to convert all the nitrate to nitrite, which was then measured by triiodide chemiluminescence. Whole blood nitrate concentrations were measured by chemiluminescence assay following reduction of nitrate to NO in a purge vessel containing vanadium III and HCl at 90 C.
Data Analysis
Data are presented as means ± standard error. Differences between study groups were detected using Student’s t-test for two group comparisons and one-way ANOVA followed by Bonferroni post hoc analysis for comparison of three or more datasets. One-way ANOVA with repeated measures was also used to detect significant changes from baseline measurements in time-course experiments. Two-way ANOVA followed by Bonferroni post hoc analysis was used to detect significant differences between groups in time-course experiments. Statistical analysis was performed using Prism 5 for Mac OS X (Graphpad Software, Inc, La Jolla, CA).
Results
Nitrite and nitrate concentrations in saliva
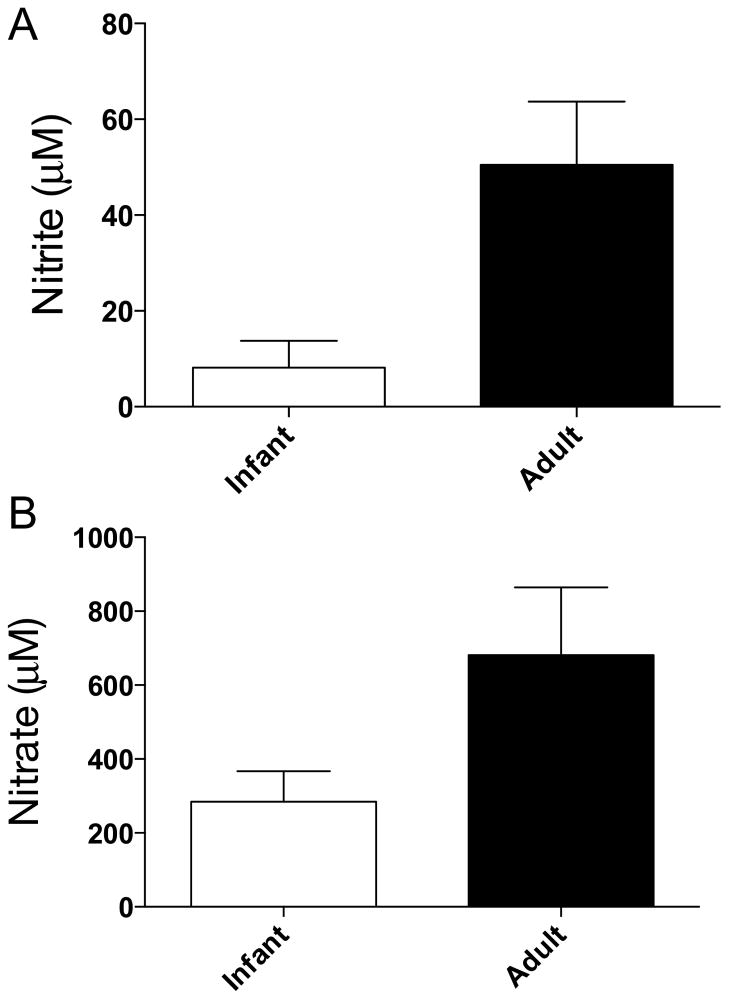
Nitrite and nitrate concentrations were measured in saliva collected from ten infants and twelve healthy adults. The results are shown in Figure 2A and participant demographics are provided in Table 2. Nitrite concentrations in infant saliva averaged 8.2 ± 5.6 μM. These concentrations were significantly lower than adult salivary nitrite concentrations which averaged 50 ± 13 μM (p=0.0501).
Figure 2.
Nitrite and nitrate concentrations in saliva of infants and adults. A) Nitrite concentrations in the saliva of infants (8 ± 5 μM) were less than adult saliva (55 ± 22 μM, ** = p<0.01). B) Salivary nitrate levels were not significantly lower in the saliva of infants (328 ± 97 μM) than in the saliva of adults (538 ± 125 μM).
Table 2.
Saliva study infant participant demographics
| Infants | |
|---|---|
| Participants, n | 10 |
| Gestational age, weeks | 39 ± 0.3 |
| Birth weight, grams | 3662 ± 170 |
| Age at time of collection, days | 3.1 ± 0.3 |
| Infants receiving antibiotics, n | 0 |
| Breast fed, n | 7 |
| Formula and breast milk fed, n | 3 |
Nitrate concentrations were measured in saliva collected from ten infants and fourteen adults and averaged 284 ± 83 μM in the saliva of infants and were not measurably different than those found in adults (681 ± 184, p=0.11) (Figure 2B). The newborn salivary nitrate concentrations were approximately ten-fold greater than previously reported newborn plasma nitrate levels [19, 22, 23], demonstrating that salivary glands are able to concentrate nitrate from the plasma into the saliva of infants, and to levels comparable to adults.
Notably, the amount of saliva obtained during the timed 90- second collection periods from infants (39 ± 8 μl) was less than the amount collected from adults (143 ± 15 μl) (p<0.001).
Conversion of nitrate to nitrite in saliva
Oral bacterial nitrate-reducing activity was measured in fresh oral swabs immediately after placing them in culture media. Swabs were obtained from 25 infants less than five days of age, from 19 infants between 14 and 40 days of age, from 9 infants approximately two months old, and from 13 healthy adults. The demographics of the infant subjects are shown in Table 3.
Table 3.
Saliva nitrate reductase study infant participant demographics
| NICU Preterm on antibiotics | NICU Term on antibiotics | NICU Preterm | Outpatient Term | NICU Term >2 weeks old | NICU Preterm >2 weeks old | Outpatient >2 weeks old | Outpatient 2 months | |
|---|---|---|---|---|---|---|---|---|
| Participants, n | 7 | 6 | 5 | 7 | 5 | 8 | 6 | 9 |
| Gestational age, weeks | 32.1 ± 0.7 | 38.6 ± 0.5 | 32.6 ± 0.7 | 39.2 ± 0.5 | 38.4 ± 0.5 | 30.8 ± 1.1 | 38.9 ± 0.7 | 39.5 ± 0.3 |
| Birth weight, grams | 1495 ± 201 | 3322 ± 86 | 1964 ± 124 | 3391 ± 140 | 3350 ± 235 | 1581 ± 174 | 3199 ± 133 | 3425 ± 127 |
| Age at time of collection, days | 3 ± 0.4 | 4.2 ± 0.3 | 4.6 ± 0.2 | 3.7 ± 0.4 | 26.6 ± 5.6 | 27.3 ± 2.9 | 17.2 ± 1.7 | 64.6 ± 1.1 |
| Breast fed, | 0 | 2 | 0 | 3 | 1 | 0 | 6 | 7 |
| Nipple/gavage breast milk fed, n | 3 | 3 | 2 | 2 | 1 | 7 | 0 | 0 |
| Formula fed only, n | 0 | 0 | 2 | 2 | 3 | 1 | 0 | 2 |
| Intravenous feeding, n | 4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
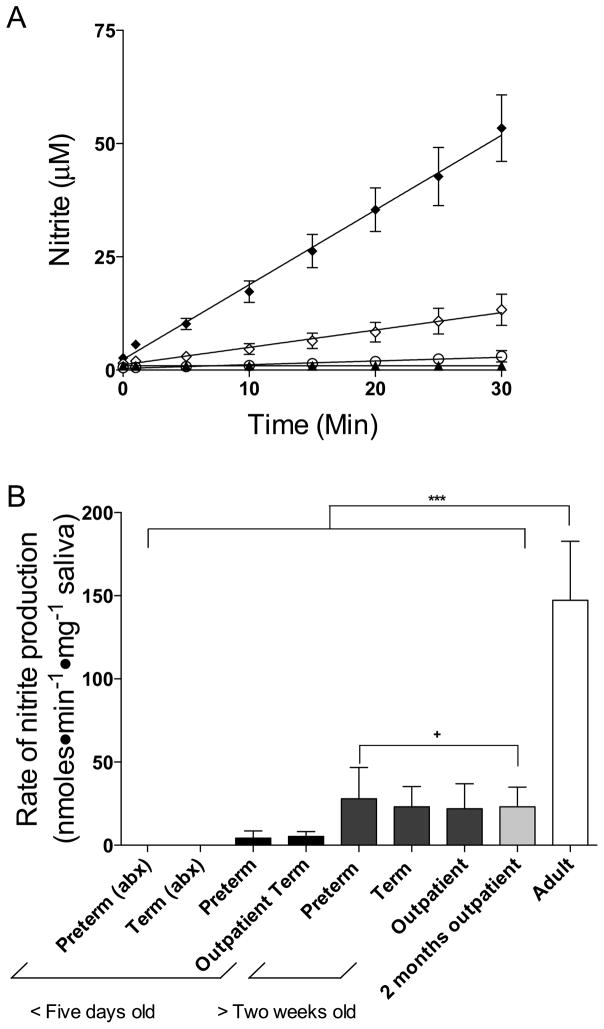
Swabs collected from infants contained similar amounts of saliva as those collected from adults (17 ± 1 vs. 17 ± 3 mg, respectively, p=0.9). The rate of nitrite production in the cultures, normalized to the weight of the saliva collected, was linear during the 30–min sampling period, as shown in Figure 3A, with the slope thus providing an index of bacterial nitrate reductase activity. In samples taken from adults nitrite was produced at a rate of 147 ± 36 nmoles⊕min−1⊕mg−1. In marked contrast, however, saliva cultures from newborns showed little or no detectable nitrite production. In the cultures collected from preterm (n = 6) and term (n = 6) infants on antibiotics, the rates of nitrite production were not significantly different than zero (−0.05 ± 0.04 nmoles⊕min−1⊕mg−1 saliva and −0.04 ± 0.11 nmoles⊕min−1⊕mg−1 saliva, respectively). In addition, there was no appreciable nitrite production in the salivary cultures taken from preterm infants in the NICU not receiving antibiotics, or from healthy term infants less than two weeks of age in the well-baby nursery and outpatient clinic (4 ± 4 nmoles⊕min−1⊕mg−1 saliva and 5 ± 3 nmoles⊕min−1⊕mg−1 saliva, respectively). Nitrite production was detectable in saliva samples collected from healthy infants between 14 and 40 days of age in the outpatient clinic (22 ± 15 nmoles⊕min−1⊕mg−1) as well as term (23 ± 12 nmoles⊕min−1⊕mg−1) and preterm infants in the NICU (28 ± 19 nmoles⊕min−1⊕mg−1), although this activity was still measurably less than that of the adults (p<0.01). Even by two months of age, the rate of nitrite production (23 ± 12 nmoles⊕min−1⊕mg−1) was significantly lower than that of adults (p<0.01, Figure 3B).
Figure 3.
A) Time course of nitrite production by nitrate-reducing bacteria cultured from saliva samples. After introduction of nitrate substrate into cultures [NO2−] was measured in samples from adults (◆), infants greater than two weeks of age (◇), newborn infants less than five days old (○), and newborn infants less than five days old receiving antibiotics (▲). B) Summary of kinetic results are shown for adults and each of the infant groups based on average rates of change of nitrite concentration with time, as shown in A. There was no significant nitrite production in the saliva of infants <5 days old, regardless of whether they were receiving antibiotics (abx). There was significant nitrite production in the infants older than two weeks (+ = p<0.01), and nitrite production was significantly greater in the adult saliva compared to infants (*** = p<0.001).
PCR detection of nitrate-reducing bacteria
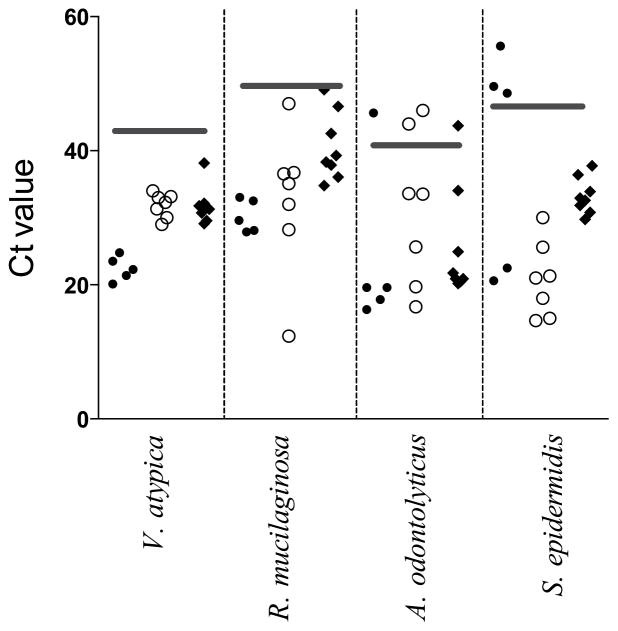
To investigate the possibility that the lack of nitrate reducing activity in the samples collected from infants was due to the absence of nitrate-reducing bacteria, PCR detection was performed on samples collected from five healthy term infants in the outpatient clinic and seven preterm infants in the NICU, who were all less than five days of age. The specificity of the amplification of target genes was confirmed by conventional PCR analysis (data not shown). The quantitative PCR demonstrated the presence of Veillonella atypica and Rothia mucilaginosa in all the infants studied. Actinomyces odontolyticus was present in four of the five term infants and five of the seven preterm infants, and Staphylococcus epidermidis was present in two of the five term infants and all of the preterm infants studied (Figure 4). These values suggest that the most abundant oral nitrate reducing bacteria in adults are also present in the mouths of neonates within a few days after birth. Thus, the lack of nitrate reductase activity in infants is more likely to be due to low abundance or activity of these bacteria as opposed to lack of inoculation. To assess this possibility further, PCR was also performed on eight adult subjects after 6 treatments with chlorhexidine, which lowers oral nitrate reductase activity by our assay to levels comparable to those of infants (see above). We found detectable presence of all four bacterial species in all adults studied, with the exception of one adult in whom Actinomyces odontolyticus was not detectable, suggesting that although chlorhexidine significantly decreases overall oral nitrate reducing activity, it does not completely eliminate the presence of nitrate-reducing bacteria.
Fig. 4.
Real time PCR detection of nitrate-reducing bacteria in newborn mouths. The presence of Veillonella atypica, Rothia mucilaginosa, Actinomyces odontolyticus, and Staphylococcus epidermidis in bacterial cultures collected from the mouths of term (●) and preterm (○) infants and adults (◆) was confirmed using primers specific to the respective bacteria. Horizontal gray bars represent the cutoff Ct value, below which the presence of the bacteria is confirmed. The data indicate the presence of most or all of these nitrate reducing bacteria in the infant mouth within the first few days of life, suggesting the lower rates of nitrate reducing activity in infants is not due to lack of inoculation.
Effect of antimicrobial mouth rinse on blood and saliva nitrite and nitrate concentrations in adults
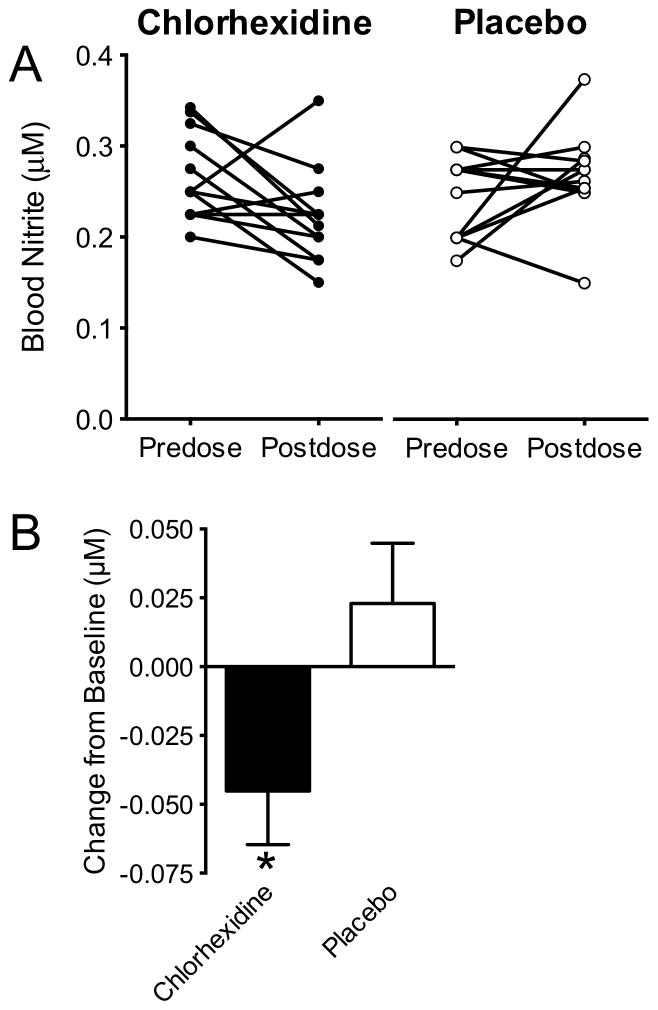
Changes in blood nitrite were examined in 24 adult subjects after three days of treatment with either anti-microbial mouth rinse or placebo. Mean blood nitrite concentration decreased by approximately 20% from 0.27±0.01 μM to 0.22±0.01 μM following chlorhexidine treatment (p<0.05), but was not affected by the placebo. As shown in Figure 5, blood nitrite concentrations decreased in 9 out of 12 subjects following chlorhexidine mouth rinse treatments, compared to only 5 out of 12 subjects with placebo mouth rinses. There were no significant differences in the blood nitrate concentrations of the study subjects before or after treatment with chlorhexidine (41 ± 8 vs 48 ± 7 μM) or placebo (35 ± 4 vs 32 ± 6 μM, data not shown).
Fig. 5.
Effect of antiseptic mouth rinse on adult blood nitrite concentrations. Blood nitrite concentrations in healthy adult volunteers were significantly decreased by ~19% compared to baseline levels following six treatments with antiseptic mouth rinse (chlorhexidine) at 12-hour intervals (*=p<0.04). No significant change was observed in a parallel group of placebo (saline) control subjects.
Saliva nitrite concentrations fell from 322 ± 70 μM to 109 ± 35 μM (p<0.01) following three days of antimicrobial mouthwash, and were unchanged (246 ± 54 μM vs 284 ± 45 μM) following treatment with placebo. Saliva nitrate concentrations increased from 189 ± 39 μM to 1083 ± 268 μM (p<0.01) following the antimicrobial mouthwash, and were again unchanged (118 ± 40 vs 141 ± 37 μM) following treatment with placebo.
Discussion
The current studies demonstrate that bacterial nitrate reductase activity in the mouth of neonates is minimal compared to that of adults. The low nitrate-reducing activity in the newborn persists for at least the first two months of life, and results in significantly lower salivary nitrite concentrations. In spite of this reduced activity, PCR evidence shows that newborn mouths do contain the major nitrate-reducing bacteria found in adults. This suggests that the diminished nitrate-reducing activity of newborns may be due to lower numbers of these bacteria, as opposed to a lack of inoculation. Thus, this study shows that oral nitrite production, which plays a prominent role in cardiovascular and gastrointestinal homeostasis of adults, has less capacity to function in neonates during the first few weeks of life.
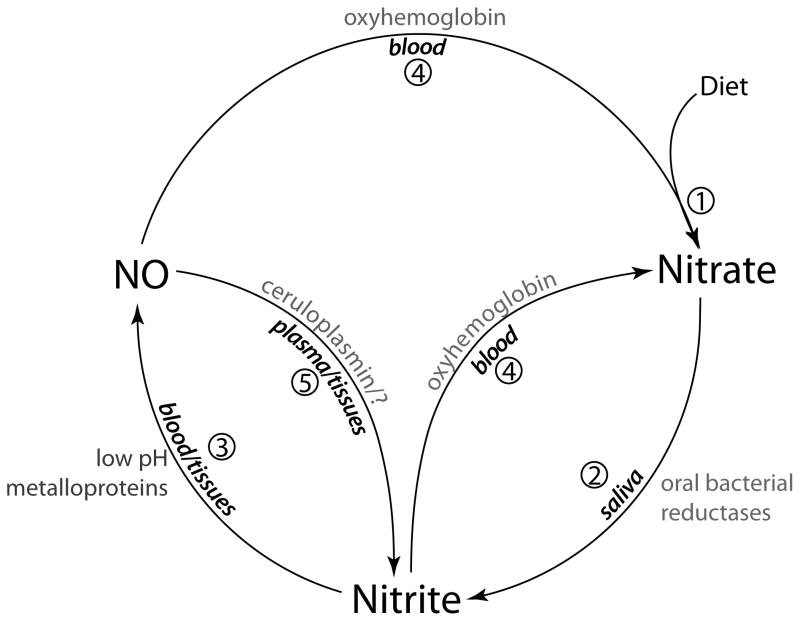
The key steps in nitrate and nitrite transport and metabolism are shown in Figure 6 and consist of 1) the introduction of nitrate into the mouth by diet or transport from plasma into the saliva by the salivary glands, 2) reduction of nitrate to nitrite by oral bacteria, 3) ingestion of nitrite, which is either converted to NO in the stomach or absorbed into the blood stream, 4) oxidation of nitrite and NO back into nitrate which can then again be secreted into the saliva, and 5) oxidation of NO into nitrite in the plasma and tissues [1].
Fig 6.
Schema showing the pathways and interconversions of nitrite and nitrate. 1) Nitrate enters the mouth from diet and from the plasma by a concentrating action of the salivary glands. 2) In the mouth nitrate is converted to nitrite by commensal bacteria on the tongue [21]. 3) In the acid milieu of the stomach nitrite is converted to NO by disproportionation [11] or absorbed into the circulation. Nitrite may also be converted to NO in blood and tissues by the action of metalloproteinases [1]. NO can be converted to 4) nitrate by reaction with oxyhemoglobin [41] or 5) nitrite by ceruloplasmin [42].
Impact of oral bacteria on nitrite ingestion
Normally, adults swallow on average 600 ml of saliva per day [24]. To our knowledge, the quantification of saliva production or ingestion in newborns has not yet been accomplished due to obvious technical challenges. However, the newborn mouth is relatively dry compared to the adult mouth, as is reflected by significantly lower volumes of saliva collected by the oral swabs in the current study. In adults, even when nitrate-reducing bacteria are present, not swallowing saliva effectively blocks the physiological effects of dietary nitrate [13]. Thus, it is reasonable to hypothesize that the relatively low rate of saliva production in newborns, coupled with a low concentration of nitrite in the saliva, has a compounded impact upon overall nitrite ingestion by the newborn infant resulting in significantly decreased nitrite intake compared to adults.
Linkage between oral bacterial activity and plasma nitrite
We have recently observed that the blood nitrite concentration of newborn infants (1.4 ± 0.5 days of age) is approximately 35% to 55% lower than that of adults [19]. The current studies found that a blockade of oral bacterial nitrate reduction activity in adults resulted in a ~20% reduction in blood nitrite concentrations, demonstrating that oral nitrate reductase activity makes a significant contribution to basal plasma nitrite concentrations. This finding is consistent with experiments in which increases in plasma nitrite, following nitrate ingestion, have been shown to require the activity of oral bacteria [13, 25]. Notably, plasma nitrite concentrations are also heavily influenced by NO derived from endothelial nitric oxide synthase (eNOS) activity [26, 27]. However, the rate of eNOS activity in newborns, relative to adults, has not been determined.
Oral bacteria
A number of specific nitrate-reducing bacteria, most of them facultative anaerobes, have been identified in the oral cavity of adult humans [21, 28]. Previous studies have reported the presence of Veillonella and Actinomyces spp. [29, 30] in infants. To our knowledge, however, this is the first study to report both the presence of nitrate reducing bacteria and measurement of oral nitrate reducing activity. The significantly lower nitrate reductase activity in newborns, despite evidence of their mouths having been colonized by these bacteria, may be due to relatively low bacterial abundance at this age, a factor not measured in the current study. This idea is supported by our observation of the presence of these bacteria in the mouths of adults following chlorhexidine treatment, concomitant with a lack of measurable nitrate reductase activity. The significantly lower bacterial nitrate reductase activity observed in infants in both an intensive care setting and the home environment, regardless of whether the infants were receiving antibiotics, serves as further evidence that the low nitrate reducing activity of newborns was not due to a delayed inoculation of these infants with nitrate-reducing bacteria. Interestingly, there is evidence that the lack of oral nitrite production in neonates may be at least partially compensated for by relatively high concentrations of nitrite found in colostrum and breast milk during the first few days of life [10].
Study Limitations. Study Limitations
We observed that salivary nitrite concentrations in adult saliva collected with cotton swabs (55 ± 22 μM) were consistently lower than concentrations measured in saliva collected by expectoration (284 ± 63 μM) in addition to being lower than most previously reported salivary nitrite concentrations which range from 90 to >670 μM. Standard curves generated by dipping swabs in small amounts of water with varying concentrations of nitrite were highly linear with significant slope. However, we observed that nitrite concentrations measured in ten expectorated adult saliva samples were significantly higher than concentrations measured from samples collected at the same time using swabs (131±32 vs 45 ±14 μM, p=0.024). This suggests that nitrite in saliva may bind to the swab or to other components of saliva that bind to the swab, resulting in an artifact of low absolute nitrite concentrations. Nevertheless, the measurement of nitrite in saliva collected in the swabs provides a relative comparison of newborn and adult concentrations where collection of expectorated samples from infants is not possible. Improved methods of neonatal saliva collection in the future may lead to more precise determinations of absolute concentrations in neonatal saliva.
It is also important to note that although our findings indicate that nitrate reducing activity is markedly less in the mouths of infants compared to adults despite the PCR detection of nitrate reducing bacteria, the PCR results cannot be used as a quantifiable comparison of bacterial abundance in infant and adult mouths due to the method of sample collection and overnight culture prior to DNA isolation.
Clinical perspective
Hypoxia and ischemia play key roles in several diseases of the newborn period. Because nitrite is protective against hypoxic and ischemic insults, questions arise as to the potential role of nitrite supplementation of the newborn infant. Such treatment may be particularly beneficial to premature infants who require prolonged periods of intubation and mechanical ventilation which has been demonstrated in adults to result in significant depletion of intragastric NO, presumably due to marked decreases in saliva production and swallowing [31]. Necrotizing enterocolitis, the most common gastrointestinal disorder to affect premature infants, likely results from a combination of decreased gastrointestinal blood flow, breakdown of the mucus barrier lining the lumen of the gut, and invasion by pathogenic bacteria [32]. Intragastric nitric oxide derived from non-enzymatic disproportionation of swallowed salivary nitrite counteracts all three of these factors in adult animal models [31, 33, 34]. However, the gastric pH of newborn infants is relatively high compared to adults [35, 36], leading to diminished NO production, which may hinder its gastroprotective effects and compound the effects of low levels of nitrite ingestion in newborn infants. Premature infants are also at significant risk of suffering intraventricular hemorrhage and episodes of inadequate systemic oxygenation. Circulating nitrite provides protection against cerebral vasospasm following subarachnoid hemorrhage in baboons [37], and can also increase cerebral blood flow [38] and decrease oxygen consumption during hypoxic stress [39]. During reperfusion, nitrite is found to protect against oxidative stress and to reduce the generation of harmful reactive oxygen species [40]. Whether these protective effects of nitrite are diminished in neonates due to the attenuation of enterosalivary nitrate-nitrite-NO metabolism calls for future study.
Highlights.
We measure the nitrate and nitrite concentrations in the saliva of newborn infants.
Oral bacterial nitrate reductase activity is lower in newborns than in adults.
The most prevalent nitrate reducing bacteria in adults are also found in newborns.
Eliminating nitrate reducing bacteria in the mouth lowers plasma nitrite levels.
Infants’ low salivary nitrite level and saliva production result in low nitrite ingestion.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr. Ravindra Rau and Cathy Rehage in recruiting and enrolling the outpatient participants, the technical assistance of Averil Austin, and the editing expertise of Nathan Jones. This work was supported by Loma Linda University and Public Health Grants DE-18664 and DE-019730 and NIDCR (to H.M.F.).
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Lundberg JO, Weitzberg E. NO-synthase independent NO generation in mammals. Biochem Biophys Res Commun. 2010;396:39–45. doi: 10.1016/j.bbrc.2010.02.136. [DOI] [PubMed] [Google Scholar]
- 2.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–50. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 3.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–8. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, Mazzon E, Cuzzocrea S, Yaqoob MM, Ahluwalia A, Thiemermann C. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007;18:570–80. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 6.Samouilov A, Kuppusamy P, Zweier JL. Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch Biochem Biophys. 1998;357:1–7. doi: 10.1006/abbi.1998.0785. [DOI] [PubMed] [Google Scholar]
- 7.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–51. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 10.Hord NG, Ghannam JS, Garg HK, Berens PD, Bryan NS. Nitrate and Nitrite Content of Human, Formula, Bovine, and Soy Milks: Implications for Dietary Nitrite and Nitrate Recommendations. Breastfeed Med. 2010;6:393–9. doi: 10.1089/bfm.2010.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–6. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1121–31. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 15.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 16.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–55. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 17.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–7. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim YI, Ninnis JR, Hopper AO, Deming DD, Zhang AX, Herring JL, Sowers LC, McMahon TJ, Power GG, Blood AB. Inhaled nitric oxide therapy increases blood nitrite, nitrate, and s-nitrosohemoglobin concentrations in infants with pulmonary hypertension. J Pediatr. 2011;160:245–51. doi: 10.1016/j.jpeds.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006;41:541–8. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113:14–9. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 22.Biban P, Zangardi T, Baraldi E, Dussini N, Chiandetti L, Zacchello F. Mixed exhaled nitric oxide and plasma nitrites and nitrates in newborn infants. Life Sci. 2001;68:2789–97. doi: 10.1016/s0024-3205(01)01086-4. [DOI] [PubMed] [Google Scholar]
- 23.Posencheg MA, Gow AJ, Truog WE, Ballard RA, Cnaan A, Golombek SG, Ballard PL. Inhaled nitric oxide in premature infants: effect on tracheal aspirate and plasma nitric oxide metabolites. J Perinatol. 2010;30:275–80. doi: 10.1038/jp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawes C. Salivary flow patterns and the health of hard and soft oral tissues. J Am Dent Assoc. 2008;139(Suppl):18S–24S. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–6. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 27.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Könönen E, Kanervo A, Takala A, Asikainen S, Jousimies-Somer H. Establishment of oral anaerobes during the first year of life. J Dent Res. 1999;78:1634–9. doi: 10.1177/00220345990780100801. [DOI] [PubMed] [Google Scholar]
- 30.Sarkonen N, Könönen E, Summanen P, Kanervo A, Takala A, Jousimies-Somer H. Oral colonization with Actinomyces species in infants by two years of age. J Dent Res. 2000;79:864–7. doi: 10.1177/00220345000790031301. [DOI] [PubMed] [Google Scholar]
- 31.Bjorne H, Govoni M, Tornberg DC, Lundberg JO, Weitzberg E. Intragastric nitric oxide is abolished in intubated patients and restored by nitrite. Crit Care Med. 2005;33:1722–7. doi: 10.1097/01.ccm.0000171204.59502.aa. [DOI] [PubMed] [Google Scholar]
- 32.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansson EA, Petersson J, Reinders C, Sobko T, Bjorne H, Phillipson M, Weitzberg E, Holm L, Lundberg JO. Protection from nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers by dietary nitrate. Free Radic Biol Med. 2007;42:510–8. doi: 10.1016/j.freeradbiomed.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Petersson J, Phillipson M, Jansson EA, Patzak A, Lundberg JO, Holm L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am J Physiol Gastrointest Liver Physiol. 2007;292:G718–24. doi: 10.1152/ajpgi.00435.2006. [DOI] [PubMed] [Google Scholar]
- 35.Kelly EJ, Newell SJ, Brownlee KG, Primrose JN, Dear PR. Gastric acid secretion in preterm infants. Early Hum Dev. 1993;35:215–20. doi: 10.1016/0378-3782(93)90108-7. [DOI] [PubMed] [Google Scholar]
- 36.Miclat NN, Hodgkinson R, Marx GF. Neonatal gastric pH. Anesth Analg. 1978;57:98–101. doi: 10.1213/00000539-197801000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. Jama. 2005;293:1477–84. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 38.Rifkind JM, Nagababu E, Barbiro-Michaely E, Ramasamy S, Pluta RM, Mayevsky A. Nitrite infusion increases cerebral blood flow and decreases mean arterial blood pressure in rats: a role for red cell NO. Nitric Oxide. 2007;16:448–56. doi: 10.1016/j.niox.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 42.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–93. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]