Abstract
Sterol regulatory element-binding protein (SREBP) transcription factors regulate cellular lipogenesis and lipid homeostasis. Recent studies reveal expanding roles for SREBPs with the description of new regulatory mechanisms, identification of unexpected transcriptional targets, and the discovery of functions for SREBPs in type II diabetes, cancer, immunity, neuroprotection and autophagy.
INTRODUCTION
Membrane-bound, basic helix-loop-helix leucine zipper (bHLH-LZ) transcription factors called sterol regulatory element-binding proteins (SREBPs) play a central role in cell metabolism by controlling synthesis of fatty acids, triglycerides, and cholesterol (Goldstein et al., 2006). Three mammalian SREBP isoforms (SREBP-1a, SREBP-1c and SREBP-2) are encoded by two genes, SREBF1 and SREBF2, and have distinct but overlapping lipogenic transcriptional programs. SREBP-1a activates fatty acid and cholesterol synthesis, SREBP-1c fatty acid synthesis, and SREBP-2 cholesterol synthesis and uptake (Goldstein et al., 2006). Early studies focused on the function of SREBPs in lipid homeostasis and regulation by cholesterol and its oxysterol derivatives. Recently however, an explosion of studies demonstrate that SREBPs integrate multiple cell signals to control lipogenesis as well as unexpected pathways important for type II diabetes, cancer, the immune response, neuroprotection and autophagy. Here, we review current knowledge regarding regulation of SREBPs and discuss the expanding cellular roles for these transcription factors.
SIGNALING TO SREBP
Sterol-dependent regulation of SREBP
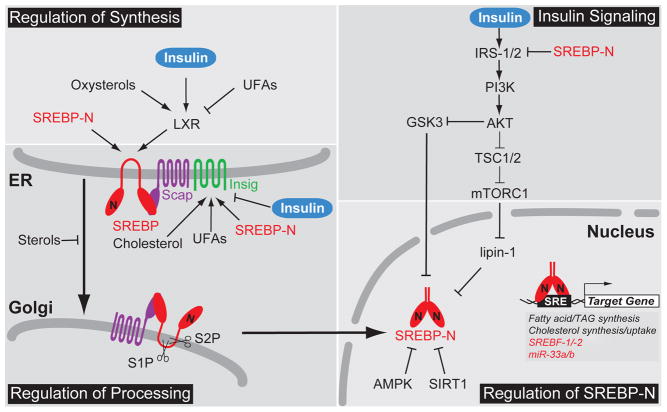
Our understanding of sterol-regulated control of SREBPs comes largely from the elegant work of Michael Brown, Joseph Goldstein and colleagues at UT-Southwestern Medical Center in Dallas. Unlike other bHLH-LZ family transcription factors, newly synthesized SREBPs are inserted into the endoplasmic reticulum (ER) membrane as inactive precursors (Fig. 1)(Osborne and Espenshade, 2009). The SREBP N-terminal bHLH-LZ transcription factor domain is released from the membrane by the sequential action of the Golgi-localized Site-1 protease (S1P) and Site-2 protease (S2P). Sterols regulate SREBP by controlling its ER-to-Golgi transport (Fig. 1). Each SREBP isoform binds the sterol-sensing protein SREBP cleavage activating protein (Scap). In cholesterol-poor cells, the SREBP-Scap complex trafficks to the Golgi via COPII vesicles where S1P and S2P proteolytically release the SREBP N-terminus (SREBP-N) from the membrane (Osborne and Espenshade, 2009). In the nucleus, SREBP-N activates genes involved in cholesterol biosynthesis and uptake, thus restoring sterol homeostasis. SREBPs are also self-regulated by transcriptional positive feedback. In addition, activation of SREBF-1/2 transcription increases expression of microRNAs-33a/b (miR-33a/b) encoded within introns of the SREBF2/SREBF1 genes, respectively. miR-33a/b negatively regulate lipid export and fatty acid oxidation to further aid the return to homeostasis (Moore et al., 2011). Excess ER cholesterol binds Scap and elicits a conformational change that promotes binding of Scap to the ER-resident protein Insig, thus blocking ER exit of the SREBP-Scap complex (Fig. 1). Oxysterol derivatives of cholesterol, such as 25-hydroxycholesterol, accumulate under conditions of excess cholesterol and independently bind to Insig to promote ER retention of SREBP-Scap (Osborne and Espenshade, 2009). Two genes, INSIG1 and INSIG2, code for Insig proteins and control tissue- and signal-specific regulation of SREBP transport. Insig-1, but not Insig-2, is subject to proteasome-dependent degradation and cholesterol additionally inhibits ER-to-Golgi transport of SREBP by stabilizing Insig-1 (Goldstein et al., 2006). INSIG1 is also a direct SREBP target gene, and thus SREBP activation both increases cholesterol levels and Insig-1 protein to provide convergent, negative feedback regulation of SREBP-Scap transport and proteolytic activation. Finally, oxysterols stimulate transcription of SREBP-1c, which stimulates fatty acid synthesis, through direct binding of the nuclear hormone receptor liver X receptor (LXR) to the SREBF1 promoter (Goldstein et al., 2006). Oxysterols are LXR agonists and it is proposed that upregulation of SREBP-1c may serve to increase the supply of unsaturated fatty acids needed for sterol esterification under conditions of cholesterol excess.
Figure 1. Multivalent regulation of SREBPs.
Regulation of SREBPs occurs at the level of SREBP synthesis, proteolytic activation, transcriptional activity, and degradation. Multiple signals regulate ER-to-Golgi transport and the proteolytic activation of SREBPs by controlling the expression and stability of Insig. In addition, nuclear SREBPs are highly regulated by post-translational modification, including phosphorylation, acetylation, and ubiquitinylation. Positive and negative regulation is indicated. Abbreviations: nuclear form of SREBP, SREBP-N; unsaturated fatty acids, UFAs; triacylglyceride, TAG.
Insulin-dependent regulation of SREBP
The liver is an important site of lipid synthesis and export, and SREBP-1c plays a major role in the upregulation of fatty acid synthesis in response to insulin (Osborne and Espenshade 2009). Insulin activates hepatic SREBP-1c both transcriptionally and post-translationally (Fig. 1). Insulin-dependent SREBP-1c transcriptional regulation requires LXR binding elements in the SREBP-1c promoter, but the detailed mechanism is unknown. Overexpression of an oxysterol catabolic enzyme, cholesterol sulfotransferase, abolishes insulin-induced SREBP-1c expression suggesting that insulin stimulates SREBP-1c transcription through LXR activation (Chen et al., 2007). Although both insulin and the LXR agonist TO-901317 stimulate SREBP-1c transcription, only insulin potently activates SREBP target genes indicating a critical post-transcriptional role for insulin (Goldstein et al., 2006).
This insulin-stimulated SREBP-1c activation is phosphatidylinositol 3-kinase (PI3K)/Akt-dependent, and the mammalian target of rapamycin (mTOR) kinase is the major PI3K/Akt downstream effector (Fig. 1). PI3K/Akt activation leads to direct phosphorylation of tuberous sclerosis complex 1/2 (TSC1/2) and PRAS40, resulting in mTOR complex 1 (mTORC1) activation. The mTOR inhibitor rapamycin is a commonly used pharmacological tool to test for mTOR dependence. However, rapamycin inhibition of mTOR varies among cell types, complicating studies of insulin’s action on SREBP. For example, rapamycin potently inhibits insulin-stimulated SREBP activation in primary rat hepatocytes (Li et al., 2010), but higher concentrations fail to block SREBP activity in mouse NIH3T3 fibroblasts (Peterson et al., 2011). Torin-1, a new mTOR inhibitor, potently blocks SREBP activation in mouse fibroblasts. Collectively, pharmacological evidence suggests that mTOR mediates insulin-stimulated SREBP activation.
Genetic mouse model experiments further demonstrate that mTORC1 is a key regulator downstream of PI3K/Akt. In Tsc1/2 null mouse embryonic fibroblasts (MEFs), mTORC1 is constitutively active and independent of upstream signals. SREBP-1 is activated in Tsc1/2 null MEFs and activation of SREBP-1 is inhibited upon rapamycin treatment, indicating that activation of mTORC1 is sufficient to stimulate SREBP-1 activity (Düvel et al., 2010). Similarly, inactivation of mTORC1 is sufficient to shut down SREBP-1 and lipogenesis. Hepatic inactivation of mTORC1 by knockout of the Raptor subunit protects mice from hepatic steatosis induced by a high fat and high cholesterol diet (Peterson et al., 2011). Inhibition of S6 kinase, a downstream target of mTORC1, blunts SREBP-1 processing in Tsc1/2 null MEFs (Düvel et al., 2010), but fails to block insulin-induced SREBP-1c activation in primary rat hepatocytes (Li et al., 2010) suggesting the existence of S6-kinase-independent SREBP-1c regulation by mTORC1. Collectively, these mouse and cultured cell studies implicate a novel downstream target of mTORC1 in regulation of SREBP-1c activity (Krycer et al., 2010).
A recent study revealed that lipin-1, a phosphatidic acid phosphatase and a transcriptional coactivator, is a direct substrate of mTORC1 and a regulator of nuclear SREBP activity (Peterson et al., 2011). Lipin-1 phosphorylation by mTORC1 blocks its nuclear localization and activates SREBP (Fig. 1). Conversely, expression of a non-phosphorylated form of lipin-1 results in lipin-1 nuclear localization, reduced levels of nuclear SREBP, and altered localization of SREBP to the nuclear periphery. Finally, knocking down lipin-1 in liver-specific Raptor knockout mice restores SREBP-1 activation. This study implicates lipin-1 as an insulin-dependent, mTORC1 downstream effector that regulates SREBP activity (Peterson et al., 2011). Interestingly, SREBP-1 directly activates LPIN1 transcription in human hepatoblastoma cells, providing a possible mechanism for negative feedback regulation of SREBP-1 activity (Csaki and Reue, 2010).
Lipin-1 is both a phosphatidic acid phosphatase required for triglyceride synthesis and transcriptional coactivator essential for adipose tissue development (Csaki and Reue, 2010). These dual functions complicate its study. How lipin-1 inhibits SREBP-1 activity and whether lipin-1 acts directly is unknown, but inhibition requires lipin-1 catalytic activity (Peterson et al., 2011). This finding is interesting as the enzymatic function of lipin-1 was previously thought to be limited to the conversion of phosphatidic acid to diacylglycerol for triglyceride and phospholipid synthesis in the cytosol. Further studies are needed to understand this nuclear, catalytic function of lipin-1. To date, studies of lipin-1 function have been carried out largely in adipose tissue where lipin-1 is the major phosphatidic acid phosphatase. However, lipin-1 is not the major enzyme in the liver, where two additional enzymes, lipin-2 and lipin-3, are expressed (Csaki and Reue, 2010). The finding that lipin-1 negatively regulates SREBP-dependent lipogenic gene expression should stimulate detailed studies of the hepatic function of the three Lpin genes and their impact on regulation of hepatic lipogenesis. Consistent with this newly discovered role for lipin-1, fatty acid synthesis was found to be upregulated in Lpin1−/− mice (Csaki and Reue, 2010). This new connection between SREBP and lipin-1 links two important regulators of lipid homeostasis and adds a new player to the list of SREBP regulators.
Insulin regulates SREBP activity through additional mechanisms other than LXR-dependent transcription and lipin-1. Inhibition of PI3K by chemical inhibitors or expression of a dominantnegative Akt inhibits SREBP ER-to-Golgi transport and proteolytic activation (Krycer et al., 2010). Consistent with this, insulin stimulates ER-to-Golgi transport of SREBP-1c by promoting its phosphorylation and association with COPII vesicles (Krycer et al., 2010). In mouse liver, transport of SREBP is additionally regulated by Akt signaling through control of Insig-2 levels (Fig. 1). Two differentially regulated mRNA transcripts (Insig-2a and Insig-2b) code for Insig-2, and Insig-2a is the predominant hepatic transcript (Goldstein et al., 2006). Insulin negatively regulates expression of Insig-2a mRNA in an Akt-dependent manner, resulting in a decreased Insig protein pool and increased ER-to-Golgi transport of SREBP (Yecies et al., 2011). Overall, insulin acts at multiple regulatory steps to control activity of SREBPs.
Additional signals to SREBPs
Sterols control ER-to-Golgi transport of SREBP by regulating Scap binding to Insig, but additional signals alter ER exit to control SREBP activity (Fig. 1). Unsaturated fatty acids decrease the proteolytic processing of SREBP-1 by stabilizing Insig-1 (Lee et al., 2008). In neurons, N-methyl-D-aspartate glutamate receptor (NMDAR) overactivation results in the accelerated degradation of Insig-1, promoting the ER-to-Golgi transport and proteolytic activation of SREBP-1 (Taghibiglou et al., 2009). ER stress also promotes the ER exit of SREBPs by decreasing Insig-1 (Osborne and Espenshade, 2009). Overexpression of GRP78, an ER chaperone, in the livers of genetically obese ob/ob mice reduces ER stress, blocks SREBP-1 processing, and prevents hepatic steatosis (Kammoun et al., 2009). In these insulin-resistant mice, Akt activity is attenuated. Thus, this study suggests that ER stress may serve as an alternative path to SREBP activation independent of Akt. Finally, bacterial pore-forming toxin treatment activates SREBPs by stimulating cellular potassium efflux, which promotes ER-to-Golgi transport of both SREBP-1 and -2 (Osborne and Espenshade, 2009). In cases where a mechanism has not been determined, the possibility exists that these signals regulate SREBP indirectly by changing sterol levels similar to how oxygen signals to SREBP in fungi (Osborne and Espenshade, 2009).
SREBP may also be regulated in an ER-to-Golgi transport-independent manner. Recently Näär and colleagues discovered that blocking phosphatidylcholine (PC) synthesis stimulates the proteolytic activation of SREBP-1 (Walker et al., 2011). An interesting model emerged from this study in which Golgi-localized S1P and S2P translocate from the Golgi to the ER upon the depletion of PC. Although further studies are needed, this study indicates that SREBP cleavage could be activated in the absence of ER-to-Golgi transport by controlling the localization of S1P and S2P.
Proteolytically activated, nuclear SREBPs integrate signals from multiple pathways through post-translational modifications including phosphorylation, ubiquitinylation, and acetylation (Fig. 1). In cultured cells, insulin additionally signals to SREBP through Akt inhibition of GSK3, which directly phosphorylates SREBP-1c (Krycer et al., 2010). In the absence of Akt signaling, GSK3 phosphorylates SREBP leading to ubiquitinylation by the SCF(Fbw7) ubiquitin ligase and proteasomal degradation of the active transcription factor. While liver-specific knockout of Fbw7 in mice resulted in steatohepatitis, the observed accumulation of liver triglyceride does not require SREBP-1, suggesting that this Fbw7 degradative mechanism may function in non-hepatic tissues (Kumadaki et al., 2011). The nutrient sensor AMP-activated protein kinase (AMPK) negatively regulates SREBP to limit lipogenesis under nutrient-limiting conditions by directly phosphorylating SREBP (Li et al., 2011). Finally, SIRT1 deacetylation of SREBP-1c increases its degradation and is regulated by feeding in mice (Walker et al., 2010). Transcriptional coactivators and cooperating transcription factors provide yet another level of regulatory control of SREBP activity (Osborne and Espenshade, 2009). Collectively, combined regulation of SREBP transcription, proteolytic activation, and nuclear activity allows SREBPs to integrate nutrient signals from multiple pathways to control metabolism.
EMERGING FUNCTIONS FOR SREBP
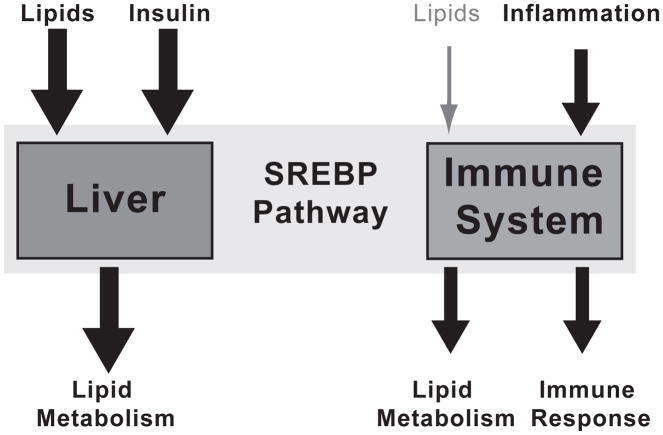
Maintaining lipid homeostasis is essential for cell viability. Given their critical role in this process, SREBPs likely function in all cells. Hepatocytes are normally non-dividing and frequently challenged by fluctuations in lipid supply and demand. Thus in the liver, lipids and insulin are the dominant signals to SREBP for control of lipogenesis (Fig. 2). However in non-hepatic cells, lipid levels may not change dramatically, thereby minimizing the sterol-dependent regulation of SREBP. In these settings, other signals may dominate, revealing new modes of sterol-independent regulation of SREBP (Fig. 2). SREBP frequently cooperates with additional transcription factors, providing a mechanism for this tissue-specific, SREBP-dependent gene expression (Osborne and Espenshade, 2009). Consistent with this, the application of ChIP-Seq technology to the SREBP pathway has revealed that SREBPs control transcriptional programs extending beyond lipid synthesis. In addition, the detailed understanding of SREBP function in lipogenesis has led to studies of SREBP function in the context of disease states.
Figure 2. SREBPs integrate multiple signals to control tissue-specific gene expression.
SREBP transcription factors respond to diverse environmental signals such as lipid supply, nutrients (via insulin), and inflammatory signals. The signals to SREBP and the target genes that SREBP activates are tissue-dependent. For example in the liver, SREBPs respond to lipid supply and insulin to control lipid metabolism. While in macrophages of the immune system, SREBP responds to inflammatory signals to promote lipid metabolism and the immune response.
Type II diabetes
The incidence of type II diabetes mellitus has increased due to the obesity epidemic, and diabetes is projected to affect one-quarter of the US population by 2050. Type II diabetes is characterized by hyperinsulinemia, hyperglycemia, and hypertriglyceridemia with associated fatty liver (hepatic steatosis), which can lead to cirrhosis and eventual liver failure. Insulin regulation of liver SREBP-1c and the fact that hyperactivation of SREBP-1 induces fatty liver suggest that SREBPs play a central role in development of hepatic steatosis. Indeed, Horton and colleagues recently demonstrated that SREBPs are essential for hepatic steatosis in genetic and dietary rodent models of obesity (Moon et al., 2012). Liver-specific knockout of the SREBP transport protein Scap eliminates activity of all three SREBP isoforms and prevents steatosis in ob/ob mice and mice on a high-fat diet. In addition, hepatic knockdown of Scap by siRNA suppresses carbohydrate-induced hypertriglyceridemia in hamsters, demonstrating the therapeutic potential of SREBP pathway inhibition. Interestingly, evidence exists that SREBPs in turn can regulate insulin signaling (Fig. 1). Insulin receptor substrate 2 (IRS-2) is a major mediator of insulin signaling in the liver, controlling insulin sensitivity. Nuclear active SREBPs can decrease IRS-2 expression, inhibit the downstream PI3K/Akt pathway, and decrease glycogen synthesis (Ide et al., 2004). Consistent with this, high SREBP-1c activity resulting from hyperinsulinemia negatively correlates with IRS-2 expression in ob/ob mice (Goldstein et al., 2006). These data suggest that the SREBP pathway may contribute to liver insulin resistance, however data from the liver Scap knockout study indicate otherwise. Liver-specific Scap knockout prevented hepatic steatosis, but loss of SREBP activity had no effect on liver insulin resistance (Moon et al., 2012). More work is required to fully understand the mechanisms underlying differential insulin signaling in the liver.
Cancer
Cancer cells are a new cell type with distinct properties compared to other cells in the body. Increased de novo lipid synthesis is one hallmark of cancer (Krycer et al., 2010). Consistent with this lipogenic character, the expression of several SREBP target genes such as fatty acid synthase and LDL-receptor are elevated in tumor cells. Since cancer cells need to maintain lipid supply to support tumor growth and SREBPs are master regulators of lipogenesis, SREBP activity is likely required for tumor growth, making the SREBP pathway a potential target for anti-cancer therapy. Studying glioblastoma, Mischel and colleagues demonstrated that epidermal growth factor receptor mutations (EGFRvIII) and hyperactivation of PI3K promote tumor growth and survival through stimulation of SREBP-1 activity. Blocking tumor cell cholesterol supply by decreasing uptake through the LDL receptor promotes tumor cell death in vivo (Guo et al., 2011). Although not directly targeting SREBPs, this study demonstrates that targeting lipid supply is a potential strategy to treat cancer. Considering that SREBPs control sterol uptake as well as de novo synthesis, inhibition of SREBP activity could be a more promising strategy.
Recently, direct connections between the tumor suppressor p53 and SREBP have been discovered. The p53 gene, TP53, is the most frequent target for mutation in tumors (>50% of human cancers exhibit mutations in TP53). The majority of TP53 mutations are missense mutations that result in expression of mutant p53 with a prolonged half-life and gain-of-function activity (Freed-Pastor et al., 2012). Using a 3D-culture breast cancer model, Prives and colleagues demonstrated that mutant p53 promotes lipid synthesis through the mevalonate pathway to support the malignant phenotype. Data suggest that p53 associates with promoters of lipid enzymes through association with SREBP and that the mevalonate pathway is a possible therapeutic target in tumors bearing p53 mutations. Together, these findings suggest that SREBPs may play an important function in tumorigenesis and highlight the SREBP pathway as a potential target for anti-cancer therapy.
Immune system
In the immune system, phagocytosis is a major mechanism used to remove pathogens such as bacteria. Phagocytosis requires membrane biogenesis to compensate for the loss of plasma membrane wrapped around the engulfed particles. Phagocytosis promotes membrane biogenesis by activating processing of both SREBP-1a and -2 (Osborne and Espenshade, 2009). SREBPs are also activated upon bacterial toxin challenge. SREBP-1a directly activates expression of the anti-apoptotic gene Api6 upon toxin challenge to promote cell survival (Im and Osborne, 2012).
Compared to SREBP-1c, expression of SREBP-1a in metabolic tissues is relatively low and its physiological regulation is largely unknown. Recently, Osborne and colleagues found that SREBP-1a is highly expressed in cells of the immune system, such as macrophages and dendritic cells (Im et al., 2011). In wild-type macrophages, lipopolysaccharide (LPS) treatment stimulates lipogenesis, but macrophages isolated from SREBP-1a-deficent mice fail to activate expression of lipogenic genes in response to LPS. Analysis of the SREBP-1a promoter identified an NF-κB binding element flanked by two Sp1 binding sites. Chromatin immunoprecipitation (ChIP) experiments in macrophages indicate that LPS enhances NF-κB/Sp1 activation of the SREBP-1a promoter to increase lipogenesis. In addition, SREBP-1a activates expression of Nlrp1, a core component of the inflammasome, and SREBP-1a deficient mice display a defective inflammatory response, showing resistance to toxic shock but sensitivity to Salmonella infection (Im et al., 2011).
Autophagy
Autophagy is a lysosomal degradative pathway that functions to remove and breakdown cellular components, such as organelles and proteins. Autophagy also mediates the breakdown of lipids stored in lipid-droplets. A genome-wide SREBP-2 ChIP-Seq analysis in mouse liver revealed that SREBP-2 occupies promoters of genes involved in autophagy (Seo et al., 2011). In cholesterol-depleted cells, knocking down SREBP-2 decreases autophagosome formation and lipid droplet association of the autophagosome protein LC3. These data provide evidence for regulation of autophagy by SREBP-2.
Neuroprotection
Neuronal excitotoxicity caused by overactivation of NMDARs is a primary neuropathological process contributing to neuronal injury after stroke and brain trauma. Activation of SREBP-1 is an essential step in NMDAR-mediated excitotoxic neuronal death (Taghibiglou et al., 2009). Overactivation of NMDARs stimulates SREBP-1 processing by accelerating the degradation of Insig-1. Systemic administration of Insig-1-derived interference peptide (Indip) blocked Insig degradation and suppressed SREBP-1 activation, reducing neuronal damage in a rat stroke model. How activation of SREBP-1 promotes neuronal damage remains to be determined, but targeting SREBP-1 downstream of NMDARs may be a neuroprotective strategy in stroke. SREBP-1 also regulates parasympathetic stimulation of the heart, which provides protection from arrhythmia and sudden death. Upon parasympathetic stimulation, the G protein-coupled inward rectifying K+ channel GIRK1/4 is activated, generating acetylcholine-sensitive K+ current (IKACh). In embryonic chick atrial myocytes, SREBP-1 activates IKACh through direct binding to the GIRK1 promoter and control of its expression (Park et al., 2008). Compared to wild-type animals, SREBP-1 knockout mice show both reduced GIRK1 expression and a reduced cardiac response to parasympathetic stimulation. Together, these data implicate SREBPs in neuronal physiology.
FUTURE CHALLENGES
Initial studies of the SREBP pathway combined powerful somatic cell genetic approaches in Chinese hamster ovary cells and in vivo experiments in mouse liver to identify pathway components and understand their regulation. In the past decade, new tools and techniques have facilitated studies in non-hepatic tissues demonstrating that SREBPs play a broader role in metabolism. Given their role as regulators of essential lipid homeostasis, SREBPs likely function in all cells. The diverse mechanisms for SREBP regulation provide ample opportunity for context-specific regulation of SREBP, and one future challenge is to understand the tissue-specific regulation and function of the SREBP-1a, -1c and -2 isoforms. Cataloging SREBP transcriptional targets in different tissues is an important first step. That we are only beginning to understand the scope of SREBP function is highlighted by the studies of SREBP-1a in the immune system (Im et al., 2011) and the discovery of miR-33a/b regulation of lipid homeostasis (Moore et al., 2011). To date, the central role for SREBPs in the expression of the LDL receptor and the cholesterol-lowering effects of statin drugs has stimulated research in the SREBP field due to therapeutic implications for cardiovascular disease. Emerging data suggest that continued study of the SREBP pathway will advance efforts to combat diseases such as diabetes, hepatic steatosis and cancer. Exciting times for SREBP indeed!
Acknowledgments
We extend a sincere apology to those whose work was not discussed or cited in this review due to limitations in space and scope. We thank Rita Brookheart and Sumana Raychaudhuri for comments on the manuscript. Research on SREBP in the Espenshade lab is supported by the National Institutes of Health (HL-077588) and an Established Investigator Award from the American Heart Association (PJE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaki LS, Reue K. Lipins: multifunctional lipid metabolism proteins. Annu Rev Nutr. 2010;30:257–272. doi: 10.1146/annurev.nutr.012809.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed-Pastor WA, Mizuno H, Zhao X, Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, Kuga D, Amzajerdi AN, Soto H, Zhu S, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1:442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T, Shimano H, Yahagi N, Matsuzaka T, Nakakuki M, Yamamoto T, Nakagawa Y, Takahashi A, Suzuki H, Sone H, et al. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat Cell Biol. 2004;6:351–357. doi: 10.1038/ncb1111. [DOI] [PubMed] [Google Scholar]
- Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG, Raffatellu M, Osborne TF. Linking lipid metabolism to the innate immune response in macrophages through Sterol Regulatory Element Binding Protein-1a. Cell Metab. 2011;13:540–549. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SS, Osborne TF. Protection from bacterial-toxin-induced apoptosis in macrophages requires the lipogenic transcription factor sterol regulatory element binding protein 1a. Mol Cell Biol. 2012;32:2196–2202. doi: 10.1128/MCB.06294-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krycer JR, Sharpe LJ, Luu W, Brown AJ. The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol Metab. 2010;21:268–276. doi: 10.1016/j.tem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kumadaki S, Karasawa T, Matsuzaka T, Ema M, Nakagawa Y, Nakakuki M, Saito R, Yahagi N, Iwasaki H, Sone H, et al. Inhibition of ubiquitin ligase F-box and WD repeat domain-containing 7α (Fbw7α) causes hepatosteatosis through Kruppel-like factor 5 (KLF5)/peroxisome proliferator-activated receptor γ2 (PPARγ2) pathway but not SREBP-1c protein in mice. J Biol Chem. 2011;286:40835–40846. doi: 10.1074/jbc.M111.235283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JN, Zhang X, Feramisco JD, Gong Y, Ye J. Unsaturated fatty acids inhibit proteasomal degradation of Insig-1 at a postubiquitination step. J Biol Chem. 2008;283:33772– 33783. doi: 10.1074/jbc.M806108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JJ, Gao B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KJ, Rayner KJ, Suárez Y, Fernández-Hernando C. The role of microRNAs in cholesterol efflux and hepatic lipid metabolism. Annu Rev Nutr. 2011;31:49–63. doi: 10.1146/annurev-nutr-081810-160756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne TF, Espenshade PJ. Evolutionary conservation and adaptation in the mechanism that regulates SREBP action: what a long, strange tRIP it’s been. Genes Dev. 2009;23:2578–2591. doi: 10.1101/gad.1854309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Georgescu SP, Du C, Madias C, Aronovitz MJ, Welzig CM, Wang B, Begley U, Zhang Y, Blaustein RO, et al. Parasympathetic response in chick myocytes and mouse heart is controlled by SREBP. J Clin Invest. 2008;118:259–271. doi: 10.1172/JCI32011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, et al. mTOR complex 1 regulates Lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YK, Jeon TI, Chong HK, Biesinger J, Xie X, Osborne TF. Genome-wide localization of SREBP-2 in hepatic chromatin predicts a role in autophagy. Cell Metab. 2011;13:367–375. doi: 10.1016/j.cmet.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghibiglou C, Martin HGS, Lai TW, Cho T, Prasad S, Kojic L, Lu J, Liu Y, Lo E, Zhang S, et al. Role of NMDA receptor-dependent activation of SREBP1 in excitotoxic and ischemic neuronal injuries. Nat Med. 2009;15:1399–1406. doi: 10.1038/nm.2064. [DOI] [PubMed] [Google Scholar]
- Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, et al. A conserved SREBP- 1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]