Summary
Phagocyte NADPH oxidase plays a key role in pathogen clearance via reactive oxygen species (ROS) production. Defects in oxidase function result in chronic granulomatous disease (CGD) with hallmark recurrent microbial infections and inflammation. The oxidase′s role in the adaptive immune response is not well-understood. Class II presentation of cytoplasmic and exogenous Ag to CD4+ T cells was impaired in human B cells with reduced oxidase p40phox subunit expression. Naturally arising mutations which compromise p40phox function in a CGD patient also perturbed class II Ag presentation and intracellular ROS production. Reconstitution of patient B cells with wild-type, but not a mutant, p40phox allele restored exogenous Ag presentation and intracellular ROS generation. Remarkably, class II presentation of epitopes from membrane Ag was robust in p40phox-deficient B cells. These studies reveal a role for NADPH oxidase and p40phox in skewing epitope selection and T cell recognition of self Ag.
Keywords: Human B cells, MHC class II presentation, NADPH oxidase
Introduction
The phagocyte NADPH oxidase plays a critical role in microbial killing by catalyzing electron transfer from NADPH to molecular oxygen giving rise to superoxide and other forms of ROS (1). This oxidase contains several phox (phagocyte oxidase) subunits including gp91phox and p22phox which comprise flavocytochrome b558 as well as cytoplasmic p40phox, p47phox, and p67phox. The binding of ligands to phagocyte receptors stimulates cytoplasmic subunit translocation to membrane-bound flavocytochrome b558 facilitating superoxide production (1). Phox subunit mutations are associated with CGD, characterized by the absence of or marked reduction in ROS production, recurrent pathogenic infections, and granulomatous inflammation (2, 3).
While the microbicidal role of phagocyte NADPH oxidase during the innate immune response to pathogenic infections is established (2), its function in APC-T cell interactions is less well-understood. In gp91phox-deficient dendritic cells, but not macrophages, NADPH oxidase tempers phagosome acidification, preserving internalized Ag for efficient MHC class I-mediated cross-presentation to CD8+ T cells (4, 5). By contrast, CD4+ T cell activation was enhanced in response to murine macrophages with p47phox mutations (6). Thus, mutation of distinct oxidase subunits may differentially affect cellular immune responses.
Evidence is conflicting regarding a direct role for NADPH oxidase in regulating MHCII Ag presentation. Presentation of exogenous ovalbumin but not tetanus toxoid Ag was altered in APC from CGD patients (7, 8). Neither study identified the defective oxidase subunits in the CGD patient-derived APC. ROS produced by NADPH oxidase can regulate autophagy in phagocytes (9), and oxidase subunits are detected in endosomes and phagosomes (10). Presentation of exogenous Ag via MHCII requires Ag transit and proteolysis in endosomes and lysosomes to yield peptide ligands (11). Cytoplasmic or nuclear Ag can also access MHCII via several autophagy pathways (12). MHCII αβ complexes are directed via the invariant chain (Ii) to endosomes where proteases fragment Ii (13). HLA-DM, whose function is regulated by HLA-DO, facilitates the removal of these fragments and antigenic peptide capture by MHCII. The resulting peptide-MHCII complexes then traffic to the cell surface for immune surveillance by CD4+ T cells.
The p40phox subunit has been associated with Crohn′s disease and rheumatoid arthritis in genome wide association studies (14–16), yet its role in Ag presentation has not been investigated. Studies here examine how loss of p40phox in human B cells affects MHCII Ag presentation and oxidase function. In the absence of functional p40phox, human B cells displayed a reduced capacity for cytoplasmic Ag presentation. Perturbations in p40phox also disrupted MHCII exogenous Ag presentation, yet presentation of membrane autoantigens was efficient. Disruption in p40phox compromised intracellular but not extracellular ROS production by B cells. These results suggest roles for NADPH oxidase and its regulatory subunit p40phox in skewing epitope selection by MHCII.
Materials and Methods
Cell lines
Human B lymphoblastoid cell lines (B-LCL) and T cells have been described (17, 18). Lentiviral shRNA targeting human p40phox (19) or β2-microglobulin (hβ2M) transcripts (Sigma-Aldrich) were used to transduce B-LCL to generate p40phox- or β2M-deficient cells. Institutional approval was obtained for human blood collection. A B-LCL, AR40 from a p40phox-deficient patient (3) (DRβ1*0101, DRβ1*0701) was generated and transduced to express DRβ1*0401 (17). Retroviruses encoding p40phox WT or p40phoxR105Q were used to transduce AR40.DR4 (3). Frev and AR40 B-LCL synthesize Igλ but not Igκ chains.
Western blotting
Cell lysates were analyzed by immunoblotting (10, 17) using Ab for p22phox (10), p40phox(Upstate), p47phox, p67phox (BD Biosciences, San Jose, CA), gp91phox (10), and GAD (Sigma-Aldrich). The mAb DA6.147 detects HLA-DRα and αβ dimers (20). The mAb PIN1.1 detects Ii (21). Membranes were stripped and reprobed for β-actin (Sigma-Aldrich) or GAPDH (Chemicon) as controls for sample loading. Quantity One®1-D Analysis Software (BioRad) was used to quantify protein expression.
Antigen presentation
APC were incubated +/− 10–20 μM human serum albumin (HSA), human IgG Ag (Sigma-Aldrich) or peptides HSA64–76, κI188–203, and κII145–159 (Quality Controlled Biochemicals) for 6 hr at 37°C. APC were washed and incubated with epitope-specific T cells for 24 hr at 37°C prior to analysis for T cell activation (17). Data shown is the average of triplicate samples for each assay, and the error bars indicate the mean T cell activation +/− the standard deviation. Statistical comparisons between two groups were performed using an unpaired t test while comparison among three groups was performed using a one-way ANOVA. In each case, p≤0.01 was considered to be significant. Adjustment for multiple comparisons was made using the Bonferroni correction.
Flow cytometry and ROS production
Cells were fixed, permeabilized, and incubated with the mAb MaP.DM1 or HLA-DO (BD Biosciences) (17). To detect intracellular ROS, viable cells were incubated with 5 μM CM-H2DCFDA (Invitrogen) and stimulated +/− 10 μg/mL PMA (Sigma) for 30 min at 37°C (22). Cellular ROS production was sensitive to the oxidase inhibitor diphenyleneiodonium.
Results and Discussion
Diminished cytoplasmic Ag presentation in B-LCL with reduced p40phox expression
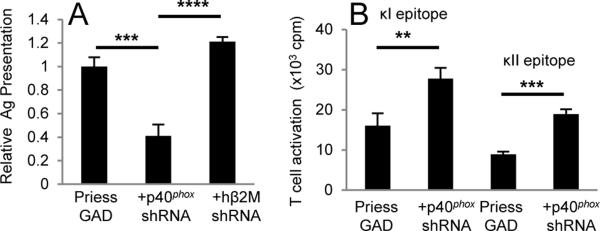
Autophagy promotes cytoplasmic Ag presentation by MHCII (12, 23). To test if p40phox plays a role in cytoplasmic GAD Ag presentation, p40phox expression was disrupted in PriessGAD B-LCL using shRNA. Expression of p40phox was reduced ~80% in these cells compared to parental PriessGAD while GAD Ag expression was unperturbed (Supplemental Fig. 1A–B). The ability of p40phox-deficient B-LCL to present GAD epitopes was substantially reduced (Fig. 1A). PriessGAD cells transduced with control hβ2M shRNA (Supplemental Fig. 1C) stimulated GAD-specific T cells comparably with parental PriessGAD (Fig. 1A). Levels of LC3-II, a marker of autophagosome formation (24), were similar in each B cell line tested, suggesting little change in autophagy with disruption of p40phox (Supplemental Fig. 1D). Surface expression of HLA-DR4 was equivalent for PriessGAD and shRNA-treated cells (Supplemental Fig. 1E–F). Notably, PriessGAD cells with reduced p40phox levels stimulated T cells specific for endogenous Ag Ig κ more efficiently than the parental PriessGAD cells (Fig. 1B). These results suggest reduced p40phox expression in B-LCL may compromise cytoplasmic Ag presentation while favoring epitope presentation from endogenous Ig κ.
FIGURE 1.
Altered Ag presentation in B-LCL with reduced p40phox expression. A, PriessGAD, PriessGAD + p40phox shRNA, or PriessGAD + hβ2M shRNA cells were cultured with GAD-specific T cells to measure cytoplasmic GAD presentation. B, PriessGAD and PriessGAD + p40phox shRNA cells were cultured with either κI- or κII-specific T cells to measure endogenous Ig κ presentation. In data not shown, APC were titrated from 1000–125 cells/well in order to determine the effect of reduced p40phox expression on endogenous Ig κ presentation. Data in A–B representative of 3 independent experiments. (**p≤0.01, ***p≤0.001, ****p≤0.0001)
Reducing B-LCL p40phox expression disrupted MHCII exogenous Ag presentation
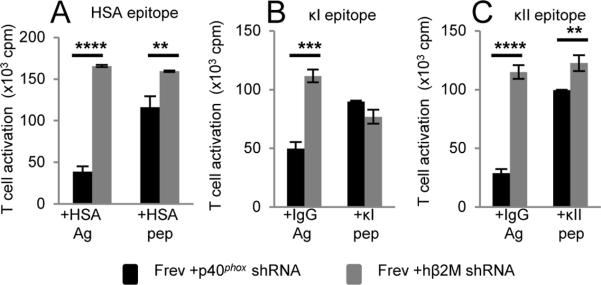
To evaluate the role of p40phox in exogenous Ag presentation, p40phox expression was diminished ~80% by treating Frev B-LCL with a p40phox-specific shRNA (Supplemental Fig. 1G). T cell responses to the exogenous Ag HSA and Frev cells with diminished p40phox levels were significantly reduced compared to Frev cells transduced with hβ2M shRNA (Fig. 2A, Supplemental Fig. 1H) or the parental line Frev (data not shown). The ability of Frev cells with reduced p40phox expression to present exogenous IgG Ag was also evaluated. Frev cells with reduced p40phox levels were less effective in presenting exogenous IgG to T cells compared with Frev transduced with control hβ2M shRNA (Fig. 2B–C) or the parental line Frev (data not shown). Surface expression of HLA-DR4 was equivalent in each cell line (Supplemental Fig. 1I–J). Only minor differences were observed in exogenous peptide presentation by Frev cells with diminished p40phox expression compared to cells treated with hβ2M shRNA (Fig. 2A–C). These results suggest that a reduction in p40phox expression in B-LCL may perturb multiple routes for Ag presentation by MHCII.
FIGURE 2.
Reduced exogenous Ag presentation in B-LCL with diminished p40phox expression. A, Frev + p40phox shRNA or Frev + hβ2M shRNA cells were incubated with HSA Ag or HSA64-76 peptide and cultured with HSA-specific T cells to measure MHCII presentation. Data representative of 2 independent experiments. B and C, Frev + p40phox shRNA or Frev + hβ2M shRNA cells were incubated with human IgG Ag, κI188-203 peptide, or κII145-159 peptide and cultured with either κI-specific T cells (B) or κII-specific T cells (C) to measure MHCII presentation. Minor differences in peptide presentation were observed using B-LCL treated with p40phox shRNA based on statistical analyses. Data in B–C representative of 3 independent experiments. (**p≤0.01, ***p≤0.001, ****p≤0.0001)
Analysis of the MHCII pathway in B-LCL with mutations in p40phox
A new genetic subgroup of CGD with mutations in the gene NCF4 encoding p40phox was described in a patient and linked to functional defects in the neutrophil NADPH oxidase (3). In this patient, one NCF4 allele harbors a frame-shift mutation with a premature stop codon while the other allele encodes a point mutation (R105Q) resulting in a nonfunctional form of p40phox. A B-LCL from this patient (AR40) expressing HLA-DR4 was further transduced with either wild-type p40phox (AR40.DR4.p40phox WT) or the R105Q mutant allele (AR40.DR4.p40phox R105Q) to evaluate the effects of p40phox mutation and reconstitution. Immunoblots demonstrated reduced p40phox expression in AR40.DR4 cells, consistent with the frame-shifted NCF4 allele as seen in patient neutrophils (3), and higher levels of wild-type or mutant p40phox in the reconstituted cells (Fig. 3A). Levels of gp91phox and p67phox were comparably reduced in the reconstituted cells relative to the patient line, likely due to clonal variation. Extracellular ROS production upon PMA stimulation was similar in the patient and wild-type p40phox reconstituted B cells (Supplemental Fig. 2A) as observed in p40phox-deficient neutrophils (3). However, basal and PMA-inducible intracellular ROS production were reduced in the AR40.DR4 cells compared to cells expressing wild-type p40phox (Fig. 3B), thus suggesting a defect in the ability of the oxidase in the patient B cells to produce intracellular ROS similar to p40phox-deficient neutrophils (3).
FIGURE 3.
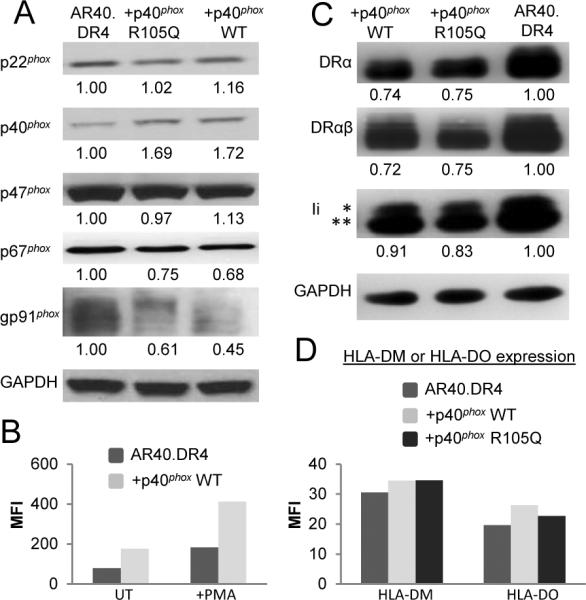
Expression of oxidase subunits and MHCII in p40phox-deficient and reconstituted B-LCL. A, Cell lysates were immunoblotted to detect oxidase subunits and GAPDH. Values indicate the ratio of oxidase subunit levels to a loading control. B, B cells were incubated with CM-H2DCFDA to detect intracellular ROS production and left untreated (UT) or PMA stimulated. C, Cell lysates were immunoblotted for HLA-DRα, HLA-DRαβ, Ii, and GAPDH. Values indicate the ratio of MHCII or Ii to GAPDH. D, B cells were stained to detect HLA-DM or HLA-DO. Data in panels A–D representative of at least 3 independent experiments.
Whether the absence of functional p40phox in AR40.DR4 B-LCL influenced the expression of molecules in the MHCII Ag presentation pathway was tested. A slight reduction in the levels of total HLA-DRα, HLA-DRαβ, and Ii were observed in AR40.DR4.p40phox WT and AR40.DR4.p40phox R105Q compared to AR40.DR4 (Fig. 3C). Maturation of Ii was not impaired in AR40.DR4 as detected by the presence of mature glycosylated forms of Ii (* in Fig. 3C) in the p40phox-deficient and reconstituted B-LCL. Changes in HLA-DM and HLA-DO can alter Ag presentation without perturbing T cell responses to synthetic peptides (25–27). AR40.DR4, AR40.DR4.p40phox WT, and AR40.DR4.p40phox R105Q expressed equivalent levels of HLA-DM and HLA-DO (Fig. 3D). Taken together, these results suggest that the absence of functional p40phox in AR40.DR4 did not substantially alter the levels of HLA-DR, -DM, -DO, and Ii.
Reconstitution of p40phox-deficient B-LCL partially restored MHCII exogenous Ag presentation
Naturally occurring mutations in p40phox impacted the ability of B-LCL to efficiently present Ag to MHCII-restricted T cells (Fig. 4). Reconstitution of the patient-derived AR40.DR4 cells with wild-type p40phox, but not the mutant p40phox R105Q allele, enhanced exogenous HSA Ag presentation (Fig. 4A). AR40.DR4 cells were unable to present either Ig κI or κII epitopes to T cells (Fig. 4B–C). Reconstitution of AR40.DR4 cells with p40phox WT restored κI epitope presentation to a greater extent than with p40phox R105Q expression (Fig. 4B). Only reconstitution of AR40.DR4 with p40phox WT facilitated presentation of the κII epitope from exogenous hIgG (Fig. 4C). Although AR40.DR4 cells were able to present exogenously added synthetic peptides to T cells (Fig. 4), the level of κI peptide presentation was reduced compared to either AR40.DR4.p40phox WT or AR40.DR4.p40phox R105Q (Fig. 4B). Surface expression of HLA-DR4 was equivalent in each cell line (Supplemental Fig. 2B). Exogenous tetanus toxoid Ag presentation was reduced not only in AR40.DR4 but in B cells deficient in another oxidase subunit gp91phox (Supplemental Fig. 2C), consistent with perturbations in oxidase function modulating MHCII Ag presentation. Reconstitution of AR40.DR4 with wild-type p40phox restored tetanus presentation (Supplemental Fig. 2C). Addition of an extracellular source of ROS failed to reconstitute tetanus presentation by B cells deficient in p40phox or gp91phox (Supplemental Fig. 2D). The ability of AR40.DR4 to endocytose a model exogenous Ag, FITC-albumin, was equivalent for p40phox-deficient or reconstituted B-LCL (Supplemental Fig. 2E). In data not shown, we observed a similar persistence of the FITC-albumin at longer time points (6–18 hr) in each cell line. These results suggest that p40phox-deficiency does not substantially affect the internalization or initial degradation of a model exogenous protein.
FIGURE 4.
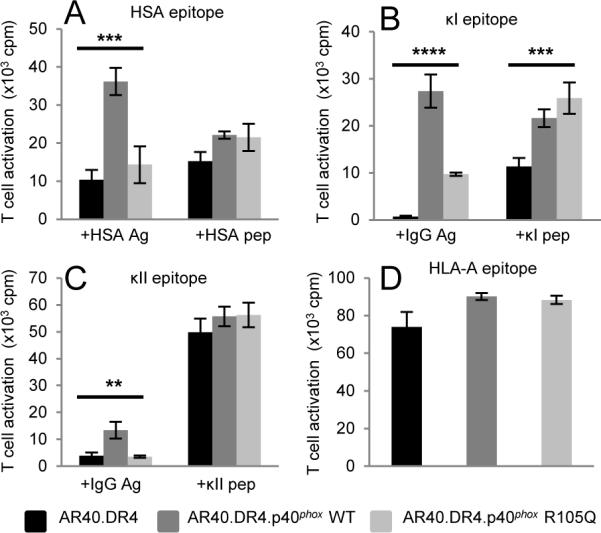
Exogenous but not endogenous Ag presentation was defective in p40phox-deficient B-LCL. A–C, B cells were incubated with HSA Ag or HSA64-76 peptide (A), human IgG Ag (B–C), κI188-203 peptide (B), or κII145-159 peptide (C) as in Fig. 2 to measure MHCII presentation. In data not shown, cells without Ag or peptide failed to activate epitope-specific T cells. (D) B cells were cultured with T cells to measure endogenous HLA-A epitope presentation. Data in A–D representative of 3 independent experiments. (**p≤0.01, ***p≤0.001, ****p≤0.0001)
The presence of assembled HLA-DRαβ dimers on the surface of AR40.DR4 suggested these MHCII may acquire peptides from a source other than exogenous Ag. The ability of these cells to present antigenic peptides derived from an endogenous transmembrane protein was evaluated using an HLA-DR4-restricted T cell recognizing an epitope from MHC class I HLA-A. AR40.DR4 cells activated the HLA-A-specific T cells while reconstitution with either the p40phox WT or R105Q mutant allele did not enhance this Ag presentation (Fig. 4D). Total MHC class I expression in each of the AR40.DR4-derived cells was equivalent (data not shown). These results suggest that while MHCII-restricted exogenous Ag presentation was impaired in the p40phox-deficient cells, the presentation of an endogenous transmembrane protein in the context of MHCII could be readily detected.
In conclusion, the microbicidal role of phagocyte NADPH oxidase in neutrophils and macrophages during the innate immune response to pathogenic infections is well-established, but the oxidase's role in APC during the adaptive immune response is less clear. Here, functional p40phox was shown to be required for the efficient presentation of cytoplasmic GAD and multiple exogenous Ag by MHCII in B cells. Additionally, studies suggest that functional gp91phox is important for MHCII presentation of exogenous Ag. In phagocytes, cytoplasmic p40phox binds to membrane phosphatidylinositol 3-phosphate (PI3P) and promotes assembly of the NADPH oxidase complex on phagosomes (19). Association of p40phox and the oxidase with PI3P found in endosomal/lysosomal membranes (3) could influence MHCII Ag processing and presentation within these organelles. The cytosolic localization of the mutant p40phox R105Q and lack of PI3P binding (3) may explain its inability to completely restore exogenous Ag presentation in p40phox-deficient B cells. In B cells, the oxidase may regulate BCR signaling (28) and as revealed here, MHCII Ag presentation. Constitutive and inducible intracellular ROS production was higher in B cells expressing functional p40phox, supporting a direct role for p40phox in regulating B cell intracellular ROS generation. Interestingly, p40phox-deficient B cells were capable of presenting epitopes derived from endogenous membrane-resident proteins suggesting that p40phox may modulate the peptide repertoire displayed by MHCII on B cells and subsequently, CD4+ T cell activation. Alterations in the function of the oxidase in B cells may therefore contribute to genetic predisposition to autoimmunity in some CGD patients. Increased incidences of rheumatoid arthritis, inflammatory bowel disease, as well as discoid lupus have been associated with oxidase subunit mutations and CGD (29).
Supplementary Material
Acknowledgments
1This work was supported by grants to J.S.B. (AI079065 and AI056097) and M.C.D. (HL45635), from the Indiana Clinical and Translational Sciences Institute and the Center of Excellence in Molecular Hematology at Indiana University (NIDDK P30 DK090948), the Children's Discovery Institute at Washington University and St. Louis Children's Hospital, and the American Heart Association (J.D.M.)
Footnotes
Disclosures The authors have no financial conflict of interest.
2Abbreviations used in this paper: B-LCL, B-lymphoblastoid cell line; CGD, chronic granulomatous disease; GAD, glutamate decarboxylase; human β2-microglobulin, hβ2M; HSA, human serum albumin; MHCII, MHC class II molecules; ROS, reactive oxygen species; PI3P, phosphatidylinositol 3-phosphate
References
- 1.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem. J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinauer MC, Newburger PE. The phagocyte system and disorders of granulopoiesis and granulocyte function. In: Nathan DG, Orkin SH, Ginsburg D, Look AT, editors. Nathan and Oski's Hematology of Infancy and Childhood. 7th ed. WB Saunders Company; Philadelphia, PA: 2008. pp. 1109–1220. [Google Scholar]
- 3.Matute JD, Arias AA, Wright NA, Wrobel I, Waterhouse CC, Li XJ, Marchal CC, Stull ND, Lewis DB, Steele M, Kellner JD, Yu W, Meroueh SO, Nauseef WM, Dinauer MC. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114:3309–3315. doi: 10.1182/blood-2009-07-231498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantegazza AR, Savina A, Vermeulen M, Perez L, Geffner J, Hermine O, Rosenzweig SD, Faure F, Amigorena S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Gelderman KA, Hultqvist M, Pizzolla A, Zhao M, Nandakumar KS, Mattsson R, Holmdahl R. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J. Clin. Invest. 2007;117:3020–3028. doi: 10.1172/JCI31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbey C, Tiercy JM, Fairweather N, Niemann H, Seger R, Corradin G. Processing and presentation of tetanus toxin by antigen-presenting cells from patients with chronic granulomatous disease (CGD) to human specific T cell clones are not impaired. Clin. Exp. Immunol. 1994;95:227–231. doi: 10.1111/j.1365-2249.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heijnen CJ, van der Meer JW, Zegers BJ. Altered antigen-presentation in the induction of the in-vitro antigen-specific T helper cell function in patients with chronic granulomatous disease. Clin. Exp. Immunol. 1986;66:111–117. [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, Glogauer M, Grinstein S, Brumell JH. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA. 2009;106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casbon AJ, Allen LA, Dunn KW, Dinauer MC. Macrophage NADPH oxidase flavocytochrome B localizes to the plasma membrane and Rab11-positive recycling endosomes. J. Immunol. 2009;182:2325–2339. doi: 10.4049/jimmunol.0803476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts C. Antigen processing in the endocytic compartment. Curr. Opin. Immunol. 2001;13:26–31. doi: 10.1016/s0952-7915(00)00177-1. [DOI] [PubMed] [Google Scholar]
- 12.Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J. Immunol. 2009;182:3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat. Immunol. 2004;5:685–692. doi: 10.1038/ni1088. [DOI] [PubMed] [Google Scholar]
- 14.Olsson LM, Lindqvist AK, Kallberg H, Padyukov L, Burkhardt H, Alfredsson L, Klareskog L, Holmdahl R. A case-control study of rheumatoid arthritis identifies an associated single nucleotide polymorphism in the NCF4 gene, supporting a role for the NADPH-oxidase complex in autoimmunity. Arthritis Res. Ther. 2007;9:R98. doi: 10.1186/ar2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, Shugart YY, Griffiths AM, Targan SR, Ippoliti AF, Bernard EJ, Mei L, Nicolae DL, Regueiro M, Schumm LP, Steinhart AH, Rotter JI, Duerr RH, Cho JH, Daly MJ, Brant SR. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts RL, Hollis-Moffatt JE, Gearry RB, Kennedy MA, Barclay ML, Merriman TR. Confirmation of association of IRGM and NCF4 with ileal Crohn's disease in a population-based cohort. Genes Immun. 2008;9:561–565. doi: 10.1038/gene.2008.49. [DOI] [PubMed] [Google Scholar]
- 17.Crotzer VL, Glosson N, Zhou D, Nishino I, Blum JS. LAMP-2-deficient human B cells exhibit altered MHC class II presentation of exogenous antigens. Immunology. 2010;131:318–330. doi: 10.1111/j.1365-2567.2010.03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lich JD, Elliott JF, Blum JS. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J. Exp. Med. 2000;191:1513–1524. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian W, Li XJ, Stull ND, Ming W, Suh CI, Bissonnette SA, Yaffe MB, Grinstein S, Atkinson SJ, Dinauer MC. Fc gamma R-stimulated activation of the NADPH oxidase: phosphoinositide-binding protein p40phox regulates NADPH oxidase activity after enzyme assembly on the phagosome. Blood. 2008;112:3867–3877. doi: 10.1182/blood-2007-11-126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruneberg U, Rich T, Roucard C, Marieke van Ham S, Charron D, Trowsdale J. Two widely used anti-DR alpha monoclonal antibodies bind to an intracellular C-terminal epitope. Hum. Immunol. 1997;53:34–38. doi: 10.1016/s0198-8859(97)00025-6. [DOI] [PubMed] [Google Scholar]
- 21.Lerner EA, Matis LA, Janeway CA, Jr., Jones PP, Schwartz RH, Murphy DB. Monoclonal antibody against an Ir gene product? J. Exp. Med. 1980;152:1085–1101. doi: 10.1084/jem.152.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JR, Koretzky GA. Production of reactive oxygen intermediates following CD40 ligation correlates with c-Jun N-terminal kinase activation and IL-6 secretion in murine B lymphocytes. Eur. J. Immunol. 1998;28:4188–4197. doi: 10.1002/(SICI)1521-4141(199812)28:12<4188::AID-IMMU4188>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 23.Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lich JD, Jayne JA, Zhou D, Elliott JF, Blum JS. Editing of an immunodominant epitope of glutamate decarboxylase by HLA-DM. J. Immunol. 2003;171:853–859. doi: 10.4049/jimmunol.171.2.853. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandra L, Kovats S, Eastman S, Rudensky AY. Variation in HLA-DM expression influences conversion of MHC class II alpha beta:class II-associated invariant chain peptide complexes to mature peptide-bound class II alpha beta dimers in a normal B cell line. J. Immunol. 1996;156:2196–2204. [PubMed] [Google Scholar]
- 27.Yi W, Seth NP, Martillotti T, Wucherpfennig KW, Sant'Angelo DB, Denzin LK. Targeted regulation of self-peptide presentation prevents type I diabetes in mice without disrupting general immunocompetence. J. Clin. Invest. 2010;120:1324–1336. doi: 10.1172/JCI40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards SM, Clark EA. BCR-induced superoxide negatively regulates B-cell proliferation and T-cell-independent type 2 Ab responses. Eur. J. Immunol. 2009;39:3395–3403. doi: 10.1002/eji.200939587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Ravin SS, Naumann N, Cowen EW, Friend J, Hilligoss D, Marquesen M, Balow JE, Barron KS, Turner ML, Gallin JI, Malech HL. Chronic granulomatous disease as a risk factor for autoimmune disease. J. Allergy Clin. Immunol. 2008;122:1097–1103. doi: 10.1016/j.jaci.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farber CM, Liebes LF, Kanganis DN, Silber R. Human B lymphocytes show greater susceptibility to H2O2 toxicity than T lymphocytes. J. Immunol. 1984;132:2543–2546. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.