Abstract
Objective
A work function measure specific for persons with prodromal Huntington disease (HD) was created to assist with workplace accommodations
Methods
A self-report HD Work Function measure (HDWF) was developed from focus group and expert validation.
Results
Pilot studies with 238 people with prodromal HD, and 185 companions; and 89 people without prodromal HD, and 70 companions indicate HDWF has acceptable internal consistency (Cronbach’s alpha = 0.77), acceptable inter-rater reliability (r = 0.58), and acceptable convergent validity with selected items from EWPS (r = −0.56), SAS (r = −0.29), and ECog (r = −0.70). The HDWF can distinguish between people with prodromal HD and people with a HD family history who do not have prodromal HD (p < 0.0001).
Conclusions
The HDWF is a brief self assessment that may be used to monitor work function.
The ability of workers with chronic illnesses to meet job expectations is a concern of employers, workers and their families, and clinicians. Work function can be described as the impact of a health condition on the output of workers, work role limitations, quality of work output, and effort required by the worker to remain productive. 1 Employment is important for financial security, social activity, personal satisfaction, and achievement.2 The ability to perform tasks in multiple domains is a component of health-related quality of life. 3 Thus, it is important for people with chronic illnesses that eventually progress to full disability to maintain employment for as long as desired, and is feasible. Some of these conditions have a prodromal period, when changes in the person’s day-to-day function are subtle, but eventually cross a threshold when a clinical diagnosis is made. Huntington disease (HD) is a progressive neurodegenerative disease that leads to severe impairment of cognitive, physical, and behavior functions that eventually interfere with work functioning. Clinical diagnosis is based on the presence of distinctive motor signs. 4 The age of onset of motor signs is associated with the length of the gene expansion that causes HD5 and typically occurs between 30–50 years old, 4 the age range when most people are at the peak of their earning potential. 6
Although people are usually not clinically diagnosed until they exhibit distinctive motor signs, 7 subtle changes in cognition 8–10 and motor function 11 can be detected up to 15 years prior to diagnosis.12 These changes are accompanied by structural and functional brain changes. 13,14 Thus, HD has a long prodromal period (prodromal HD). As signs and symptoms become more pronounced, both individuals and family members report increasing interference with work function. 15 These changes eventually result in reduced work hours or cessation of work for people with HD. Changes in work function ability might be one of the most reliable initial indicators of functional decline in persons with prodromal HD. 16 Therefore, psychometrically sound measures are needed to quantify work function for patients with prodromal HD. These measures may be useful in assisting workers with prodromal HD and their employers to adapt work demands to fit the person’s level of health and day-to-day function and to examine the effect of these adaptations.
Current scales do not adequately measure self-assessment of skills needed to complete one’s employment responsibilities in this population and thus cannot detect early declines in work function. The Unified Huntington’s Disease Rating Scale (UHDRS) contains a Total Functional Capacity (TFC) scale developed to be used with individuals after they receive a clinical HD diagnosis. The scale includes items that address ability to work, 16 but it does not identify specific work-related functions that are most affected in prodromal HD. An assessment of 486 people in the prodromal period of HD found that work and managing finances were the most common function limitations reported in this sample. However, despite these findings, over 88% of participants scored at the ceiling on these measures at baseline, and new measures that are sensitive to real-life function are needed. 17 Others are developing measures that address functioning in day-to-day activities that include work but are not specific to work function. 18 The HDWF instrument was developed to measure workers’ and companions’ perceptions of role limitations and effort for job performance in prodromal HD. Although employer data may be a preferred indicator of work function, this is not feasible for some people with prodromal HD, as the potential for genetic discrimination is an important concern for this population. 19
The instrument was created based on Federal Drug Administration guidelines 20 for patient-reported outcome (PRO) measures in order to make it potentially useful for future clinical trials. A PRO measure is “any report of the status of a patient’s health condition that comes directly from the patient without the interpretation of the patient’s responses by a physician or anyone else,” 20 (p. 2). A collateral, or proxy measure of work function, to be completed by companions of persons with prodromal HD, was also created. Collateral data may be useful as a second source of data in prodromal HD due to the possibility of impaired insight in some people with prodromal HD. 21,22 The determination of inter-rater reliability with information from a proxy is consistent with assessments of function or disability in people with other neurologic conditions including stroke 23 and Alzheimer disease. 24
The purpose of the present study was to create a measure, the HD Work Function (HDWF) survey that may be useful for clinical and workplace assessments, which captures perceptions of work function as reported by individuals with prodromal HD and their companions. The long-term goal for this effort is to develop a measure that may be useful for longitudinal documentation of change in work function over the prodromal period of HD. The aims are to 1) develop a measure that focuses on work role limitations and effort, two components of work function that may be affected by cognitive, behavioral, and motor changes in people with prodromal HD; and 2) determine internal consistency, inter-rater reliability, content, construct, and convergent validity of the measure.
METHODS
The study was approved by the University of Iowa IRB and all procedures to protect human subjects were followed throughout the study. The HDWF survey was created using the first three of four PRO instrument development and modification processes: 20 1) identify concepts and develop conceptual framework; 2) create instrument; 3) assess measurement properties; 4) modify instrument. Methods proceeded in three parts. First, instrument development methods included identifying concepts and developing the conceptual framework through collection of data from individuals with prodromal HD and their companions, and review of the literature. Second, items were evaluated for content validity by experts and by people with prodromal HD and their companions. Third, internal consistency, inter-rater reliability with companions, convergent validity, and the ability of the instrument to distinguish people with prodromal HD from those who don’t have prodromal HD were analyzed.
Identify Concepts and Develop Conceptual Framework
The first stage in creating a PRO measure is to identify the concepts and domains to be measured. 20 This includes: identifying the population and how the measure will be applied in this population; identifying appropriate content to measure intended constructs; and identifying a hypothesized structure for how content categories are to be related. This procedure is consistent with standard survey and test development methodology. 25 For the purposes of creating the HDWF survey, the work role limitation component of work function was defined as the cognitive, behavioral, and physical changes that limit the ability to perform expected tasks related to paid or unpaid work. Data triangulation—the comparison of data from the prodromal HD literature, existing measures, and focus groups and interviews—was used to determine the appropriate content to be measured on the HDWF survey. 20,26
Review of the Prodromal HD Literature
Researchers report changes in cognition and behavior for persons with prodromal HD, including executive function, 27 memory, 28 psychomotor processing, 11 and moodiness and depression, 29,30 prior to clinical diagnosis. 12 Data from persons with prodromal HD and their family members suggest that work function may be compromised. 31 Beglinger and colleagues 16 reported that up to two thirds of persons with prodromal HD reported some occupational decline as measured by The Total Functional Capacity Scale32 and the Functional Assessment Scale; 33 however, those measures were not able to identify specific areas of decline. The hypothesized structure of the HDWF reflected motor, cognitive, and psychiatric or behavioral manifestations that eventually are apparent in Huntington disease. This structure is consistent with the framework for the functional measure, UHDRS, used in diagnosed HD, in which progressive deterioration of motor and cognitive abilities, and development of psychiatric symptoms contribute to loss of day-to-day function.
Review of Existing Measures
Existing measures of functional status for people with HD were developed as clinical rating scales. The Total Functional Capacity Scale contains one item on occupation, with scoring as “unable,” “marginal work only,” “reduced capacity for job” or “normal.” 32 The Functional Assessment Survey includes questions on accustomed work and volunteer work. 33 Neither measure is sufficient to assess work-related function in the workplace, or functional outcomes in clinical trials. 17 A literature search was conducted to identify other measures that address work function. Of the 16 citations found, we extensively reviewed three that address work function and health: the Endicott Work Productivity Scale (EWPS), 34 the World Health Organization Health and Work Performance Questionnaire, 35 and the Work Limitations Questionnaire. 26 The EWPS is a self-report measure administered to patients for the purpose of determining how the individual’s specific medical condition impacts their ability to complete daily work tasks. The Health Performance Questionnaire is a self-report measure that takes job performance, absence, and work-related accidents into account to estimate the cost of health problems in the workplace. The Work Limitations Questionnaire is intended to measure work ability as a function of health status. While each measure addresses some aspects of work function, no single measure addresses motor, behavioral/psychiatric, cognitive domains, and compensation efforts that reflect work function among people with prodromal HD. Thus, a comprehensive measure of work function that captures all the domains of possible work function impairment in prodromal HD was needed.
Focus Groups and Interviews
A semi-structured interview guide was developed after reviewing literature 12,16,36 related to prodromal HD, work function in other chronic illnesses, 37–39 and existing work function measures. Focus group participants were recruited at an annual Huntington’s Disease Society of America meeting by providing a description of the study, and inviting people who had the gene mutation but had not received a diagnosis of HD, and their companions to participate. Eight persons and three companions participated. Topics discussed included perspectives and experiences regarding maintaining their work. Audio-recorded data were transcribed verbatim and analyzed along with focus group assistant’s field notes to identify common themes. 40
Subsequent to the focus groups, individuals with prodromal HD and companions were recruited from the HD Registry maintained by the University of Iowa HD Center for Excellence. Participants at varying time points from predicted onset of clinical diagnosis based on age and CAG repeat 41 were selected in order to enhance the generalizability of the data. Nine persons with prodromal HD, or who were recently diagnosed, and eight of their companions participated in telephone interviews. Occupations of these participants were in the same categories as the prior participants, with one person who was an active volunteer. Interviews ranged in length from 10 to 30 minutes. Data saturation 42 was reached after 17 interviews. Participants were asked to describe any changes in work function related to cognitive, motor, or behavioral changes they had noticed in themselves or their companions with prodromal HD. Interviews were audio recorded and transcribed verbatim.
Procedures for developing the measure included: generating items; choosing an administration method, recall period, and response scales; drafting instructions and formatting the instrument; and drafting procedures for scoring and administration.20 Potential items were drafted for each domain based on comments, concepts, or topics that were identified from the focus groups and interviews. Whenever possible, items and response options were written to resemble participants’ wording as closely and accurately as possible. A glossary of quotes from interviews was kept in a log.
Create Instrument
A provisional form of the HDWF was created based on the literature review, focus group, and interview results. Content identified during focus groups and interviews concerning work function comprised four domains — motor, behavioral, cognitive, and compensatory strategies. These domains are consistent with the prodromal HD literature and with two of four potential dimensions of work function: work role limitations, and extra effort required by the worker to remain productive. 1 See Table 1 for the domains and participant comments identified from analyses of focus group and interview data.
TABLE 1.
Symptom Domains Supported by Narrative Data
| SYMPTOM DOMAIN Examples | NARRATIVE DATA |
|---|---|
| Cognitive | |
|
Difficulty multitasking I can do more than one thing at a time without getting confused |
I can get stressed out if I’ve got…two things [I have to do] [010] [H]e’s failed to multi-task [111] |
| Motor | |
|
Loss of strength: weakness I drop things because I lose my grip on them |
My wrists and ankles are not strong as they used to be. I have a fear of going up and down ladders because my ankles are not strong [006] Like a 15 year old learning how to drive a car [001] …she’s becoming quite uncoordinated, spilling stuff, dropping stuff [104] |
| Behavior | |
|
Irritability I become irritated when things at work don’t go as I wish I am often impatient with the people I work with |
Things would upset me more than they used to [019] I would say she has gotten moodier…you know that’s another tough one too, cause she’s having a terrible menopause….she gets much more stressed out like easier, more so than I do [110] |
| Compensatory Strategies | |
|
List making/visual reminders If I don’t make “to-do lists,” I forget things |
I have to make notes while I’m talking to someone or I’ll forget. Sticky notes all over the place [004] [R]ight before he stopped working, he would…keep a pad of paper by the phone, and you know, write things down and double check things a lot more just to make sure that he was not forgetting anything [111] |
The team determined what percentage of total items should be linked to each domain based on the frequency with which each domain appeared in the focus groups, interviews, and the literature. Although roughly evenly distributed, fewer motor changes were reported than in other domains; this was expected because the onset of classic motor changes (e.g., chorea, dystonia, involuntary movements) 7 would indicate that the person is no longer in the prodromal phase of HD. Thus, the items were distributed in the following way: six behavior items, six cognitive items, five compensatory strategy items, and four motor items.
The provisional form of the HDWF survey consisted of 21 items, each linked to one of four domains. The HDWF consists of two sections: a demographics section and the measurement device. The survey follows a 7-point Likert-type format, with scale descriptors provided only at both extremes (“Not at all like me” and “Very much like me”) to allow enough points to capture change in work function if used as a repeated measure, yet not so many as to introduce random noise. Some items were written in order that a response on the high end of the scale represents high-quality work function (e.g., “I rarely feel tired when I’m supposed to be working”). Other items were written in order that a response on the high end of the scale represents low-quality work function (e.g., “I am often impatient with the people I work with”). The latter items are reverse scored so that higher scores on the HDWF indicate higher levels of work function. The total score is then computed as the sum of all the responses across all items.
The use of negative language was avoided. Thus, “I prefer to keep to myself rather than working or socializing with others” was used in place of “I don’t like to work or socialize with others.” This variation in perspective and the avoidance of negative language where possible was used to control for respondents who tend to answer survey questions according to a specific response set, such as always agreeing with an item, always choosing a response at an extreme value of the scale, or always choosing a response near the center of the scale. 43 See Table 2 for sample items. The instrument is designed to provide information from people who have paid employment, but is also suitable for those who perform volunteer work, are students, or homemakers.
TABLE 2.
Sample HDWF Survey Items—Worker Version
| HDWF Survey Item | Not at all like me |
Very much like me |
|||||
|---|---|---|---|---|---|---|---|
| If I don’t use reminders such-as “to, list” I forget things | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| It is difficult for me to get back on track when I have been distracted | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| I am on time for appointments and meeting | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| I prefer to keep to myself rather than working or socializing with others | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
Assess Measurement Properties
Assessment of measurement properties included content validity determination of the provisional HDWF that was assessed via expert evaluation. After modifications were made based on results of the expert evaluation, assessment of measurement properties of the 20-item final version of the HDWF survey included cognitive interviews within and outside the US, internal consistency, inter-rater reliability, convergent validity, and ability of the measure to distinguish those with prodromal HD from those who do not have prodromal HD.
Content Validity: Expert Evaluation
Six content experts were asked to evaluate the provisional HDWF survey in order to provide content-related validity data for perceived role limitation components of work function. Four evaluators had expertise in HD; one was an expert in occupational health; and one was an expert in occupational human resources. Experts evaluated each item individually and the survey as a whole. Experts were asked to rate if the item was worded appropriately, if it possessed the ability to capture deterioration in work function, and if it was important. Response scale ranged from 1–5, with a 1 indicating “No,” a 3 indicating “Maybe” and a 5 indicating “Yes.” Experts also selected which domain or domains (identified previously via focus groups, interviews, and prodromal HD literature review) they thought each item was measuring; and if experts believed items measured more than one domain, they were asked to rank domains in order of representativeness. This allowed them to analyze the total HDWF survey in terms of content balance and representativeness. Finally, experts had the opportunity to make suggestions for improving each item.
The comprehensiveness evaluation section of the expert survey review consisted of seven open-ended questions; experts provided suggestions concerning appropriate content balance, critical features not represented by the HDWF survey, items that should be added and/or deleted, format, length, and appropriateness of demographics items. Means, medians, and standard deviations on the expert evaluation item review were used as indicators of item quality.
Responses to the question regarding which domain(s) the items belonged were analyzed to provide an indication of congruence between the domain(s) the experts thought the item was measuring and the domain(s) the instrument design team thought the item was measuring. Open-ended questions were analyzed in conjunction with the quantitative analysis in order to modify existing items on the HDWF survey. Three items received a median rating of less than four on the question, “is the wording appropriate for this item.” These items were revised using suggested wording in the narrative response section of the expert ratings.
Content Validity: Cognitive Interviews
Content validity of the final 20-item HDWF survey was evaluated using cognitive interview techniques. 20 A cognitive interview guide was constructed based on established procedures for cognitive interviewing. 44 People with prodromal HD and companions who either had previously participated in the focus groups or interviews, or were new to the study were invited to participate in cognitive interviews via telephone. Participants were invited from the US and in three English-speaking countries: Canada, England, and Australia. Participants in the US were first informed about the purpose and intended use of the instrument and were mailed copies of the HDWF. Participants completed the HDWF survey prior to the interview and recorded time to complete the survey. Participants outside the US participated in individual or group cognitive interviews of participants with prodromal HD or companions that began with participants completing the HDWF then responding to interview items.
Participants provided feedback on their understanding of both individual items and response items, and whether they considered items to be relevant to themselves or their partners with prodromal HD, 45 or, in the case of participants outside the US, whether items were culturally relevant. They were asked to paraphrase items in their own words to clarify discrepancies between what the items were designed to measure and what the subjects thought the items were trying to measure. Item-specific probes were issued to ensure that specific words or phrases in each item were correctly interpreted. For example, “What does the term ‘socializing’ mean to you in this statement?” Participants stated whether the directions were clear and if they understood the scale. The interviewer took notes and results were organized into a cognitive interview grid that included: paraphrase, lexical problems, inclusion/exclusion problems, temporal problems, logical problems, and computational problems. 46
Following the cognitive interviews, the final 20-item HDWF was administered to people with prodromal HD, their companions, and at-risk people who tested negative for the HD gene-mutation and their companions as a component of the PREDICT-HD study. PREDICT-HD is a longitudinal observation study of people who elected to complete predictive testing for the CAG expansion in the gene for HD, but do not have a clinical HD diagnosis. 17 Participants self-administered the measure with explanation by a researcher or clinician.
Convergent Validity
Convergent validity of the HDWF was evaluated using the PREDICT-HD samples by computing Pearson correlation coefficients between the HDWF and other measures currently in the PREDICT-HD battery. Participants self-administered the measure with explanation by a researcher or clinician. Data collection was conducted either with a paper and pencil survey, or with a computer tablet.
Convergent validity was expected with the EWPS measure, 34 an instrument designed specifically to assess work function. Convergent validity was expected with the Everyday Cognition (EC0g), a measure of everyday cognitive function, 47 and the Social, Leisure, and Family Relationships (SAS-SR), a measure of social adjustment. 48 Each of these measures addresses components of day-to-day function relying on cognitive and social interaction skills. Lower scores on each of these measures indicate better levels of function. While work function and social adjustment are different constructs, some components may overlap. The SAS-SR has been used as a measure of work role limitations and normally includes work-related items.1 In the PREDICT-HD study, from which the sample was recruited for this study, 11 items from the SAS-SR are used that reflect day-to-day social interactions.
Ability to Distinguish Prodromal HD from Other Populations
The ability of the measure to distinguish between scores for persons with prodromal HD and gene mutation negative participants in the PREDICT-HD sample and worker scores in a normative sample was assessed using analysis of variance and analysis of covariance while controlling for age and gender, with follow-up t-tests. Effect sizes were calculated using Cohen’s d. Data were obtained from a community sample to help establish normative values for the HDWF. An advertisement to recruit employed members of the community and their companions was run for three consecutive days (Sunday, Monday, Tuesday) and two days in a single week (Sunday, Wednesday) one month later. The newspaper in which the ad ran is circulated in two counties in a Midwestern state with a circulation of over 60,000 for the daily edition and 75,000 for the Sunday edition. The ad invited people to participate if they were between ages 18–50, had worked for at least two years, and had a spouse or partner who was also willing to complete a survey. Pairs were asked to select one person to complete the survey as the worker, and the other to complete the survey as the companion to evaluate the partner’s work function.
Inter-rater Reliability
Correlation analyses and paired t-tests were conducted to compare worker and companions as proxy ratings. The observed correlations provide an indication of the relative congruence between each pair of respondents. The paired t-test results provide an indication of whether workers as a group tended to rate their own work function higher or lower than companions’ ratings. Therefore, the paired t-test provides an indication of group differences, whereas the observed correlations provide an indication of congruence between worker and companion ratings. These analyses were conducted separately for the normative sample and for the PREDICT-HD samples.
RESULTS
Aim 1: Instrument Development
The final HDWF contains 20 items. The response categories are on a seven point Likert scale with verbal anchors only at the lowest end (1), “not at all like me”, and at the highest end (7) “very much like me”. The instrument includes a checklist for the employment level that best matches the worker’s current situation, and what workplace accommodations, if any, have been made. Higher scores on the HDWF indicate better function.
Aim 2: Measurement Properties
Content Validity: Expert Evaluation
Twelve of the 21 items on the provisional HDWF survey received an overall median score of 5.0, on a scale of 1 (lowest)-5 (highest), indicating experts rated these items highly according to the three criteria (appropriate wording, ability to detect change, and importance for measuring work function). Means and standard deviations were heavily subject to outliers in this study; thus median values were mostly used to indicate item quality. Only two of the 21 items received a combined median score less than 4.0.
Across the three criteria, items received the lowest median score values on the criterion “ability to detect change.” This was due to the fact that some items contained an internal comparison (e.g., “I can do my work as well as I could a year ago”). As a result, all items were changed to be stationary as opposed to incorporating an internal comparison (e.g., “I do my work as well as I ever could”) to capture work function for the participant at a specific point in time.
For 10 items, experts selected the intended domain over 50% of the time. For six other items experts selected the intended domain over 40% of the time. For five items, experts selected other domains more often than the intended domain. This appeared to be related to placing items intended as compensatory strategies into the domains in which the strategy was being used (for example, use of “to-do” lists was rated most often as belonging to the cognitive domain. Nine wording changes were made based on the expert validation. One item was deleted the measure used in the cognitive interviews included 20 items, with a total possible score range from 20–140. The 20-item final HDWF version was used for all further assessments of measurement properties.
Content Validity: Cognitive Interviews
The sample for cognitive interviews is shown in Table 3. In general, participants stated the HDWF items made sense, were relevant to their situations, and they were able to select responses for each item. The survey took all participants, except one, 10–15 minutes or less to complete. Suggestions to improve the clarity of items included rewording the item about using “to-do lists” to be inclusive enough of other visual reminders, and replacing the word multitask, because the word had negative connotations to them. Participants outside the US did not identify cross-cultural language problems in the survey, indicating it is appropriate for use in English-speaking countries outside the US.
TABLE 3.
Cognitive Interview Sample
| USA | Canada | UK | Australia | Total | |
|---|---|---|---|---|---|
| Participant | 3 | 4 | 8 | 3 | 18 |
| Companion | 5 | 4 | 4 | 2 | 15 |
Pilot Study Sample
A total of 238 individuals with prodromal HD and 185 companions comprised the PREDICT-HD sample. In addition, 89 people who were gene mutation negative and 70 companions of people who were gene mutation negative who are enrolled in PREDICT-HD as comparison subjects also completed the HDWF measure.
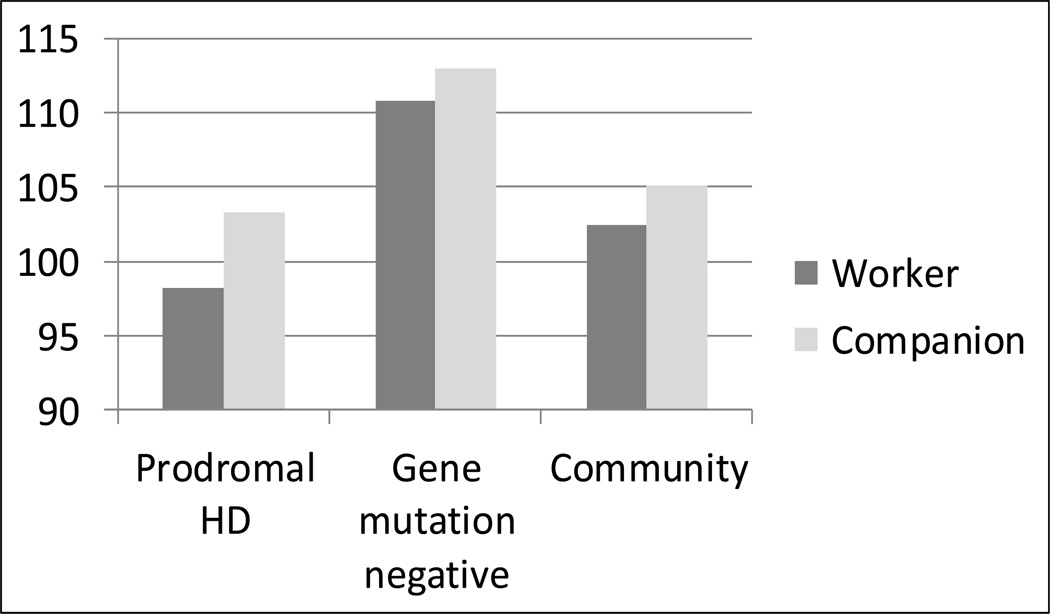
The mean total HDWF score for people with prodromal HD in the PREDICT-HD sample was 98.27 (SD = 18.59; range = 42–139); mean HDWF companion scores for people with prodromal HD was 103.27 (SD = 20.51; range = 50–136). The paired t-test was significant (t184 = 4.26; p < 0.0001; d = 0.44). The mean total HDWF score for gene mutation negative comparison participants was 110.84 (SD = 12.94; range = 71–134); mean HDWF companion scores for gene mutation negative participants was 112.94 (SD = 14.82; range = 80–139). The paired t-test was not significant (t69 = 0.88; p = 0.38). Mean HDWF scores for persons with prodromal HD were lower than gene mutation negative participants (t325 = −5.87; p < 0.0001; d = −0.73). Companions of persons with prodromal HD rated partners lower on the HDWF than companions of gene mutation negative participants (t253 = −3.61; p < 0.001; d = −0.51). (Figure 1)
FIGURE 1.
Comparison of HDWF Total Score across Pilot Samples (possible range = 20–140)
The normative sample was comprised of 108 individuals (54 pairs) from the community. For three pairs, both the worker and the companion completed the survey in view of their own work function as opposed to the companion completing the survey in view of the worker’s work function. Thus, aspects of the data analysis which focus on worker and companion congruence only include 102 participants (51 pairs). Fifteen respondents had one or more missing values (<0.5% of all data). Missing data were replaced through single imputation using the EM-algorithm.49
Mean total score for workers was 102.40 (SD = 12.79; range = 71–127), and for companion ratings mean total score was 105.06 (SD = 12.96; range = 65–130). The correlation between worker and companion scores for the normative sample was low, but statistically significant (r = 0.28, p < 0.05), whereas the paired t-test was not statistically significant (t50 = 0.96, p < 0.35). These results indicate that, as a group, workers do not significantly rate their own work function as being significantly better or worse than companions do, but worker and companion scores might not necessarily be used interchangeably.
Internal Consistency
Internal consistency coefficients for the normative sample (workers and companions combined) were calculated to test the reliability of the HDWF: Cronbach’s alphas were calculated for each of the four domains well as for the measure as a whole. Internal consistency for the 20 items of the HDWF survey was 0.77. Cronbach’s alphas for the motor, behavioral, cognitive, and compensatory strategies domains were 0.68, 0.54, 0.69, and 0.22, respectively. The coefficients for the subscales were expected to be lower than the overall coefficient due to the small number of items per subscale. We also anticipated the alpha for the compensatory strategies subscale would be low because items relate to strategies used to compensate for deficits in the other domains and therefore were less internally homogeneous.
Ability to Distinguish between Prodromal HD and not Prodromal HD and Normative Sample
The analysis of variance to test for differences between means of workers’ HDWF scores in the normative and PREDICT-HD samples was significant (F2, 375 = 18.37; p < 0.0001)(Fig. 1). Follow-up t-tests between the groups found a significant difference between PREDICT-HD workers with prodromal HD and gene mutation negative workers (t375 = −6.05; p < 0.001; d = 0.77) and between normative workers and gene mutation negative workers (t375 = 2.87; p = 0.004; d = 0.80); however, the difference between the community sample of workers and the prodromal HD workers was not significant (t375 = 1.60; p = 0.11; d = 0.21). Differences between means of workers’ HDWF scores in the normative and PREDICT-HD samples remained significant after adjusting for age and gender using analysis of covariance (F2, 373 = 19.97; p < 0.0001). The difference between the community sample of workers and the prodromal HD workers was still not significant (t373 = 1.38; p = 0.17).
Inter-rater Reliability
The correlation between PREDICT-HD worker and companion scores was statistically significant (r = 0.58, p < 0.0001). The correlation between gene mutation negative workers and companion scores was also significant (r = 0.29; p = 0.01).
Convergent Validity
Selected items from the ECog scale,47 the EWPS,34 and the SAS-SR,50 which had been edited for grammar to fit this population in the PREDICT-HD study, were completed by this sample. Scores for persons with prodromal HD were strongly negatively correlated with 34 items from the ECog scale (r = −0.70; p < 0.0001) and moderately correlated with 10 items from the EWPS (r = −0.56; p < 0.0001). Scores on the HDWF for persons with prodromal HD were moderately correlated with 11 items adapted from the SAS-SR (r = −0.29; p < 0.0001).
HDWF scores for prodromal HD companions were significantly correlated with the SAS-SR (r = −0.25; p = 0.0006). For gene mutation negative workers there were moderate correlations between the HDWF total and the ECOG (r = −0.47; p < 0.0001) and the 10 items from the EWPS (r = −0.35; p = 0.003). There were no statistically significant correlations between gene mutation negative companion scores on the HDWF and the other measures.
DISCUSSION
The purpose of this study was to develop and analyze selected psychometric properties of a new instrument to detect work function ability related to perceived role limitation and effort, as provided by self report by people with prodromal HD and their companions. Testing of this measure in the current analyses demonstrates the instrument has acceptable internal consistency and inter-rater reliability between people with prodromal HD and their companions. The measure has acceptable convergent validity with selected items from the ECog, 47 EWPS, 34 and SAS-SR. 48 The measure distinguished between those with prodromal HD and those who have a family history of HD but do not have the gene mutation. The measure did not distinguish those with prodromal HD in the sample in this study from a community population. The lack of difference between prodromal participants and normative samples may reflect to inclusion of people in all phases of prodromal HD in this sample. People with prodromal HD can be categorized according to variables including the length of the trinucleotide repeat and by age.51 The stage of prodromal HD, and other factors, such as employer accommodation, may be associated with changes in work function in this population. Studies that examine variables influencing work function over time are needed to clarify these variables.
The HDWF appears to distinguish differences in work function between people with prodromal HD and gene mutation negative comparison participants. Studies are needed to document sensitivity to change over time to determine if the measure will be useful for clinical trials. The HDWF also appears to be effective as a collateral measure of work function since companions of persons with prodromal HD were able to provide adequate proxy data on partners’ work function. This may become more important in longitudinal studies of people with prodromal HD where insight may become impaired among some prodromal HD participants as they approach diagnosis. 21,22
Clinical Significance
It is clear that a prodromal period exists in HD, although the pattern of cognitive, behavioral, and motor decline is difficult to predict for individuals.12 Furthermore, capacity for self-insight may be limited in the later stages of prodromal HD. 21 The HDWF can be a useful addition to the existing functional assessment options when documentation of perceptions of the individual and proxy regarding role limitations and changes in effort are needed for workplace accommodations or clinical assessments. The management of disclosure of prodromal HD status to employers is a sensitive topic for which persons with prodromal HD may fear loss of their jobs or other forms of workplace discrimination, as reported by approximately 6% of persons in an international sample.19 In cases where workplace accommodations can be made, some individuals with prodromal HD report that they can continue to be productive in their work environment. 18
Potential for Use in Other Neurodegenerative Disorders
Measures of work function, such as the HDWF, may be useful in other slowly progressing neurodegenerative disorders. With brain imaging and other biomarkers of Alzheimer disease progression, there is the potential to document presymptomatic Alzheimer disease, as well as monitor outcomes of treatments. 52 However, limitations of current data based evidence include short duration, variability in quality of measures, and cross sectional studies. 53 This measure may be of use for monitoring work function in early stages of other neurodegenerative conditions such as multiple sclerosis that affect younger individuals. A presymptomatic period is recognized in Parkinson’s disease. Treatments are available which may result in early symptom improvement and positive influences on overall quality of life. Detection of early changes is a priority and will be critical when neuroprotective therapies become available. 54
In conclusion, the HDWF is a brief self-administered survey for people with prodromal HD and their companions to document work role function and limitations. It may offer a useful model for documenting functional changes in the workplace for people in the earliest stages of neurodegenerative conditions in which there is anticipated cognitive decline, but for which new therapies may alter the current course of these conditions. The HDWF can be obtained by contacting Dr. Jane Paulsen, (jane-paulsen@uiowa.edu).
Acknowledgments
Funding: This research is supported by the National Institutes for Health, National Institute of Neurological Disorders and Stroke (NS40068) and CHDI Foundation, Inc. We thank the PREDICT-HD sites, the study participants, and the National Research Roster for Huntington Disease Patients and Families. See the Appendix for the full list of PREDICT-HD Investigators, Coordinators, Motor Raters, Cognitive Raters.
PREDICT-HD Investigators, Coordinators, Motor Raters, Cognitive Raters
Active: September 2010–August 2011
Thomas Wassink, MD, Stephen Cross, BA, Mycah Kimble, BA, Patricia Ryan, MSW, LISW, MA, Jessica Wood, MD, PhD, Eric A. Epping, MD, PhD, and Leigh J. Beglinger, PhD (University of Iowa, Iowa City, Iowa, USA);
Edmond Chiu, MD, Olga Yastrubetskaya, PhD, Joy Preston, Anita Goh, D.Psych, Chathushka Fonseka, Stephanie Antonopoulos and Samantha Loi (St. Vincent’s Hospital, The University of Melbourne, Kew, Victoria, Australia);
Phyllis Chua, MD, and Angela Komiti, BS, MA (The University of Melbourne, Royal Melbourne Hospital, Melbourne, Australia);
Lynn Raymond, MD, PhD, Rachelle Dar Santos, BSc, Kimberley Carter, BSc, and Joji Decolongon, MSC, CCRP (University of British Columbia, Vancouver, British Columbia, Canada);
Adam Rosenblatt, MD, Christopher A. Ross, MD, PhD, Barnett Shpritz, BS, MA, OD, Nadine Yoritomo, RN and Claire Welsh (Johns Hopkins University, Baltimore, Maryland, USA);
William M. Mallonee, MD, Greg Suter, BA, and Judy Addison (Hereditary Neurological Disease Centre, Wichita, Kansas, USA);
Ali Samii, MD, and Alma Macaraeg, BS (University of Washington and VA Puget Sound Health Care System, Seattle, Washington, USA);
Randi Jones, PhD, Cathy Wood-Siverio, MS, Stewart A. Factor, DO, and Claudia Testa, MD, PhD (Emory University School of Medicine, Atlanta, Georgia, USA);
Roger A. Barker, BA, MBBS, MRCP, Sarah Mason, BSC, Anna Goodman, PhD, and Anna DiPietro (Cambridge Centre for Brain Repair, Cambridge, UK);
Elizabeth McCusker, MD, Jane Griffith, RN, Clement Loy, MD, and David Gunn, BS (Westmead Hospital, Sydney, Australia);
Bernhard G. Landwehrmeyer, MD, Michael Orth MD, PhD, Sigurd Süβmuth, MD, RN, Katrin Barth, RN, and Sonja Trautmann, RN (University of Ulm, Ulm, Germany);
Kimberly Quaid, PhD, Melissa Wesson, MS, and Joanne Wojcieszek, MD (Indiana University School of Medicine, Indianapolis, IN);
Mark Guttman, MD, Alanna Sheinberg, BA, and Irita Karmalkar, BSc (Centre for Addiction and Mental Health, University of Toronto, Markham, Ontario, Canada);
Susan Perlman, MD, Brian Clemente, and Arik Johnson, PsyD (University of California, Los Angeles Medical Center, Los Angeles, California, USA);
Michael D. Geschwind, MD, PhD, Jon Gooblar, BA, and Gail Kang, MD (University of California San Francisco, California, USA);
Tom Warner, MD, PhD, Maggie Burrows, RN, BA, Marianne Novak, MD, Thomasin Andrews, MD, BSC, MRCP, Elisabeth Rosser, MBBS, FRCP, and Sarah Tabrizi, MD, PhD (National Hospital for Neurology and Neurosurgery, London, UK);
Anne Rosser, MD, PhD, MRCP, Kathy Price, RN, and Sarah Hunt, BSc (Cardiff University, Cardiff, Wales, UK);
Frederick Marshall, MD, Amy Chesire, LCSW-R, MSG, Mary Wodarski, BA, and Charlyne Hickey, RN, MS (University of Rochester, Rochester, New York, USA);
Oksana Suchowersky, MD, FRCPC, Sarah Furtado, MD, PhD, FRCPC, and Mary Lou Klimek, RN, BN, MA (University of Calgary, Calgary, Alberta, Canada);
Peter Panegyres, MB, BS, PhD, Joseph Lee, and Steve Andrew (Neurosciences Unit, Graylands, Selby-Lemnos & Special Care Health Services, Perth, Australia);
Joel Perlmutter, MD, Stacey Barton, MSW, LCSW, and Amy Schmidt (Washington University, St. Louis, Missouri, USA);
Zosia Miedzybrodzka, MD, PhD, Daniela Rae, RN, and Mariella D’Alessandro, PhD (Clinical Genetics Centre, Aberdeen, Scotland, UK);
David Craufurd, MD, Ruth Fullam, BSC, Judith Bek, PhD, and Elizabeth Howard, MD (University of Manchester, Manchester, UK);
Pietro Mazzoni, MD, PhD, Karen Marder, MD, MPH, and Paula Wasserman, MA (Columbia University Medical Center, New York, New York, USA);
Rajeev Kumar, MD and Diane Erickson, RN (Colorado Neurological Institute, Englewood, Colorado, USA);
Vicki Wheelock, MD, Terry Tempkin, RNC, MSN, Lisa Kjer, MSW, and Kathleen Baynes, PhD (University of California Davis, Sacramento, California, USA);
Joseph Jankovic, MD, Christine Hunter, RN, CCRC, and William Ondo, MD (Baylor College of Medicine, Houston, Texas, USA);
Wayne Martin, MD, Pamela King, BScN, RN, Marguerite Wieler and Satwinder Sran, BSC (University of Alberta, Edmonton, Alberta, Canada);
Anwar Ahmed, PhD, Stephen Rao, PhD, Christine Reece, BS, Janice Zimbelman, PhD, PT, Alexandra Bea, BA, Emily Newman, BA, Alex Bura, BA, Lyla Mourany, and Juliet Schulz (Cleveland Clinic Foundation, Cleveland, Ohio, USA).
Steering Committee
Jane Paulsen, PhD, Principal Investigator, Eric A. Epping, MD, PhD, Hans Johnson, PhD, Megan Smith, PhD, Janet Williams, PhD, RN, FAAN, Leigh Beglinger, PhD, Jeffrey D. Long, PhD, James A. Mills, MS (University of Iowa, Iowa City, IA); Elizabeth Aylward, PhD (Seattle Children's Research Institute, WA); Kevin Biglan, MD (University of Rochester, Rochester, NY); Blair Leavitt, MD (University of British Columbia, Vancouver, BC, Canada); Marcy MacDonald, PhD (Massachusetts General Hospital); Martha Nance, MD (Hennepin County Medical Center, Minneapolis, MN); and Cheryl Erwin, JD, PhD (University of Texas Medical School at Houston).
Scientific Sections
Bio Markers: Blair Leavitt, MDCM, FRCPC (Chair) and Michael Hayden, PhD (University of British Columbia); Stefano DiDonato, MD (Neurological Institute “C. Besta,” Italy); Ken Evans, PhD (Ontario Cancer Biomarker Network); Wayne Matson, PhD (VA Medical Center, Bedford, MA); Asa Peterson, MD, PhD (Lund University, Sweden), Sarah Tabrizi, MD, PhD (National Hospital for Neurology and Neurology and Neurosurgery, London); Beth Borowsky, PhD (CHDI); Andrew Juhl, BS, James A. Mills, MS, Kai Wang, PhD (University of Iowa); and David Weir, BSc (University of British Columbia).
Brain: Jean Paul Vonsattell, PhD (Chair), and Carol Moskowitz, ANP, MS (Columbia University Medical Center); Anne Leserman, MSW, LISW, Lynn Schaul, BA, and Stacie Vik, BA (University of Iowa).
Cognitive: Deborah Harrington, PhD (Chair), Gabriel Castillo, BS, Jessica Morison, BS, and Jason Reed, BS (University of California, San Diego), Michael Diaz, PhD, Ian Dobbins, PhD, Tamara Hershey, PhD, Erin Foster, OTD, and Deborah Moore, BA (Washington University Cognitive Science Battery Development); Holly Westervelt, PhD (Chair, Quality Control and Training, Alpert Medical School of Brown University), Jennifer Davis, PhD, and Geoff Tremont, PhD, MS (Scientific Consultants, Alpert Medical School of Brown University); Megan Smith, PhD (Chair, Administration), David J. Moser, PhD, Leigh J. Beglinger, PhD, Kelly Rowe, Gloria Wenman, and Danielle Theriault, BS (University of Iowa); Carissa Gehl, PhD (VA Medical Center, Iowa City, IA); Kirsty Matheson (University of Aberdeen); Karen Siedlecki, PhD (Fordham University); Marleen Van Walsem (EHDN); Susan Bonner, BA, Greg Elias, BA, Mary Gover, Rachel Bernier, and Melanie Faust, BS (Rhode Island Hospital); Beth Borowski, PhD (CHDI); Noelle Carlozzi (University of Michigan); Kevin Duff, PhD (University of Utah); Nellie Georgiou-Karistianis (St. Vincent’s Hospital, The University of Melbourne, Australia); Julie Stout, PhD (Monash University, Melbourne, Australia); Herwig Lange (Air-Rahazentrum); and Kate Papp (University of Connecticut).
Functional: Janet Williams, PhD (Chair), Leigh J. Beglinger, PhD, Anne Leserman, MSW, LISW, Eunyoe Ro, MA, Lee Anna Clark, Nancy Downing, RN, PhD, Joan Laing, PhD, Kristine Rees, BA, Michelle Harreld, BS, and Stacie Vik, BA (University of Iowa); Rebecca Ready, PhD (University of Massachusetts); Anthony Vaccarino, PhD (Ontario Cancer Biomarker Network); Sarah Farias, PhD (University of California, Davis); Noelle Carlozzi, PhD (University of Michigan); and Carissa Gehl, PhD (VA Medical Center, Iowa City, IA).
Genetics: Marcy MacDonald, PhD (Co-Chair), Jim Gusella, PhD, and Rick Myers, PhD (Massachusetts General Hospital); Michael Hayden, PhD (University of British Columbia); Tom Wassink, MD (Co-Chair) Eric A. Epping, MD, PhD, Andrew Juhl, BA, James Mills, MS, and Kai Wang, PhD (University of Iowa); Zosia Miedzybrodzka, MD, PhD (University of Aberdeen); and Christopher Ross, MD, PhD (Johns Hopkins University).
Imaging: Administrative: Ron Pierson, PhD (Chair), Kathy Jones, BS, Jacquie Marietta, BS, William McDowell, AA, Greg Harris, BS, Eun Young Kim, MS, Hans Johnson, PhD, and Thomas Wassink, MD (University of Iowa); John Ashburner, PhD (Functional Imaging Lab, London); Steve Potkin, MD (University of California, Irvine); and Arthur Toga, PhD (University of California, Los Angeles). Striatal: Elizabeth Aylward, PhD (Chair, Seattle Children's Research Institute). Surface Analysis: Eric Axelson, BSE (University of Iowa). Shape Analysis: Christopher A. Ross (Chair), MD, PhD, Michael Miller, PhD, and Sarah Reading, MD (Johns Hopkins University); Mirza Faisal Beg, PhD (Simon Fraser University). DTI: Vincent A. Magnotta, PhD (Chair, University of Iowa); Karl Helmer, PhD (Massachusetts General Hospital); Kelvin Lim, MD (University of Ulm, Germany); Mark Lowe, PhD (Cleveland Clinic); Sasumu Mori, PhD (Johns Hopkins University); Allen Song, PhD (Duke University); and Jessica Turner, PhD (University of California, Irvine). fMRI: Steve Rao, PhD (Chair), Erik Beall, PhD, Katherine Koenig, PhD, Michael Phillips, MD, Christine Reece, BS, and Jan Zimbelman, PhD, PT (Cleveland Clinic); April Bryant, Andrew Juhl, BS, Kathy Jones, and Gloria Wenman (University of Iowa).
Motor: Kevin Biglan, MD (Chair) (University of Rochester); Karen Marder, MD (Columbia University); Jody Corey-Bloom, MD, PhD (University of California, San Diego); Michael Geschwind, MD, PhD (University of California, San Francisco); Ralf Reilmann, MD and Zerka Unds (Muenster, Germany); and Andrew Juhl, BS (University of Iowa).
Psychiatric: Eric A. Epping, MD, PhD (Chair), Nancy Downing, RN, PhD, Jess Fiedorowicz, MD, Robert Robinson, MD, Megan Smith, PhD, Leigh Beglinger, PhD, James Mills, MS, Kristine Rees, BA, Michelle Harreld, BS, Adam Ruggle, Stacie Vik, BA, Janet Williams, PhD, Dawei Liu, PhD, David Moser, PhD, and Kelly Rowe (University of Iowa); Karen Anderson, MD (University of Maryland); David Craufurd, MD (University of Manchester); Mark Groves, MD (Columbia University); Anthony Vaccarino, PhD and Ken Evans, PhD (Ontario Cancer Biomarker Network); Hugh Rickards, MD (Queen Elizabeth Psychiatric Hospital); Eric van Duijn, MD (Leiden University Medical Center, Netherlands); Phyllis Chua (The University of Melbourne, Royal Melbourne Hospital); and Kimberly Quaid, PhD (Indiana University School of Medicine).
Core Sections
Statistics: Jeffrey D. Long, PhD, Ji-In Kim, PhD, James A. Mills, MS, Blair Harrison, MPH, Ying Zhang, PhD, Dawei Liu, PhD, Wenjing Lu, and Spencer Lourens (University of Iowa).
Recruitment/Retention: Martha Nance, MD (Chair, University of Minnesota); Anne Leserman, MSW, LISW, Nicholas Doucette, BA, Mycah Kimble, BA, Patricia Ryan, MSW, LISW, MA, Kelli Thumma, BA, Elijah Waterman, BA, and Jeremy Hinkel, BA (University of Iowa).
Ethics: Cheryl Erwin, JD, PhD, (Chair, McGovern Center for Health, Humanities and the Human Spirit); Eric A. Epping, MD, PhD Janet Williams, PhD, Nicholas Doucette, BA, Anne Leserman, MSW, LISW, James Mills, MS, Lynn Schaul, BA, and Stacie Vik, BA (University of Iowa); Martha Nance, MD (University of Minnesota); and Lisa Hughes, MEd (University of Texas Medical School at Houston).
IT/Management: Hans Johnson, PhD (Chair), R.J. Connell, BS, Karen Pease, BS, Ben Rogers, BA, BSCS, Jim Smith, AS, Shuhua Wu, MCS, Roland Zschiegner, Erin Carney, Bill McKirgan, Mark Scully, and Ryan Wyse (University of Iowa); Jeremy Bockholt (AMBIGroup).
Program Management
Administrative: Chris Werling-Witkoske (Chair), Greg Ennis, MA, Stacie Vik, BA, Karla Anderson, BS, Jennifer Rapp, BA, Sean Thompson, BA, Leann Davis, Machelle Henneberry, Ann Dudler, Jamy Schumacher, and Craig Stout (University of Iowa).
Financial: Steve Blanchard, MSHA, Kelsey Montross, BA, and Phil Danzer (University of Iowa).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none declared.
Contributor Information
Bradley Brossman, Boston College, Chestnut Hill, MA 02467.
Janet K. Williams, The University of Iowa, Iowa City, IA 52242.
Nancy Downing, The University of Iowa, Iowa City, IA 52242.
James A. Mills, University of Iowa, Iowa City, IA 52242.
Jane S. Paulsen, The University of Iowa, Iowa City, IA 52242.
References
- 1.Nieuwenhuijsen K, Franche RL, van Dijk FJ. Work functioning measurement: Tools for occupational mental health research. J Occup Environ Med. 2010;52(8):778–790. doi: 10.1097/JOM.0b013e3181ec7cd3. [DOI] [PubMed] [Google Scholar]
- 2.Warr P. Work values: Some demographic and cultural correlates. Journal of Occupational and Organizational Psychology. 2008;81(4):751–775. [Google Scholar]
- 3.Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. J Nurs Scholarsh. 2005;37(4):336–342. doi: 10.1111/j.1547-5069.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 4.Roos RA. Huntington's disease: A clinical review. Orphanet J Rare Dis. 2010;5(1):40. doi: 10.1186/1750-1172-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinsztein DC, Leggo J, Chiano M, et al. Genotypes at the GluR6 kainate receptor locus are associated with variation in the age of onset of huntington disease. Proc Natl Acad Sci U S A. 1997;94(8):3872–3876. doi: 10.1073/pnas.94.8.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bureau of Labor Statistics. [Accessed August/29, 2011];Earnings by age and sex. http://www.bls.gov/opub/ted/2010/ted_20101025.htm. Updated 2010.
- 7.Sturrock A, Leavitt BR. The clinical and genetic features of huntington disease. J Geriatr Psychiatry Neurol. 2010;23(4):243–259. doi: 10.1177/0891988710383573. [DOI] [PubMed] [Google Scholar]
- 8.Duff K, Beglinger LJ, Theriault D, Allison J, Paulsen JS. Cognitive deficits in Huntington's disease on the repeatable battery for the assessment of neuropsychological status. J Clin Exp Neuropsychol. 2009:1–9. doi: 10.1080/13803390902926184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulsen JS, Zhao H, Stout JC, et al. Clinical markers of early disease in persons near onset of huntington's disease. Neurology. 2001;57(4):658–662. doi: 10.1212/wnl.57.4.658. [DOI] [PubMed] [Google Scholar]
- 10.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of huntington's disease decades before diagnosis: The predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biglan K, Ross C, Langbehn D, et al. Motor abnormalities in premanifest persons with huntington's disease: The PREDICT-HD study. Movement Disorders. 2009;24(12):1763–1772. doi: 10.1002/mds.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulsen JS. Early detection of huntington's disease. Future Neurology. 2010;5(1):85–104. doi: 10.2217/fnl.09.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulsen JS. Functional imaging in huntington's disease. Experimental Neurology. 2009;216(2):272–277. doi: 10.1016/j.expneurol.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulsen JS, Nopoulos PC, Aylward E, et al. Striatal and white matter predictors of estimated diagnosis for huntington disease. Brain Res Bull. 2010;82(3–4):201–207. doi: 10.1016/j.brainresbull.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCabe MP, Roberts C, Firth L. Work and recreational changes among people with neurological illness and their caregivers. Disabil Rehabil. 2008;30(8):600–610. doi: 10.1080/09638280701400276. [DOI] [PubMed] [Google Scholar]
- 16.Beglinger LJ, O'Rourke JJ, Wang C, et al. Earliest functional declines in huntington disease. Psychiatry Res. 2010;178(2):414–418. doi: 10.1016/j.psychres.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulsen JS, Wang C, Duff K, et al. Challenges assessing clinical endpoints in early huntington disease. Mov Disord. 2010;25(15):2595–2603. doi: 10.1002/mds.23337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccarino AL, Sills T, Anderson KE, et al. Assessment of day-to-day functioning in prodromal and early huntington disease. PLoS Curr. 2011;3:RRN1262. doi: 10.1371/currents.RRN1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erwin C, Williams JK, Juhl AR, et al. Perception, experience, and response to genetic discrimination in huntington disease: The international RESPOND-HD study. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(5):1081–1093. doi: 10.1002/ajmg.b.31079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food and Drug Administration. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims. 2009 doi: 10.1186/1477-7525-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duff K, Paulsen JS, Beglinger LJ, et al. "Frontal" behaviors before the diagnosis of huntington's disease and their relationship to markers of disease progression: Evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci. 2010;22(2):196–207. doi: 10.1176/appi.neuropsych.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoth KF, Paulsen JS, Moser DJ, Tranel D, Clark LA, Bechara A. Patients with huntington's disease have impaired awareness of cognitive, emotional, and functional abilities. J Clin Exp Neuropsychol. 2007;29(4):365–376. doi: 10.1080/13803390600718958. [DOI] [PubMed] [Google Scholar]
- 23.Schlote A, Richter M, Wunderlich MT, et al. WHODAS II with people after stroke and their relatives. Disabil Rehabil. 2009;31(11):855–864. doi: 10.1080/09638280802355262. [DOI] [PubMed] [Google Scholar]
- 24.Villanueva V, Garcia AM. Validity and reliability of surrogate information for controls in a case-control study on alzheimer's disease. J Alzheimers Dis. 2006;10(4):409–416. doi: 10.3233/jad-2006-10410. [DOI] [PubMed] [Google Scholar]
- 25.Brennan RL, editor. Educational measurement. 4th ed. Westport, CT: American Council on Education/Praeger; 2006. [Google Scholar]
- 26.Lerner D, Amick BC, 3rd, Rogers WH, Malspeis S, Bungay K, Cynn D. The work limitations questionnaire. Med Care. 2001;39(1):72–85. doi: 10.1097/00005650-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Robins Wahlin TB, Lundin A, Dear K. Early cognitive deficits in swedish gene carriers of huntington's disease. Neuropsychology. 2007;21(1):31–44. doi: 10.1037/0894-4105.21.1.31. [DOI] [PubMed] [Google Scholar]
- 28.Solomon AC, Stout JC, Johnson SA, et al. Verbal episodic memory declines prior to diagnosis in huntington's disease. Neuropsychologia. 2007;45(8):1767–1776. doi: 10.1016/j.neuropsychologia.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC Predict-HD Investigators of the Huntington Study Group. Psychiatric symptoms in huntington's disease before diagnosis: The predict-HD study. Biol Psychiatry. 2007;62(12):1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 30.Williams JK, Hamilton R, Nehl C, et al. "No one else sees the difference:" family members' perceptions of changes in persons with preclinical huntington disease. American journal of medical genetics. Part B, Neuropsychiatric genetics. 2007;144B(5):636–641. doi: 10.1002/ajmg.b.30479. [DOI] [PubMed] [Google Scholar]
- 31.Downing NR, Williams JK, Paulsen JS. Couples' attributions for functional changes in prodromal huntingtion disease. Journal of Genetic Counseling. 2010;19(4):343–352. doi: 10.1007/s10897-010-9294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoulson I, Fahn S. Huntington disease: Clinical care and evaluation. Neurology. 1979;29(1):1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Huntington Study Group. Unified huntington's disease rating scale: Reliability and consistency. Mov Disord. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 34.Endicott J, Nee J. Endicott work productivity scale (EWPS): A new measure to assess treatment effects. Psychopharmacol Bull. 1997;33(1):13–16. [PubMed] [Google Scholar]
- 35.Kessler RC, Barber C, Beck A, et al. The world health organization health and work performance questionnaire (HPQ) J Occup Environ Med. 2003;45(2):156–174. doi: 10.1097/01.jom.0000052967.43131.51. [DOI] [PubMed] [Google Scholar]
- 36.Duff K, Paulsen J, Mills J, et al. Mild cognitive impairment in prediagnosed huntington disease. Neurology. 2010;75(6):500–507. doi: 10.1212/WNL.0b013e3181eccfa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Escorpizo R, Bombardier C, Boonen A, et al. Worker productivity outcome measures in arthritis. J Rheumatol. 2007;34(6):1372–1380. [PubMed] [Google Scholar]
- 38.Mattke S, Balakrishnan A, Bergamo G, Newberry SJ. A review of methods to measure health-related productivity loss. Am J Manag Care. 2007;13(4):211–217. [PubMed] [Google Scholar]
- 39.Michalek EE, Yatham LN, Maxwell V, Hale S, Lam W. The impact of bipolar disorder upon work functioning: A qualitative analysis. Bipolar Disord. 2007;9(1–2):126–143. doi: 10.1111/j.1399-5618.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 40.Sandelowski M. Focus on research methods. whatever happened to qualitative description? Research in nursing health. 2000;23(4):334–340. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 41.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR International Huntington's Disease Collaborative Group. A new model for prediction of the age of onset and penetrance for huntington's disease based on CAG length. Clin Genet. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 42.Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 43.Pettit FA. A comparison of world-wide-web and paper-and-pencil personality questionnaires. Behavior Methods Research. 2002;34(1):50–54. doi: 10.3758/bf03195423. [DOI] [PubMed] [Google Scholar]
- 44.Willis GB. Cognitive interviewing: A tool for improving questionnaire design. Thousand Oaks, CA: Sage Publications; 2005. [Google Scholar]
- 45.Speight J, Barendse SM. FDA guidance on patient reported outcomes. BMJ. 2010;340:c2921. doi: 10.1136/bmj.c2921. [DOI] [PubMed] [Google Scholar]
- 46.Conrad F, Blair J. From impressions to data: Increasing the objectivity of cognitive interviews. 1996 Proceedings of the Section on Survey Research Methods. 1996;1:1–9. [Google Scholar]
- 47.Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiatry. 1976;33(9):1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- 49.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. New York: John Wiley & Sons; 2002. [Google Scholar]
- 50.Weissman MM, Olfson M, Gameroff MJ, Feder A, Fuentes M. A comparison of three scales for assessing social functioning in primary care. Am J Psychiatry. 2001;158(3):460–466. doi: 10.1176/appi.ajp.158.3.460. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Long JD, Mills JA, et al. Indexing disease progression at study entry with individuals at-risk for huntington disease. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(7):751–763. doi: 10.1002/ajmg.b.31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reiman EM, Langbaum JB, Tariot PN. Alzheimer's prevention initiative: A proposal to evaluate presymptomatic treatments as quickly as possible. Biomark Med. 2010;4(1):3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daviglus ML, Bell CC, Berrettini W, et al. National institutes of health state-of-the-science conference statement: Preventing alzheimer disease and cognitive decline. Ann Intern Med. 2010;153(3):176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 54.Postuma RB, Gagnon JF, Montplaisir J. Clinical prediction of parkinson's disease: Planning for the age of neuroprotection. J Neurol Neurosurg Psychiatry. 2010;81(9):1008–1013. doi: 10.1136/jnnp.2009.174748. [DOI] [PubMed] [Google Scholar]