Abstract
The transcriptional co-activators PGC-1α and PGC-1β are master regulators of oxidative phosphorylation and fatty acid oxidation gene expression. Pressure overload hypertrophy and heart failure are associated with repressed PGC-1α and PGC-1β gene expression. Maintaining expression of PGC-1α and β preserves contractile function in response to a pathological increase in workload. Here we discuss the regulation of PGC-1 proteins under conditions of pressure overload hypertrophy and heart failure.
PGC-1 Transcriptional Coactivators
The family of PGC-1 (peroxisome proliferator activated receptor γ (PPARγ) coactivator) proteins plays a key role in the regulation of mitochondrial biogenesis and metabolism. Three members of the PGC-1 family of transcriptional coactivators have been described: PGC-1α was first identified in a yeast 2-hybrid screen as a PPARγ interaction protein following cold exposure in brown adipose tissue (Puigserver et al. 1998). Two structural homologues of PGC-1α were subsequently identified by sequence homology: PGC-1β (also called PERC, PGC-1 related estrogen receptor coactivator), and PRC (PGC-1-related coactivator 1) (Andersson and Scarpulla 2001; Kressler et al. 2002; Lin et al. 2002). PGC-1α and PGC-1β are mainly expressed in tissues with high content of mitochondria and high oxidative capacity such as the heart, brown adipose tissue, skeletal muscle, and kidney. PGC-1α expression is induced by conditions that increase energy demand and mitochondrial ATP-production such as fasting, exercise, and cold exposure (Kelly and Scarpulla 2004; Lehman et al. 2000; Puigserver et al. 1998). PGC-1α expression in the heart is induced at birth coincident with the increase in mitochondrial oxidative capacity (Lehman et al. 2000).
Coactivators are proteins that bind to transcription factors and amplify the activity of the transcriptional machinery. Both, PGC-1α and PGC-1β, regulate the expression of genes involved in oxidative phosphorylation via coactivation of the transcription factors NRF (nuclear respiratory factor) 1 and 2, TFAm (mitochondrial transcription factor A), and ERR (estrogen-related receptor)-α. NRF-1 and 2 regulate the expression of nuclear encoded genes that are required for mitochondrial oxidative phosphorylation. They also bind to the TFAm promoter thereby coordinating the transcription of the nuclear and mitochondrial genome (Kelly and Scarpulla 2004). TFAm binds to both strands of mitochondrial DNA (mtDNA) to coordinate its transcription and replication. The mitochondrial genome contains 37 genes encoding 22 tRNAs, 2 rRNAs and 13 subunits involved in the electron transport chain that are present in complexes I, III, IV and V (not II). This accounts for less than 2 % of the > 1500 genes that encode mitochondrial proteins. Deletion of TFAm in cardiac tissue resulted in decreased electron transport capacity, decreased mitochondrial DNA content, cardiomyopathy, and heart failure (Larsson et al. 1998; Parisi et al. 1993) highlighting the critical importance of these specific oxidative phosphorylation (OXPHOS) subunits that are encoded by mtDNA. PGC-1α also regulates the expression of genes involved in fatty acid metabolism by coactivating the nuclear receptors (NRs) PPARs and ERRs. PPARα and PPARβ regulate fatty acid uptake and oxidation in the heart. PPARs form a heterodimeric complex with the retinoid X receptor (RXR). This complex mediates transcriptional activation of their target genes following recruitment of coactivators, such as PGC-1α, and direct binding of ligands such as long chain fatty acids or their derivatives to their cognate NRs (Lehman and Kelly 2002) (For summary see Figure 1).
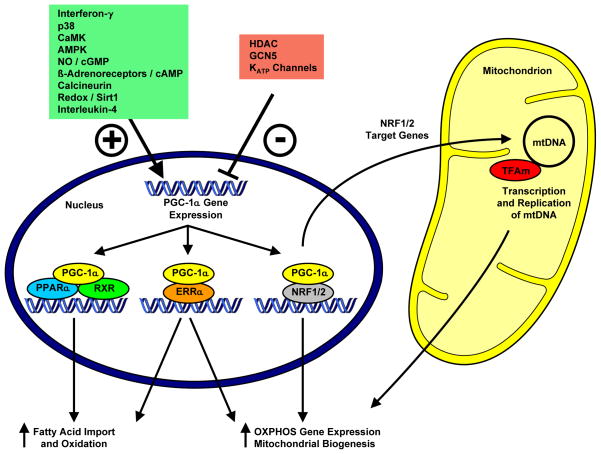
Figure 1.
Multiple stimuli increase (indicated by +) or decrease (indicated by −) PGC-1α expression. PGC-1α coactivates PPAR and ERR transcription factors and thereby regulates the expression of genes involved in mitochondrial fatty acid import and oxidation. In addition, PGC-1α regulates the expression of nuclear encoded oxidative phosphorylation (OXPHOS) subunits via coactivation of ERRs, NRF-1 and NRF-2. NRF-1 and NRF-2 also bind to the promoter of the mitochondrial transcription factor A (TFAm) and coordinates the transcription of the nuclear and mitochondrial genome.
Furthermore, PGC-1α modulates the activity of the transcriptional machinery by docking to the Cdk7/Cyclin H/ménage-à-trois 1 (MAT1) heterotrimer. PGC-1α directly interacts with MAT1/Cdk7 and Cdk7-mediated phosphorylation of RNA polymerase increases transcriptional activity. Knockdown of MAT1 resulted in PGC-1 mediated metabolic defects in isolated working heart perfusions and isolated mitochondria respirations (Sano et al. 2007a). PGC-1α interacts with the splicing machinery and the SRC-1 histone acetyl transferases resulting in increased transcription. Also, PGC-1α assembles with the TRAP/DRIP transcription initiation complex facilitating the interaction between RNA polymerase II and the coactivator complex (Wallberg et al. 2003).
Transgenic Mouse Models for PGC-1 Proteins
Overexpression Models
The role of PGC-1 proteins in the heart has been extensively studied using transgenic mouse models. PGC-1α induces cardiac mitochondrial biogenesis in vivo. Cardiomyocyte-specific overexpression of PGC-1α using the αMHC (α-myosin heavy chain) promoter starting at embryonic day 11.5 leads to uncontrolled mitochondrial biogenesis, ultimately causing loss of sarcomeric structure and a dilated cardiomyopathy (Lehman et al. 2000). Mitochondria occupy about 40 % of the volume in adult cardiomyocytes (cytosol only about 5 %). Thus, any increase in mitochondrial volume might compromise the content of structures in the already low cytosolic volume fraction, displacing the sarcomeric apparatus and impairing contractile function. Inducing PGC-1α overexpression in adult mouse hearts resulted in a more modest increase in mitochondrial number that ultimately precipitated a cardiomyopathy, which completely resolved when PGC-1α transgene expression was turned off (Russell et al. 2004). In vitro, both PGC-1α and PGC-1β have been shown to stimulate mitochondrial biogenesis and O2 consumption (Lehman et al. 2000; St-Pierre et al. 2003). However, no mouse-model has been described yet showing increased mitochondrial biogenesis following PGC-1β overexpression.
Knockout Models
Two independently generated PGC-1α-KO models have been described (Arany et al. 2005; Lehman et al. 2008; Leone et al. 2005). One model exhibited normal contractile function under basal conditions and the other showed age-dependent contractile dysfunction. PGC-1α deletion resulted in impaired expression of genes involved in oxidative phosphorylation and fatty acid oxidation. Isolated working heart experiments demonstrated lower cardiac power, reduced palmitate oxidation, and increased glucose oxidation. This was paralleled by impaired maximal mitochondrial oxygen consumption (VADP) with palmitoyl-L-carnitine as substrate and increased pyruvate-mediated respirations. Mitochondrial ATP-synthesis was impaired coincident with mitochondrial uncoupling. PGC-1α-KO hearts exhibited impaired inotropic and chronotropic response both in vivo in response to exercise and ex vivo following dobutamine challenge using Langendorff preparations. Mitochondrial volume density was normal in either model. Together, these studies highlight that PGC-1α expression is required for maintaining normal mitochondrial function and its deficiency manifests as modest age-dependent contractile dysfunction in non-stressed hearts. However, more substantial defects in contractile function became apparent in the face of superimposed stressors both in vivo and ex vivo.
Four independently generated PGC-1β-KO models have been recently published (Lelliott et al. 2006; Sonoda et al. 2007; Vianna et al. 2006). PGC-1β-KO hearts exhibit normal contractile function under non-stressed conditions. Inotropic responses were relatively preserved in PGC-1β-KO hearts in response to the selective β1α1-agonist dobutamine, but similar to PGC-1α KO mice, the chronotropic responses were impaired (Lelliott et al. 2006). Also, left ventricular contractility was persevered following exercise-induced stress response in PGC-1β-KO mice of similar age (Lai et al. 2008). Absence of PGC-1β resulted in repressed OXPHOS gene expression. In contrast to PGC-1α-KO hearts, fatty acid oxidation (FAO) gene expression was relatively unchanged. Similarly, FAO was not impaired in isolated working PGC-1β-KO hearts (Riehle et al. 2011). This suggests an isoform-specific contribution of PGC-1 proteins to FAO gene expression and fatty acid metabolism.
Mouse models with single deletion of PGC-1α or PGC-1β suggest that contractile function can be maintained under non-stressed conditions despite mitochondrial dysfunction, altered gene expression and cardiac metabolism, as long as the other isoform is normally expressed. These models therefore suggest redundant or overlapping roles for PGC-1 proteins in the regulation of mitochondrial gene expression and biogenesis. The extensive redundancy was demonstrated by generating mice lacking both PGC-1α and PGC-1β in the heart (Lai et al. 2008), which died shortly after birth because of heart failure. Analysis of electron microscopy sections obtained from hearts with combined deficiency for PGC-1α and PGC-1β revealed decreased mitochondrial number and size. This was associated with ultrastructural abnormalities including impaired cristae density consistent with a defect in mitochondrial biogenesis. Transcriptional analysis showed impaired fatty acid oxidation and oxidative phosphorylation gene expression. Hexokinase 2 levels were increased and pyruvate dehydrogenase kinase 4 (Pdk4) levels were decreased. This suggested increased reliance on glucose metabolism and that the postnatal fuel shift towards fatty acid oxidation was blocked. Expression of the fetal genes Nppa and Nppb was increased, whereas expression of the adult sarcomeric isoform β-myosin heavy chain (Mhy6) was decreased further supporting an arrest of the physiological maturation program. Table 1 summarizes the cardiac characteristics of gain-of-function and loss-of-function models for PGC-1 proteins in non-stressed hearts.
Table 1.
Cardiac Phenotypes of Gain-of-Function and Loss-of-Function Models for PGC-1 Proteins under Basal Conditions
| Overexpression Models | PGC-1α | PGC-1β | PGC-1α / PGC-1β |
|---|---|---|---|
| Parameter | |||
| Mitochondrial biogenesis | ↑↑ (overexpression starting ED 11.5) | ND | ND |
| ↑ (following induction in adult heart) | |||
| Contractile function | ↓↓ (overexpression starting ED 11.5) | ND | ND |
| ↓ (induction in adult heart: reversible by repression of transgene) | |||
| References | Lehman et al. 2008, Russell et al. 2004 | ||
|
| |||
| Knockout Models | PGC-1α | PGC-1β | PGC-1α / PGC-1β |
|
| |||
| Parameter | |||
| Heart size | = | = | ↓↓ |
| Contractile performance and capacity | |||
| Echocardiography | = / ↓ age-dependent | = | ↓↓ (perinatal heart failure) |
| LV Cath | ↓ max dP/dt; ↓ min dP/dt | = | ND |
| Cardiac output (IWH) | ↓ | = | ND |
| Cardiac power (IWH) | ↓ | = | ND |
| Inotropic response | ↓ (dobutamine response + post exercise) | = / ↓ (dobutamine response) | ND |
| Chronotropic response | ↓ (dobutamine response + post exercise) | ↓ (dobutamine response) | ND |
| Arrhythmia | ND | ND | bradycardia, atrio-ventricular block II |
| Mitochondrial volume density | = | = / ↓ age-dependent | ↓↓ |
| Mitochondrial function / Energetic reserves | |||
| VADP | ↑ Pyr, ↓ PC, = Glu | = Pyr, ↓ PC | ND |
| ATP-synthesis | ↓ Pyr, ↓ PC, ↓ Glu | = Pyr, ↓ PC | |
| ATP/O | ↓ Pyr, ↓ PC, ↓ Glu | = Pyr, = PC | |
| ↓ ATP-concentrations (NMR) | |||
| Fibrosis | = | = | = |
| Substrate metabolism | |||
| Glucose oxidation | ↑ | ↑ | ND |
| Glycolysis | ND | = | |
| Palmitate oxidation | ↓ | ↑ | |
| FAO gene expression | = / ↓ | = | ↓ |
| OXPHOS gene expression | = / ↓ | ↓ | ↓ |
| ANP, BNP gene expression | = / ↑ | = | ↑ |
| References | Arany et al. 2005, Arany et al. 2006, Lehman et al. 2008, Leone et al. 2005, Lu et al. 2010 | Lai et al. 2008, Lelliott et al. 2006, Riehle et al. 2011, Sonoda et al. 2007 | Lai et al. 2008 |
ND, not determined. ED, embryonic day; FAO, fatty acid oxidation; IWH, isolated working heart preparations; LV Cath, left ventricular catheterization; max dP/dt, rate of maximal increase in pressure; NMR, nuclear magnetic resonance spectroscopy; OXPHOS, oxidative phosphorylation; VADP, maximal mitochondrial oxygen consumption obtained by incubating saponin permeabilized cardiac fibers with ADP and pyruvate (Pyr), palmitoyl-L-carnitine (PC) or glutamate (Glu) as substrates respectively, each combined with malate. No transgenic mouse model with modulated PRC expression in the heart has been yet reported.
PGC-1 Proteins in Pathological Cardiac Hypertrophy and Heart Failure
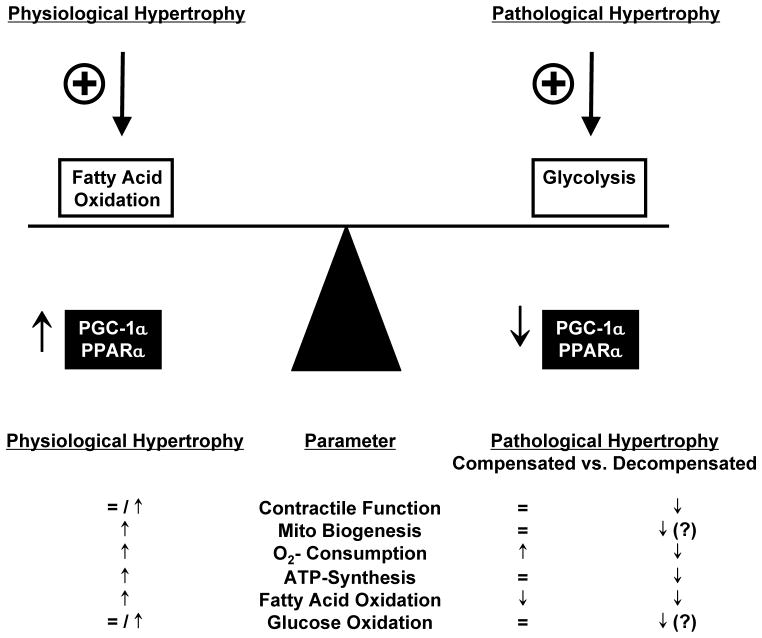
Cardiac hypertrophy is defined as an increase in ventricular mass and can be broadly characterized as physiological or pathological hypertrophy. Physiological cardiac hypertrophy is considered if the hypertrophic response does not impair contractile function and the heart is able to adapt to the increased hemodynamic load (“athlete’s heart”). This is characterized by increased mitochondrial biogenesis (Rimbaud et al. 2009; White et al. 1987), oxygen consumption, ATP synthesis and PGC-1α gene expression (Duncan et al. 2007; Finck and Kelly 2007; O'Neill et al. 2007) that compensates for the increased energy demand. In contrast, pathological hypertrophy is characterized by an initial phase of compensation, which can progress to contractile dysfunction (decompensation) that ultimately leads to heart failure. Common causes of pathological hypertrophy are aortic valve stenosis or regurgitation and arterial hypertension (Abel and Doenst 2011). A common model which is used to induce pathological hypertrophy in rodents is the induction of left ventricular pressure overload (POH) by surgical constriction of the transverse aorta between the brachiocephalic trunk and the left carotid artery (TAC) (Doenst et al. 2010; Zaha et al. 2003). POH and heart failure are associated with repressed gene expression of PGC-1α and its transcriptional partner PPAR-α leading to decreased expression of genes involved in fatty acid oxidation. This has been described for numerous rodent models of POH (Arany et al. 2006; Garnier et al. 2003; Huss et al. 2007; Riehle et al. 2011). Down regulation of the PGC-1α/PPARα complex has been suggested as a potential mechanism for the switch in substrate utilization from mainly fatty acid oxidation toward glucose oxidation (Lehman and Kelly 2002) resulting in more efficient ATP production (O2 consumed / ATP produced). This substrate switch has been described both in patients with nonischemic cardiomyopathy and in animal models of pressure overload and heart failure (Davila-Roman et al. 2002; Razeghi et al. 2001; Sack et al. 1996). PGC-1β expression is also repressed in rodent models of pressure overload hypertrophy (Riehle et al. 2011). PRC expression has been reported to be unchanged following pressure overload (Arany et al. 2006). The key features of physiological and pathological cardiac hypertrophy are summarized in Figure 2.
Figure 2.
Key features of myocardial energy metabolism in response to physiological and pathological hypertrophy.
In contrast to previous reports, a recent study reported no decrease of PGC-1α mRNA levels following pressure overload hypertrophy in rodents (Hu et al. 2008). Differences in the duration of pressure overload and the degree of left ventricular dysfunction might account for the discrepancy between these results and those of previous reports. Similarly, recent studies showed no decrease in PGC-1α protein levels in samples from human failing heart (Hu et al. 2011; Karamanlidis et al. 2010). This was associated with down regulation of ERRα and defects in mitochondrial DNA replication and maintenance. Thus mechanisms that are independent of PGC-1 may exist that result in impaired mitochondrial DNA content. Differences between studies in rodents and humans might could also be influenced by pharmacological treatment of human subjects compared to untreated animals. For instance, phosphodiesterase inhibitors and catecholamines, common drugs for the treatment of end-stage heart failure, might stimulate PGC-1α expression via cAMP-mediated mechanisms (Puigserver et al. 1998). Differences in age when comparing samples from patients with heart failure with control subjects might also confound results, as PGC-1α levels decrease with aging (Ling et al. 2004).
Hearts with Deletion of PGC-1α or PGC-1β are More Susceptible to Heart Failure in Response to Pressure Overload
Both, PGC-1α and PGC-1β deficient hearts showed accelerated heart failure in response to TAC (Arany et al. 2006; Lu et al. 2010; Riehle et al. 2011). The underlying mechanisms are complex and other mechanisms besides energetic starvation might exist. Accelerated heart failure in PGC-1α-KO hearts was associated with further repression of OXPHOS and fatty acid oxidation gene expression (Arany et al. 2006). Importantly, PGC-1β expression was maintained, suggesting PGC-1 independent mechanisms exist that regulate OXPHOS and FAO gene expression. This also suggests a threshold of mitochondrial capacity exists, which cannot be sustained by PGC-1β in the absence of PGC-1α under conditions of pressure overload. A number of defects became apparent in PGC-1β-KO hearts following TAC that ultimately resulted in heart failure. This includes failure to increase glycolysis and glucose oxidation, reduced cardiac efficiency and further impairment of mitochondrial function. Both, PGC-1α (Lu et al. 2010) and PGC-1β deficient hearts (Riehle et al. 2011) exhibited increased oxidative stress following TAC indicating an overlapping role for the PGC-1 proteins in regulating antioxidant mechanisms. Together, these studies suggest multiple levels of redundancy in the functions of PGC-1α and PGC-1β. A threshold for PGC-1 proteins might be required to sustain mitochondrial and contractile function. This hypothesis is supported by the study of hearts with combined deletion of PGC-1α and PGC-1β that died from early onset heart failure (Lai et al. 2008). Table 2 summarizes the characteristics of PGC-1α and PGC-1β-KO hearts following pressure overload induced by TAC.
Table 2.
Characteristics of Loss-of-Function Models for PGC-1 Proteins in Response to Pressure Overload
| Knockout Models | PGC-1α-KO | PGC-1β-KO |
|---|---|---|
| Parameter | ||
| Heart size | ↑ | = |
| Fibrosis | = / ↑ | ↑ |
| Contractile performance and capacity | ||
| Echocardiography | ↓ | ↓ |
| LV Cath | ↓ | ↓ |
| Cardiac output (IWH) | ND | = (3 wk post TAC) |
| Cardiac power (IWH) | ND | = (3 wk post TAC) |
| Mitochondrial volume density | ND | = |
| Mitochondrial function / Energetic reserves | ||
| VADP | ND | = Pyr, ↓ PC |
| ATP-synthesis | ND | = Pyr, ↓ PC |
| ATP/O | ND | = Pyr, = PC |
| ↓ succinate dehydrogenase / cytochrome oxidase (in situ activity stains) | ||
| ↓ Citrate synthase | ||
| Substrate metabolism | ||
| Glucose oxidation | ND | ↓ (3 wk post TAC) |
| Glycolysis | ND | ↓ (3 wk post TAC) |
| Palmitate oxidation | ND | = (3 wk post TAC) |
| FAO gene expression | = / ↓ | = |
| OXPHOS gene expression | = / ↓ | = / ↓ |
| ANP, BNP expression | ↑ | = |
| Oxidative stress | ↑ | ↑ |
| References | Arany et al. 2006; Lu et al. 2010 | Riehle et al. 2011 |
ND, not determined. FAO, fatty acid oxidation; IWH, isolated working heart preparations; LV Cath, left ventricular catheterization; OXPHOS, oxidative phosphorylation; VADP, maximal mitochondrial oxygen consumption following incubation of saponin permeabilized cardiac fibers to ADP with pyruvate (Pyr) or palmitoyl-L-carnitine (PC) as substrate respectively, each combined with malate. Characteristics for PGC-1β-KO mice are presented vs. TAC operated WT controls 8 weeks post TAC unless otherwise indicated. No gain-of-function models for PGC-1 proteins in response to pressure overload have been described yet. No transgenic mouse model with modulated PRC expression in the heart has been yet reported.
Modulation of PGC-1α Activity
PGC-1α is induced by exercise, cold exposure and fasting, which are conditions that promote oxidative metabolism. Signaling pathways associated with those stimuli include NO, AMPK, p38 MAPK, β-adrenergic receptor signaling / cAMP, and calcium-calmodulin kinase, which all increase PGC-1α expression or transactivation. These mechanisms are the subject of previous reviews (Huss and Kelly 2005; Rowe et al. 2010; Schilling and Kelly 2011; Ventura-Clapier et al. 2008) and are summarized in Figure 1. The mechanisms for impaired PGC-1α expression and PGC-1α downstream targets under conditions of pressure overload and heart failure are incompletely understood. Previous studies suggested roles for cyclin-dependent kinase-9 (Cdk9) (Sano and Schneider 2004) and Akt (Cook et al. 2002): Akt directly phosphorylates PGC-1 α and prevents the recruitment of PGC-1α to its cognate promoter regions in hepatocytes (Li et al. 2007). Cdk9 phosphorylates RNA-Polymerase II (RNAPII). This blocks the recruitment of RNAPII and the general transcription factor TATA-binding protein (TBP) to the endogenous PGC-1 promoter and decreases the assembly of the PGC-1 pre-initiation complex. Also, mice with disrupted KATP activity (SUR1-transgenic mice) or Kir 6.2 knockdown (Kir6.2 KO) showed decreased PGC-1α levels. Using rat neonatal cardiomyocytes, disruption of KATP activity decreased the promoter activity and expression of PGC-1α in response to hypoxia (Hu et al. 2008). Thus various upstream signaling mechanisms likely regulate PGC-1α levels under stress conditions.
A recent study has identified Mitochondrial Endonuclease G (ENDOG) as a direct target of ERRα and PGC-1α (McDermott-Roe et al. 2011). ENDOG is a nuclear-encoded member of family of DNA/RNA nucleases (Schafer et al. 2004). The best-characterized function of ENDOG is its contribution in nucleosome degradation during programmed cell death. ENDOG-KO mice exhibit increased ROS production and cardiac hypertrophy. Therefore, it will be of interest to determine the contribution of ENDOG in PGC-1 deficient hearts under conditions of pressure overload in the context of increased oxidative stress and accelerated transition to heart failure.
PGC-1 Proteins and Angiogenesis
Angiogenesis occurs both under physiological conditions such as exercise, embryogenesis, and pregnancy as well as under pathological conditions i.e. tumor growth. Cardiac tissue is highly vascularized based on its high energy and oxygen demand. Recent studies suggested microvascular rarefaction as a cause for accelerated heart failure in response to pressure overload (Izumiya et al. 2006; Sano et al. 2007b). Both, PGC-1α and PGC-1β regulate angiogenesis as shown in skeletal muscle (Arany et al. 2008; Rowe et al. 2011). This is facilitated via PGC-1 / ERRα-mediated induction of vascular endothelial growth factor (VEGF) and is independent of hypoxia inducible factor (HIF) signaling. It will be of great interest to determine the impact of PGC-1α and PGC-1β mediated angiogenesis on the development of heart failure under conditions of pathologically increased workload, however a recent report provided strong evidence that PGC-1α mediated angiogenesis is essential for the cardiac adaptation to pregnancy and its absence leads to peripartum cardiomyopathy (Patten et al. 2012).
Summary and Clinical Perspective
Absence of PGC-1 proteins accelerated the transition to heart failure following pressure overload. Even though definitive proof is missing, the positive effect of exercise training in patients with heart failure might involve induction of PGC-1α expression. It would be of interest to determine if overexpression of PGC-1α or PGC-1β in the physiological range has beneficial effects under conditions of pressure overload. If true, then pharmacological modulation of PGC-1 protein activity might be a promising future target for retarding the transition from compensated pressure overload hypertrophy to heart failure.
Footnotes
Conflict of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res. 2011;90:234–42. doi: 10.1093/cvr/cvr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Scarpulla RC. Pgc-1-related coactivator, a novel, serum-inducible coactivator of nuclear respiratory factor 1-dependent transcription in mammalian cells. Mol Cell Biol. 2001;21:3738–49. doi: 10.1128/MCB.21.11.3738-3749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–12. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–71. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–91. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem. 2002;277:22528–33. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–7. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- Doenst T, Pytel G, Schrepper A, Amorim P, Farber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res. 2010;86:461–70. doi: 10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–17. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–8. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, Nichols CG, Bache RJ, Chen Y. Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload. Circ Res. 2008;103:1009–17. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Xu X, Lu Z, Zhang P, Fassett J, Zhang Y, Xin Y, Hall JL, Viollet B, Bache RJ, et al. AMP activated protein kinase-alpha2 regulates expression of estrogen-related receptor-alpha, a metabolic transcription factor related to heart failure development. Hypertension. 2011;58:696–703. doi: 10.1161/HYPERTENSIONAHA.111.174128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–55. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–93. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–8. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–68. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- Kressler D, Schreiber SN, Knutti D, Kralli A. The PGC-1-related protein PERC is a selective coactivator of estrogen receptor alpha. J Biol Chem. 2002;277:13918–25. doi: 10.1074/jbc.M201134200. [DOI] [PubMed] [Google Scholar]
- Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP. Transcriptional coactivators PGC-1{alpha} and PGC-l{beta} control overlapping programs required for perinatal maturation of the heart. Genes Dev. 2008;22:1948–61. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–6. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–56. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED, et al. The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol. 2008;295:H185–96. doi: 10.1152/ajpheart.00081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29:339–45. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, et al. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol. 2006;4:e369. doi: 10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–6. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta ), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem. 2002;277:1645–8. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest. 2004;114:1518–26. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, et al. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal. 2010;13:1011–22. doi: 10.1089/ars.2009.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott-Roe C, Ye J, Ahmed R, Sun XM, Serafin A, Ware J, Bottolo L, Muckett P, Canas X, Zhang J, et al. Endonuclease G is a novel determinant of cardiac hypertrophy and mitochondrial function. Nature. 2011;478:114–8. doi: 10.1038/nature10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J, Guo A, Zaha VG, Davis DK, Schell JC, et al. A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab. 2007;6:294–306. doi: 10.1016/j.cmet.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA, Xu B, Clayton DA. A human mitochondrial transcriptional activator can functionally replace a yeast mitochondrial HMG-box protein both in vivo and in vitro. Mol Cell Biol. 1993;13:1951–61. doi: 10.1128/mcb.13.3.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–8. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–39. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–31. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- Riehle C, Wende AR, Zaha VG, Pires KM, Wayment B, Olsen C, Bugger H, Buchanan J, Wang X, Moreira AB, et al. PGC-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circ Res. 2011;109:783–93. doi: 10.1161/CIRCRESAHA.111.243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbaud S, Garnier A, Ventura-Clapier R. Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol Rep. 2009;61:131–8. doi: 10.1016/s1734-1140(09)70015-5. [DOI] [PubMed] [Google Scholar]
- Rowe GC, Jang C, Patten IS, Arany Z. PGC-1beta regulates angiogenesis in skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301:E155–63. doi: 10.1152/ajpendo.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res. 2010;107:825–38. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–33. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–42. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- Sano M, Izumi Y, Helenius K, Asakura M, Rossi DJ, Xie M, Taffet G, Hu L, Pautler RG, Wilson CR, et al. Menage-a-trois 1 is critical for the transcriptional function of PPARgamma coactivator 1. Cell Metab. 2007a;5:129–42. doi: 10.1016/j.cmet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007b;446:444–8. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- Sano M, Schneider MD. Cyclin-dependent kinase-9: an RNAPII kinase at the nexus of cardiac growth and death cascades. Circ Res. 2004;95:867–76. doi: 10.1161/01.RES.0000146675.88354.04. [DOI] [PubMed] [Google Scholar]
- Schafer P, Scholz SR, Gimadutdinow O, Cymerman IA, Bujnicki JM, Ruiz-Carrillo A, Pingoud A, Meiss G. Structural and functional characterization of mitochondrial EndoG, a sugar non-specific nuclease which plays an important role during apoptosis. J Mol Biol. 2004;338:217–28. doi: 10.1016/j.jmb.2004.02.069. [DOI] [PubMed] [Google Scholar]
- Schilling J, Kelly DP. The PGC-1 cascade as a therapeutic target for heart failure. J Mol Cell Cardiol. 2011;51:578–83. doi: 10.1016/j.yjmcc.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci U S A. 2007;104:5223–8. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278:26597–603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1{alpha} Cardiovasc Res. 2008;79:208–17. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- Vianna CR, Huntgeburth M, Coppari R, Choi CS, Lin J, Krauss S, Barbatelli G, Tzameli I, Kim YB, Cinti S, et al. Hypomorphic mutation of PGC-1beta causes mitochondrial dysfunction and liver insulin resistance. Cell Metab. 2006;4:453–64. doi: 10.1016/j.cmet.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg AE, Yamamura S, Malik S, Spiegelman BM, Roeder RG. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha. Mol Cell. 2003;12:1137–49. doi: 10.1016/s1097-2765(03)00391-5. [DOI] [PubMed] [Google Scholar]
- White FC, McKirnan MD, Breisch EA, Guth BD, Liu YM, Bloor CM. Adaptation of the left ventricle to exercise-induced hypertrophy. J Appl Physiol. 1987;62:1097–110. doi: 10.1152/jappl.1987.62.3.1097. [DOI] [PubMed] [Google Scholar]
- Zaha V, Grohmann J, Gobel H, Geibel A, Beyersdorf F, Doenst T. Experimental model for heart failure in rats--induction and diagnosis. Thorac Cardiovasc Surg. 2003;51:211–5. doi: 10.1055/s-2003-42264. [DOI] [PubMed] [Google Scholar]