Abstract
To maintain tolerance, autoreactive B cells must regulate signal transduction from the B cell receptor and Toll-like receptors. We recently identified that dendritic cells and macrophages regulate autoreactive cells during TLR4 activation by releasing IL-6 and soluble CD40L (sCD40L). These cytokines selectively repress antibody secretion from autoreactive, but not antigenically naïve, B cells. How IL-6 and sCD40L repress autoantibody production is unknown. In this paper, we show that IL-6 and sCD40L are required for low-affinity/avidity autoreactive B cells to maintain tolerance through a mechanism involving receptor crosstalk between the BCR, TLR4, and the IL-6 receptor or CD40. We show that acute signaling through IL-6 receptor or CD40 integrates with chronic BCR-mediated ERK activation to restrict pERK from the nucleus and repress TLR4-induced Blimp-1 and XBP-1 expression. Tolerance is disrupted in 2-12H/MRL/lpr mice where IL-6 and sCD40L fail to spatially restrict pERK and fail to repress TLR4-induced Ig secretion. In the case of CD40, acute signaling in B cells from 2-12H/MRL/lpr mice is intact, but the chronic activation of pERK emanating from the BCR is attenuated. Re-establishing chronically active ERK through retroviral expression of constitutively active MEK1 restores tolerance upon sCD40L, but not IL-6, stimulation indicating that regulation by IL-6 requires another signaling effector. These data define the molecular basis for the regulation of low-affinity autoreactive B cells during TLR4 stimulation, they explain how autoreactive but not naïve B cells are repressed by IL-6 and sCD40L, and they identify B cell defects in lupus-prone mice that lead to TLR4-induced autoantibody production.
Introduction
Tolerance mechanisms that eliminate or inactivate autoreactive B and T cells prevent adaptive immune responses to self-antigens. Elimination or inactivation of self-reactive B cells occurs during development through a series of checkpoints involving receptor editing, clonal deletion, anergy, and competition for growth factors [1–3]. Additional mechanisms limit self-antigen presentation, co-stimulation, proliferation, and participation in germinal centers[4]. Tolerance mechanisms also regulate autoreactive B cells activated by pathogen associated molecular patterns (PAMPS) through Toll-like receptors (TLRs) [5–8]. Regulating TLR-induced immunoglobulin (Ig) secretion is important in maintaining tolerance because gene deletion and overexpression studies have identified TLR2, TLR4, and TLR7 as contributing to autoantibody titers, renal disease, and the heightened cytokine production found in autoimmune disease [9–16]. Further, cell surface expression of endogenous self-antigens such as the TLR4/TLR9 chaperone molecule gp96, promote lupus-like autoimmune disease in mice [10]. Thus, activation of TLR4by endogenousligands,[17, 18]can potentially activate autoreactive B cells.
Since antigenically naïve and autoreactive B cells express TLRs, maintaining tolerance requires that B cells acutely stimulated by foreign antigen be regulated differently from those chronically stimulated by self-antigen. We recently identified dendritic cell (DC)/macrophage (MF)-mediated tolerance as a mechanism that selectively represses Ig secretion from autoreactive B cells in response to TLR4 stimulation. We found that IL-6 and sCD40L, secreted by TLR4-activated DCs and MFs, repress TLR4-induced Ig secretion in autoreactive B cells, while these soluble mediators fail to repress antigenically naïve B cells [5, 6]. This finding suggests that acute stimulation of the IL-6 receptor or CD40 in cells chronically stimulated through the BCR attenuates TLR4 activation.
The molecular mechanisms underlying B cell unresponsiveness rely on chronic binding of self-antigen to the B cell receptor (BCR) [19]. Mechanistically, constitutive BCR engagement induces low-level calcium oscillations that sustain continuous ERK activation through KSR2, a protein scaffold that links the Ca2+ pathway to the Ras/MAPK pathway [20–23]. This low-level ERK activation has been referred to as tolerogenic ERK [8, 21], and is insufficient to activate key signaling effectors required for complete B cell activation and Ig secretion. How chronic low-level ERK activation regulates Ig secretion has not been defined; however, biological significance is ascribed to changes in ERK activation in other systems [24]. For example in fibroblasts, sustained but not transient ERK activation leads to entry into S phase [25]. In the immune system, the amplitude of the ERK response and the spatial localization of pERK impact the decision between T cell activation and anergy [26, 27]. In the nervous system, sustained ERK activation promotes neuronal cell differentiation through the stabilization of immediate early gene products such as c-fos [28].
In this report, we show that the ability of DCs and MFs to repress LPS-induced antibody secretion from autoreactive B cells relies on two ERK signals originating from different receptors. The first signal is the chronic basal pERK induced by constitutive self-antigen ligation of the BCR. The second is the acute ERK signal derived from theIL-6 receptor or CD40. Integration of these two signals excludes pERK from the nucleus and represses Blimp-1 and XBP-1 expression. We find that in addition to pERK, a second (unidentified) signaling effector is required for IL-6to regulate TLR4. In contrast, repression by sCD40 requires only pERK. Regulating TLR4 through chronic and acute pERK is important in disease because IL-6 and sCD40L fail to repress anti-nucleosome production in lupus-prone MRL/lpr and anti-Sm in 2-12H/MRL/lpr mice. Loss of tolerance is associated with loss of the high basal pERK (tolerogenic ERK) and coincident with the inability to exclude pERK from the nucleus despite normal signaling through CD40 and TLR4. This suggests that loss of tolerogenic ERK by the BCR is at least in part responsible for the loss of tolerance during TLR4 stimulation. Collectively, the findings illustrate the three-way integration of signals from the BCR, CD40/ IL-6 receptor and TLR4 that allow autoreactive, but not antigenically naïve, B cells to be repressed during TLR4-induced immune responses.
Materials and Methods
Mice
2-12H, 2-12H/Vκ8, 2-12H/MRL/lpr,2-12H/MRL/MpJ, MRL/lprand Ars/A1 transgenic mice have been previously described [19, 29–32]. C57BL/6 (B6), MD4 (HEL-Ig), and MD4 × ML5 (HEL-Ig × sHEL) mice were purchased from The Jackson Laboratory. Female 2-12H/MRL/lpr, 2-12H/MRL/MpJ, and MRL/lprmice were used at 5–9 weeks of age. All other mice were 8 to 14 weeks of age at the time of analysis. Animals were maintained in an accredited animal facility at the University of North Carolina.
Reagents
Hen egg lysozyme (HEL) and double-stranded DNA (dsDNA) were purchased from Sigma. dsDNA was boiled and rapidly chilled to make single-stranded DNA (ssDNA). p-azophenylarsonate conjugated to BSA (Ars5BSA) was provided by Larry Wysocki (National Jewish Research Center, Denver, CO). Small nuclear riboprotein (snRNP) complexes were purified from HeLa cells as previously described [33]. Fluorochrome- or biotin-labeled antibodies specific for CD3 (145-2C11), CD9 (KMC8), CD11b (Ml/70), CD11c (HL3), CD19 (1D3), CD21 (7G6), CD23 (B3B4), CD138 (281-2), and B220 (RA3-6B2) were purchased from BD Bioscience, phospho-p44/42 MAPK (T202/Y204) from Cell Signaling, and goat anti-rabbit IgG-Alexa 647 from Molecular Probes. Recombinant IL-6 (rIL-6) was purchased from eBioscience, and recombinant soluble CD40L (rsCD40L) from R&D Systems. HB100 (anti-IgMa/κ), 187.1 (anti-κ),RS3.1 (anti-IgMa), 33-60 (anti-IgM), B7.6 (anti-IgM), and 2.4G2 (anti-CD16/32) were purified from hybridoma culture supernatant using Protein G Sepharose (GE Healthcare), or MEP HyperCel (BioSepra). The MEK1 inhibitors U0126 and PD98059 were purchased from Calbiochem (San Diego, CA), resuspended in DMSO, and used at 0.5 µM (U0126) and 20 µM (PD98059).
B cell purification
Splenic B cells were isolated by negative selection (StemCell Technologies). B cells were 70–95% pure (with fewer than 5% DCs and MFs). In some experiments, marginal zone B cells were depleted from B6 splenocytes by the addition of biotinylated anti-CD9. Ficoll-Paque PLUS (GE biosciences) separation was used prior to negative selection to increase the purity of B cells from MRL/lpr, and 2-12H/MRL/lpr mice.
Retroviral transduction of B cells
Dr. Harvey Lodish (Whitehead Institute) and Dr. Chris Marshall (Institute of Cancer Research, London, UK) provided constitutively active MEK1 (CA-MEK1) and dominant negative MEK1 (DN-MEK1), respectively. These cDNA constructs were subcloned into MSCV-LTR. The retroviral constructs were transfected into Plat-E ecotropic packaging cells (Cellbiolabs) and cultured for 48 hrs. Supernatants were harvested and stored at −80°C. To express DN-MEK1 and CA-MEK1, we LPS-stimulated B cells for 18 hours in the absence or presence of rIL-6 or rsCD40L. Cells were washed and viral supernatants (1ml) were added with polybrene (5µg/ml) for 2 hours then washed and re-cultured with LPS in the absence or presence of rIL-6 or rsCD40L. GFP expression was evident by 24 hours but cells were cultured 48 hours before Ig secretion was measured. The efficiency of retroviral transduction ranged from 15%(2-12H/MRL/lpr) to 50–65% (B6 and 2-12H/Vκ8).
Cell sorting
Follicular (FO; CD19+CD23+CD21/35loCD138−), marginal zone (MZ; CD19+CD23−CD21/35+CD138−), and pre-plasma cells (pre-PC; CD19+CD138int) were sorted from 2-12H and 2-12H/MRL/lpr mice (>90% purity). GFPhi cells were sorted from CA-MEK-transduced B cells on day 4 (> 98% purity), and from DN-MEK1-transduced B cells on day 2 (> 98% purity).
LPS stimulation
Purified B cells (1 × 105/per well) were cultured with LPS from E. coli 055:B5 (Sigma; 30 µg/ml) or purified LPS (Invitrogen; 15 µg/ml) for 4 days in the absence or presence of rIL-6 (30 ng/ml), rsCD40L (75 ng/ml), HEL (100 µg/ml), ssDNA (500 ng/ml), Ars5BSA (2 µg/ml), Sm (50 U/ml), or snRNP (50 µg/ml). The different sources of LPS (Sigma or Invitrogen) did not yield different results in any assay but simply reflect availability. Immunoglobulin secretion was measured by ELISA from cell-free culture supernatants.
ELISPOT Assays
Since allelic exclusion is >93% in the 2-12H/Vκ8 (H+L restricted) B cells we used ELISA analysis (IgMa/κ) to quantitate Ig secretion [5, 29].To detect antibody from 2-12H B cells (no light chain restriction), we used ELISPOT to ensure that only Sm-specific antibody secreting cells (ASCs) were enumerated [6]. For ELISPOT analysis, cells were cultured for 3 days (or for retroviral infection, sorted for GFP expression, then cultured for 3 days), washed and transferred to plates coated with 1 U/well Sm antigen (Immunovision) and incubated for 8 hrs. The ASCs were detected using biotinylated anti-IgMa followed by streptavidin-horseradish peroxidase (BD Biosciences). The plates were analyzed using an ImmunoSpot Analyzer and software package (Cellular Technology).
ELISA
IgM secretion by B6 mice was detected using anti-mouse IgM (33–60) and biotin-labeled anti-mouse IgM (B7.6). IgMa/κ ELISA was used to measure IgM secretion by Ars/A1 mice. Mouse anti-HEL (IgMa) (MD4 × ML5) was measured as described previously [5, 34].Mouse anti-Sm (IgMa) was detected using anti-mouse IgMa (RS3.1) and biotinylated anti-mouse IgM (B7.6). Mouse anti-nucleosome Ig was capturedon plates coated overnight at 4°C with mouse histones(4 µg/well; Immunovision). Plates were washed and blocked with BSA containing dsDNA (10 µg/ml Sigma-Aldrich) for 3 h at room temperature. Samples and standards (PL2-6) were loaded and incubated overnight at 4°C then detected with biotinylated goat anti-mouse Ig. PL2-6 (anti-nucleosome hybridoma) was used to generate a standard curve.
Real-time PCR
Total RNA was extracted from 2 × 106 B cells using TRIZOL (Invitrogen). cDNA was prepared with random hexamers and M-MLV reverse transcriptase (Invitrogen) andanalyzed in triplicate real-time PCR reactions. Primers for xbp-1 were as follows: (forward, 5'- ACACGCTTGGGAATGGACAC -3';reverse, 5' CCATGGGAAGATGTTCTGGG -3'). Primers for prdm1 (Blimp-1) and 18s RNA have been described [35, 36]. Quantitative PCR (SYBR Green) reactions were performed on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems).Quantification of transcripts relative to 18s RNA transcripts was determined using the ΔΔ Ct method [37].
Immunoblotting
Cell lysates were prepared from 3 × 106 cells in buffer containing 1% NP-40, 150 mM NaCl, 10 mM Tris (pH 7.5), 2 mM sodium orthovanadate, 1 mM PMSF, 0.4 mM EDTA, 10 mM NaF, and 1 µg/ml each of aprotinin, leupeptin, and α1-anti-trypsin (Sigma). SDS-PAGE separated proteins were immunoblotted for phospho-ERK and total ERK (Cell Signaling Technologies), Blimp-1 (Novus Biologicals), XBP-1, lamin A, or β-tubulin (Santa Cruz Biotechnology, Inc). Proteins were detected using chemiluminescence (GE Biosciences).
Nuclear and cytoplasmic extracts
Purified splenic B cells were stimulated with LPS alone or in combination with rIL-6 or rsCD40L for 30 minutes. To collect cytoplasmic extracts, 15 × 106 cells were resuspended in 200 µl lysis buffer (10 mM HEPES (pH 7.9), 10 mM MgCl2, 0.5 mM DTT, protease and phosphatase inhibitors[38, 39]) for 15 minutes on ice followed by 5 minute centrifugation at 6600 × g. To collect nuclear extracts, nuclei were washed (as above) then resuspended in 10 µl of buffer D (20 mM HEPES (pH 7.9), 430 mM NaCl, 0.2 mM EDTA, 1.5 mM MgCl2, 0.5 mM DTT, 25% glycerol, protease and phosphatase inhibitors). After gentle mixing, 50 µl of buffer C (20 mM HEPES (pH 7.9), 0.2 mM EDTA, 1.5 mM MgCl2, 0.5 mM DTT, 25% glycerol, protease and phosphatase inhibitors) was added and incubated for 15 minutes at 4°C, then supernatant was collected following centrifugation. Protein concentrations were determined by the Bradford method (Bio-Rad). Cytoplasmic (5µg) and nuclear (10 µg) extracts were separated by SDS-PAGE and immunoblotted as above with phospho-ERK, total ERK, lamin A, and beta-tubulin.
Intracellular phospho-flow
Phosphorylated and total protein levels were determined by flow cytometry as previously described[40]. Briefly, splenic B cells were purified by negative selection and stimulated with LPS in the absence or presence of rIL-6 or rsCD40L for various time points. At indicated times, cells were fixed with 2 % PFA for 15 min, permeabilized for 30 min on ice with cold MeOH, washed with PBS containing 2%FCS, and then FcR blocked with mAb 2.4G2 (10 µg/ml) for 15 min. Cells were stained with anti-phospho-ERK(p44/42) or anti-ERK(p44/42) (Cell signaling Technology) for 1hr, washed 3 times then stained with CD19 (BioLegend) and F(ab')2 fragment of goat anti-rabbit IgG (H+L) (Invitrogen) for 1hr. ERK phosphorylation levels were determined by flow cytometry.
Confocal Microscopy
B cells purified by negative selection were fixed in 2% PFA (room temperature, 15 minutes), permeabilized in ice-cold methanol (10 minutes at −20°C), and then blocked in 2.4G2 for 1 hr. Cells were stained with Hoechst 33342 (1 µg/ml; 15 minutes at room temperature), washed, and stained for pERK (1 hr) followed by goat anti-rabbit IgG-Alexa 647 (1 hr). Images were captured using a Zeiss 710 confocal microscope with a 63 × 1.4 NA (oil) PLAN APO lens and Zeiss Zen software. Randomly chosen cells were analyzed in Image J. The nuclear localization of pERK was quantified by calculating the Mander’s coefficient of colocalization (ratio of colocalized pixels/total fluorescent pixels) between red and blue fluorescence (ratio=50%, threshold red=50, threshold blue=30).
Statistical Analysis
The one-sample t test was used when comparing antibody secretion in treated and untreated cultures and for the quantitation of confocal microscopy data. The exact Wilcoxon rank-sum test was used to compare antibody secretion between experimental groups. Statistical analyses were performed with GraphPad Prism.
Results
Smith (Sm) antigen fails to regulate TLR4-induced Ig secretion
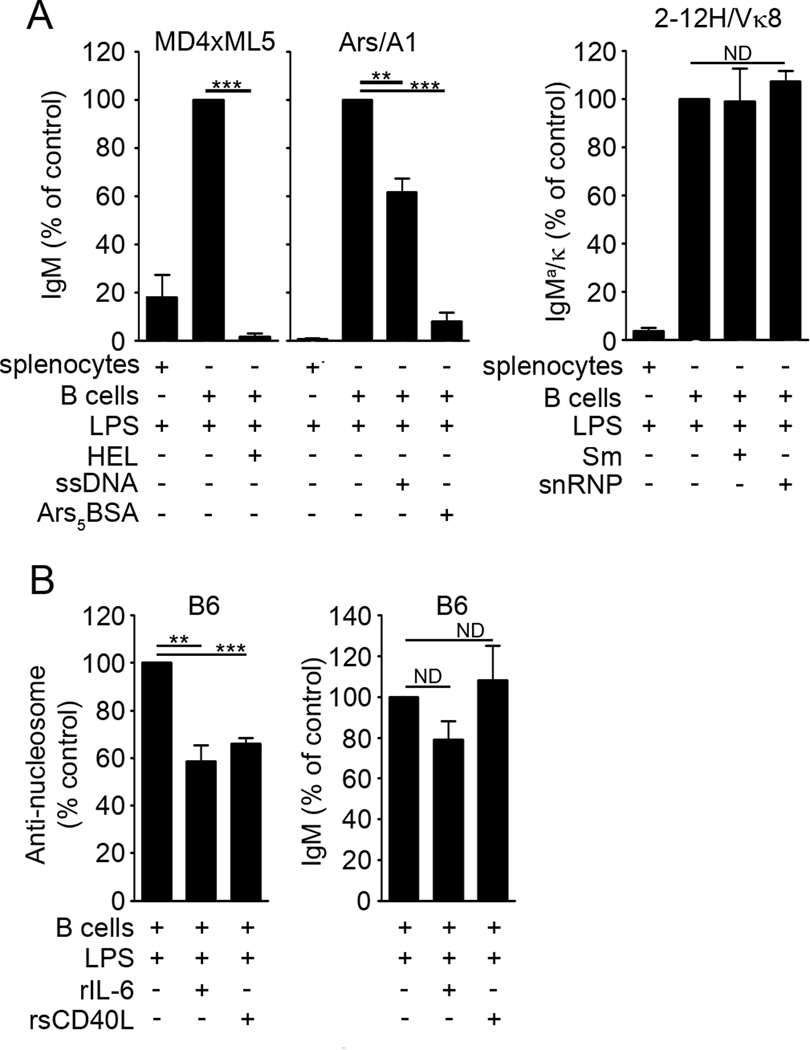
Regulating TLR-induced Ig secretion by autoreactive B cells is important in maintaining tolerance. Studies using hen egg lysosyme (HEL)-specific BCR transgenic mice showed that binding of high-affinity HEL to B cells from HEL-Ig × sHEL (MD4 × ML5) mice repressed TLR4- and TLR9-induced Ig secretion [8, 41]. Using a lower affinity model in which B cells were specific for Smith (Sm) antigen (2-12H/Vκ8), we showed that DC/MF-derived IL-6 and sCD40L repressed TLR4-induced Ig secretion [5, 6]. To assess whether repression of Ig secretion from lower affinity B cells was regulated in the same way as HEL-specific B cells, we cultured HEL-, Ars- and Sm-specific B cells with their cognate antigens and LPS (Figure 1A). We included Ars/A1 B cells because the Ars-specific BCR allowed us to directly compare a low affinity nuclear self-antigen (ssDNA) to a higher avidity multivalent neo-self-antigen (Ars5BSA). Consistent with previous findings[5], LPS stimulation of unpurified splenocytes from HEL-, Ars-, and Sm-specific immunoglobulin transgenic mice showed markedly reduced Ig secretion due to the presence of DCs and MFs. Upon separation of the B cells from DC/MFs, LPS-stimulated B cells from all of the models lost their unresponsive phenotype and secreted Ig (Figure 1A). Co-culture of anergic HEL-specific B cells (sHEL × HEL-Ig) with soluble HEL repressed LPS-induced Ig secretion, as previously shown [41]. The co-culture of Ars-specific B cells with Ars5BSA reduced Ig secretion by 90%, while ssDNA repressed 38% of secretion (Figure 1A, middle panel). In contrast, neither recombinant Sm nor snRNPs repressed Ig secretion from 2-12H/Vκ8 B cells (Figure 1A, right panel). These data indicate that chronic BCR exposure to HEL and Ars5BSA confers TLR4 unresponsiveness, and suggests that low-affinity/avidity nuclear self-antigens only partially modulate (ssDNA), or fail to modulate(Sm/snRNPs) TLR4-induced activation. However, low-affinity antigens are sufficient to induce susceptibility of autoreactive B cells to repression mediated by IL-6 and sCD40L, soluble factors delivered by TLR4-activated DCs and MFs [5, 6]. Thus, BCR ligation is important in regulating TLR4-induced autoantibody production either by directly regulating TLR4, or by indirectly impacting TLR4 through IL-6 receptor and CD40.
Figure 1. Low-affinity/avidity antigens fail to regulate TLR-induced Ig secretion.
Splenic B cells (1 × 105) from MD4 × ML5 (A, left panel), Ars/A1 (A, middle panel), and 2-12H/Vκ8 (A, right panel) mice were stimulated for 4 days with LPS (30 µg/ml) in the absence or presence of HEL (100 µg/ml), ssDNA (500 ng/ml), Ars5BSA (2 µg/ml), Sm (50 U/ml), snRNP (50 µg/ml). IgM or IgMa/κ antibody levels were quantitated by ELISA. In the LPS-stimulated controls, 100% reflects Ig in the range of 8–16 µg/ml (MD4 × ML5), 2–5µg/ml (Ars/A1), and 2–3 µg/ml (2-12H/Vκ8). Splenic B cells (1 × 105) from B6 mice (B) were stimulated for 4 days with LPS (30 µg/ml) in the absence or presence of rIL-6 (30 ng/ml), or rsCD40L (75 ng/ml). Anti-nucleosome Igand total IgM levels were quantitated by ELISA. In the LPS-stimulated controls, 100% reflects 18–42 µg/ml anti-nucleosome Ig, and 14–44 µg/ml total IgM. The data shown represent at least 3 experiments.(*** P<0.001, **P<0.01, ND: no difference)
To determine whether the repression of TLR4-induced Ig secretion by IL-6 and sCD40L was unique to B cells from immunoglobulin transgenic mice, we tested whether these soluble mediators repressed nucleosome-specific B cells from C57BL/6 (B6) mice. As shown in Figure 1B (left panel), recombinant IL-6 (rIL-6) repressed 41%, while recombinant soluble CD40L (rsCD40L) repressed 34%, of anti-nucleosome secretion. Diminished Ig secretion was specific to autoreactive cells because neither rIL-6 nor rsCD40L significantly repressed total IgM (Figure 1B, right panel). Collectively, the data show that IL-6 and sCD40L repress transgenic and non-transgenic B cells of multiple autoreactive specificities, and in contrast to the direct regulation of TLR4 by high-affinity antigens, low-affinity antigens require the products of activated DCs and MFs.
rIL-6 and rsCD40L regulate transcription factors involved in plasma cell differentiation
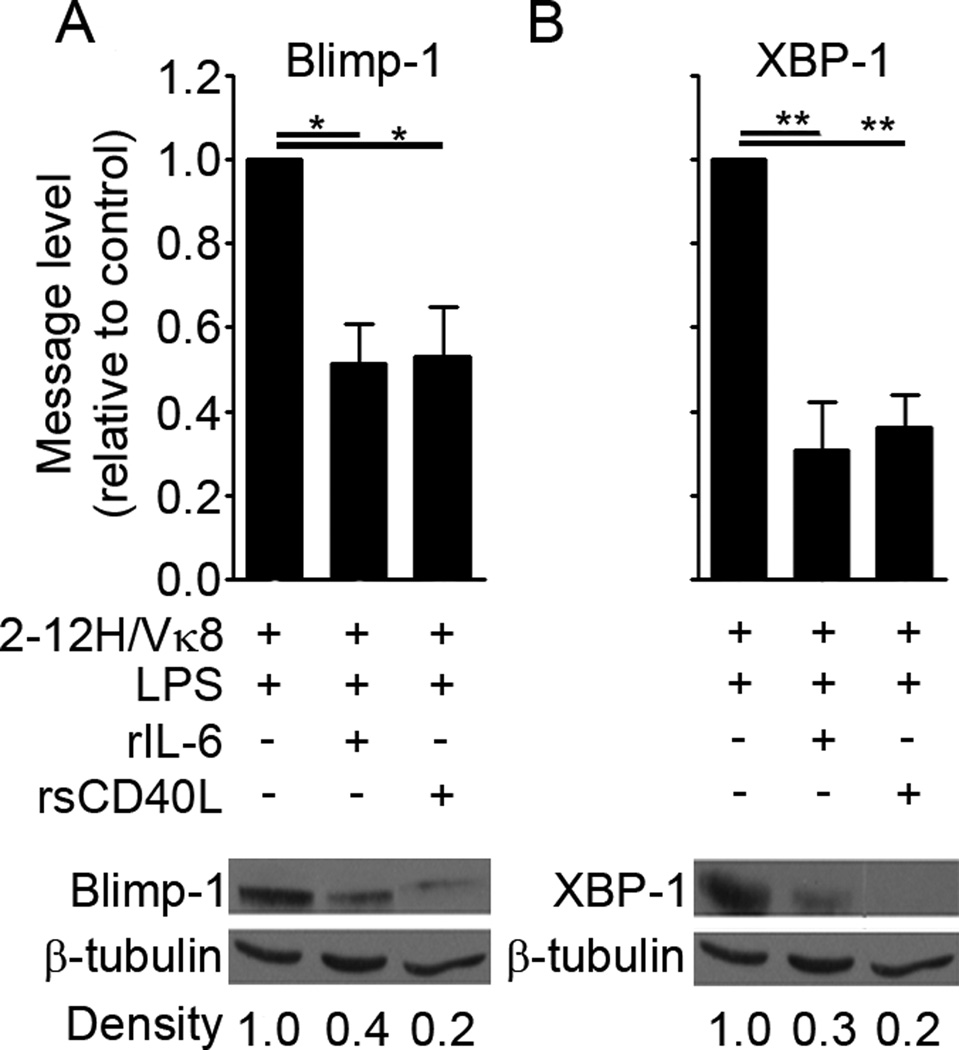
To establish the molecular basis for the repression of autoreactive B cells by IL-6 and sCD40L, we examined whether the transcription factors that promote plasma cell differentiation are repressed by rIL-6 or rsCD40L. Blimp-1 represses Bcl-6, Pax5, and c-myc while promoting Ig secretion by upregulating XBP-1.When Sm-specific B cells were LPS-stimulated in the presence of rIL-6 or rsCD40L, prdm1 (Blimp-1) message levels were reduced by approximately 50% (Figure 2A, upper panel). rIL-6 and rsCD40L also reduced Blimp-1 protein levels by 60% and 80% respectively (Figure 2A, lower panel). Similarly, rIL-6 reduced the message levels of XBP-1 by 70% in LPS-stimulated Sm-specific B cells, while rsCD40L reduced message levels by 64% (Figure 2B, upper panel). rIL-6 and rsCD40L also repressed XBP-1 protein levels by 70% and 80%, respectively (Figure 2B, lower panel). This indicates that when B cells are chronically exposed to self-antigen, IL-6 and sCD40L interfere with the TLR4-induced transcriptional program that directs plasma cell differentiation.
Figure 2. IL-6 and sCD40L decrease the levels of Blimp-1 and XBP-1.
2-12H/Vκ8 B cells were stimulated for 3 days with LPS (30 µg/ml) or LPS combined with rIL-6 (30 ng/ml) or rsCD40L (75 ng/ml). The relative levels of Blimp-1 (A; upper left) and XBP-1 (B; upper right) message were measured by real-time PCR (**P–0.01, *P<0.05). 2-12H/Vκ8 B cells were cultured for 3 days with LPS or LPS combined with rIL-6 or rsCD40L. Lysates from 3 × 106 cells were immunoblotted for Blimp-1 (A; lower left) and XBP-1 (B; lower right).The density of each band was quantitated using Image J, and the fold changes were calculated relative to β-tubulin. The data shown represent at least 3 experiments.
2-12H/Vκ8 B cells exhibit heightened levels of basal pERK
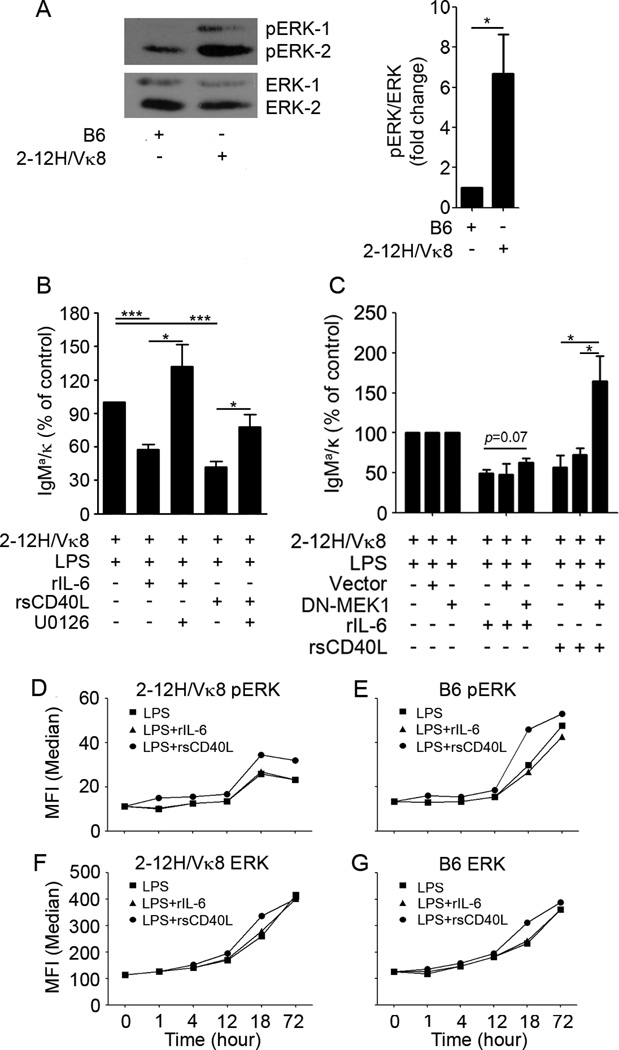
Compared to naïve B cells, autoreactive cells exhibit increased basal ERK phosphorylation (tolerogenic ERK) [19, 21, 22]. To begin to understand how IL-6 and sCD40L regulate TLR4-induced Ig secretion we assessed whether low-affinity Sm-specific B cells (2-12H/Vκ8) exhibit heightened basal phospho-ERK (pERK). As shown in Figure 3A, 2-12H/Vκ8 B cells (anergic) exhibited a 6.9-fold higher level of basal ERK phosphorylation compared to B6 B cells (naïve). When compared in side-by-side analysis, these levels were comparable to those found in B cells from HEL-Ig × sHEL mice (data not shown). This suggests that heightened basal pERK may be important in the regulation of 2-12H/Vκ8 B cells.
Figure 3. IL-6 and sCD40L repress TLR4-induced Ig in a MEK-dependent manner.
3 × 106 purified ex vivo B6 and 2-12H/Vκ8 B cells were lysed and immunoblotted for pERK and total ERK1/2. The density of each band was quantitated with ImageJ, and the ratio of pERK to total ERK1/2 was calculated and expressed as fold change (A). 2-12H/Vκ8 B cells were stimulated for 4 days with LPS or LPS combined with rIL-6 or rsCD40L, in the absence or presence of U0126 (0.5 µM)(B). 3 × 106 splenic B cells from 2-12H/Vκ8 mice were stimulated with LPS (30 µg/ml) in the absence or presence of rIL-6 (30 ng/ml)or rsCD40L (75 ng/ml) for 18–24 hrs followed by DN-MEK1 gene transfer. Two days later, GFP+ B cells (1 × 105) were sorted and stimulated under the same conditions for an additional 48 hrs. IgMa/κ was quantitated by ELISA (C). 1 × 106 splenic B cells from B6 (E and G), or 2-12H/Vκ8 (D and F) mice were stimulated with LPS (30 µg/ml) in the absence or presence of rIL-6 or rsCD40L for various time periods followed by FACS analysis of intracellular pERK and total ERK staining. The data shown represent at least 3 experiments.
The ability of rIL-6 and rsCD40L to repress TLR4-induced Ig is ERK-dependent
Since constitutive ERK phosphorylation is central to anergy [21–23], we sought to determine whether rIL-6 and rsCD40L regulate TLR4-induced Ig secretion via pERK. We treated LPS-stimulated 2-12H/Vκ8 B cells with U0126, a pharmacological inhibitor of MEK1 (Figure 3B). We found that when ERK activation was inhibited, rIL-6 was unable to repress TLR4-induced Igsecretion and sCD40L repressed only 25% of Ig secretion. To corroborate these results, we used retroviral gene transfer to introduce DN-MEK1 into 2-12H/Vκ8 B cells (Figure 3C). When cells were transduced with DN-MEK1, rIL-6 only repressed 37% of Ig secretion, while it repressed 52% of Ig secretion in LPS-stimulated 2-12H/Vκ8B cells. Although not statistically different (ρ=0.07), we consistently saw that cells expressing DN-MEK had higher Ig secretion. In contrast, DN-MEK1 expressing2-12H/Vκ8 B cells were not repressed by rsCD40L, and actually showed enhanced Ig secretion (165% of controls). This enhanced secretion reflected B cell proliferation (data not shown). It remained unclear why U0126 had a much greater effect on IL-6 mediated repression than did DN-MEK1.To ensure that the ability of U0126 to restore Ig secretion during IL-6 costimulation was not due to non-specific effects, we assessed whether PD98059 restored Ig secretion (supplemental Figure 1). Like U0126, LPS stimulated 2-12H/Vκ8 B cells costimulated with IL-6 in the presence of PD98059 restored Ig secretion suggesting that the MEK/ERK pathway plays a role in the regulation of Ig secretion by IL-6. Taken together, these results indicate that repression of TLR4-induced Ig secretion by sCD40L, and likely IL-6, is dependent on ERK activation.
Regulation of TLR4-induced Ig secretion by rIL-6 and rsCD40L does not alter the magnitude or kinetics of ERK phosphorylation
In most signal transduction pathways, the activation of ERK occurs rapidly (within 10 minutes) and is not sustained. However, in anergic B cells the low-level Ca2+ oscillations occurring as a consequence of constitutive self-antigen ligation of the BCR promote a low level of continuously active ERK. Thus, when other ERK-coupled surface receptors, such as IL-6R and CD40 become activated, the acute ERK signal they induce is superimposed on the chronic, sustained pERK emanating from the BCR. To begin to understand how IL-6 and sCD40L repress TLR4-induced Ig secretion, we used flow cytometry to assess whether they promoted altered patterns of ERK phosphorylation over time. As shown in Figure 3D, the levels of pERK increased slightly during the first 12 hours of LPS stimulation but were substantially increased between 12 and 18 hours. It is noteworthy that LPS-stimulated B6 B cells (Figure 3E) achieved higher levels of pERK by 18 hours that increased by 72 hours, while pERK in 2-12H/Vκ8 B cells plateaued after 12 hours. Regardless, these levels were not significantly altered by treatment with rIL-6 or rsCD40L in B6 or 2-12H/Vκ8 mice. The levels of total ERK were also not different between B6 and 2-12H/Vκ8 B cells, and total ERK increased over time in both groups (Figure 3F and 3G). Similarly, immunoblotting did not detect changes in pERK upon IL-6 or rsCD40L costimulation (data not shown).Thus, the ability of IL-6 and sCD40L to repress LPS-induced Ig secretion cannot be explained by changes in ERK phosphorylation.
rIL-6 and rsCD40L alter the subcellular localization of pERK in 2-12H/Vκ8 B cells
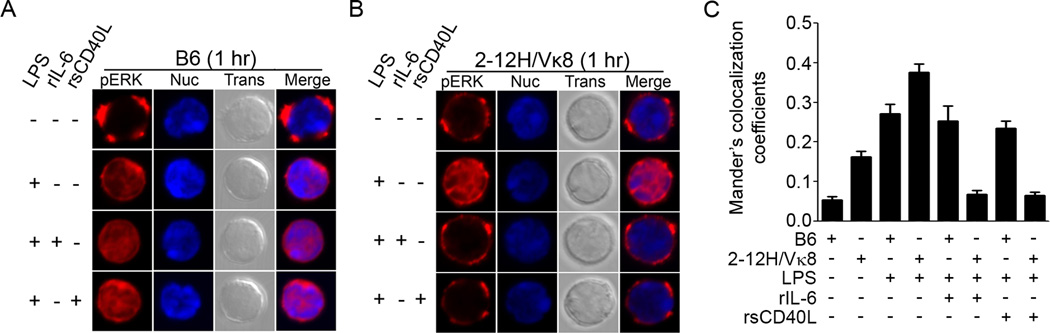
In addition to phosphorylation, ERK can be regulated by subcellular location. Spatial restriction of pERK has been shown to contribute to T cell activation while a diffuse distribution of pERK is associated with anergy [26, 27, 42]. To test whether rIL-6 orrsCD40L altered the intracellular location of pERK, we analyzed cells by confocal microscopy. We found that unstimulated B6 B cells retained pERK in the cytoplasm, but upon LPS stimulation, pERK was translocated to the nucleus within 1 hour (Figure 4A and compilation in 4C). The location of pERK in the nucleus did not change when LPS-stimulated B6 B cells were treated with rIL-6 or rsCD40L. Similar to the unstimulated B6 B cells, unstimulated 2-12H/Vκ8 B cells retained pERK in the cytoplasm and upon LPS stimulation, translocated pERK to the nucleus (Figure 4B and compilation in 4C). However, treatment of the 2-12H/Vκ8 cells for 1 hour with either LPS+rIL-6, or LPS+rsCD40L excluded pERK from the nucleus coincident with failure to secrete Ig. Over time (18 hr), the intensity of pERK staining diminished and the remaining pERK entered the nucleus of 2-12H/Vκ8 B cells. Except for aunique punctate staining in the cytoplasm of 2-12H/Vκ8 B cells, the distribution of pERK was similar in B6 and 2-12H/Vκ8 B cells (supplemental Figure 2A, 2B, and compilation in 2C). By day 3 (when early Ig secretion is detected) both B6 and 2-12H/Vκ8 B cells once again showed strong pERK staining; however, it was restricted to the nucleus regardless of treatment with rIL-6 or rsCD40L (supplemental Figure 2D, 2E, and compilation in 2F).
Figure 4. IL-6 and sCD40L alter the subcellular localization of pERK in B cells chronically exposed to self-antigen.
1 × 106 purified B6 and 2-12H/Vκ8 B cells were stimulated with LPS (30 µg/ml) or LPS in combination with rIL-6 (30 ng/ml) or rsCD40L (75 ng/ml) for 1 hr. Cells were fixed, stained for pERK (red) and dsDNA (blue), and imaged using confocal microscopy (A,B). The co-localization of pERK with the nucleus was quantified in 37–92 cells by calculating the Mander’s coefficient between red and blue fluorescence (Ratio=50%, Threshold Red = 50, Threshold Blue = 30) (C).
To corroborate this finding, we prepared B cell nuclear extracts (lamin A+; β-tubulin-) from B6 and 2-12H/Vκ8 mice B cells (supplemental Figure 3). Stimulation with LPS increased nuclear pERK in both B6 and 2-12H/Vκ8 B cells. However, treatment with LPS+rIL-6 or LPS+rsCD40L reduced the levels of nuclear pERK in 2-12H/Vκ8 B cells, but not B6 B cells. Collectively, the data show that within 1 hour following co-stimulation of IL-6 receptor or CD40 in combination with TLR4, pERK is excluded from the nucleus of autoreactive B cells coincident with the repression of Ig secretion.
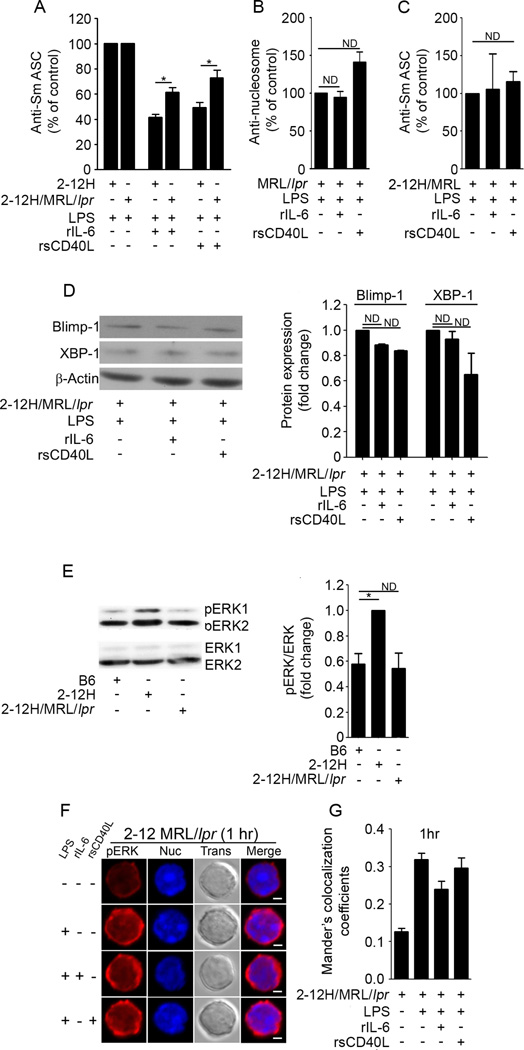
rIL-6 and rsCD40L fail to repress Sm-specific B cells from 2-12H/MRL/lpr mice
Our findings that DC/MF-mediated tolerance regulates autoreactive B cells during TLR4 stimulation identify a potential point of dysregulation in lupus-prone mice. We reasoned that a B cell defect that disrupted chronic BCR ligation might prevent the heightened levels of basal pERK and/or attenuate the spatial restriction of pERK, thereby allowing autoantibody secretion. To test this possibility, we co-cultured 2-12H and 2-12H/MRL/lpr B cells with LPS in the absence or presence of rIL-6 or rsCD40L (Figure 5A). As expected, rIL-6 and rsCD40L repressed Ig secretion from 2-12H B cells(58% and 50%, respectively). However, B cells from 2-12H/MRL/lpr mice were significantly less repressed by rIL-6 and rsCD40L (39% and 27%, respectively). This is not due to Fas deficiency because rIL-6 and rsCD40L were unable to repress B cells from 2-12H/MRL/MpJ mice (Figure 5C). To ensure that the failure to regulate autoreactive B cells in the 2-12H/MRL/lprmice was not unique to the 2-12H transgene, we tested whether nucleosome-specific B cells in MRL/lpr mice were regulated by IL-6 and sCD40L during TLR4 responses. As shown in Figure 5B, neither rIL-6 nor rsCD40L repressed anti-nucleosome Igin MRL/lpr mice. Coincident with the loss of regulation of Ig secretion, rIL-6 and rsCD40L did not diminish LPS-induced Blimp-1 and XBP-1 levels (Figure 5D). This reveals that the regulatory mechanisms controlling TRL4-induced Ig secretion are disrupted in lupus-prone mice.
Figure 5. IL-6 and sCD40L fail to repress B cells from autoimmune-prone mice.
1 × 105 splenic B cells from 2-12H and 2-12H/MRL/lpr mice (5–9 weeks old) were stimulated with LPS (30 µg/ml) in the absence or presence of rIL-6 (30ng/ml) orrsCD40L (75 ng/ml) for 4 days. ASCs were enumerated using ELISPOT assays (A). 1 × 105 splenic B cells from MRL/lpr mice (5–10 weeks old) were stimulated with LPS in the absence or presence of rIL-6 orrsCD40L for 4days. Anti-nucleosome Ig secretion was quantitated by ELISA (B). 1 × 105 splenic B cells from 2-12H/MRL/MpJ (2-12H/MRL)mice (5 –9 weeks old) were stimulated with LPS in the absence or presence of rIL-6 orrsCD40L for 4days. The number of Sm secreting cells was quantitated by ELISPOT (C). 2-12H/MRL/lpr B cells were stimulated for 3 days with LPS (30 µg/ml) in the absence or presence of rIL-6 (30 ng/ml), or rsCD40L (75 ng/ml). Lysates from 3 × 106 cells were immunoblotted for Blimp-1 and XBP-1. The density of each band was quantitated with ImageJ, and the fold change was calculated relative to β-actin (D). 3 × 106 purified B6, 2-12H/Vκ8,and 2-12H/MRL/lpr B cells were lysed and immunoblotted for pERK and total ERK1/2. The density of each band was quantitated with ImageJ and the ratio of pERK/ERK from 3 experiments was calculated (E). 1 × 106 purified ex vivo2-12H/MRL/lpr B cells were stimulated with LPS in the absence or presence of rIL-6 orrsCD40L for 1 hr. Cells were fixed, stained for pERK (red) and dsDNA (blue), and imaged using confocal microscopy (F). Colocalization of pERK with the nucleus at the 1 hr time point was quantified in 40 cells by calculating the Mander’s coefficient between red and blue fluorescence (Ratio=50%, Threshold Red = 50, Threshold Blue = 30) (G). The data shown represent at least 3 experiments.
The inability of rIL-6 and rsCD40L to repress Ig secretion is coincident with loss of basal pERK, and the translocation of pERK to the nucleus
Our data show that Sm-specific B cells exhibit heightened levels of basal ERK phosphorylation, and upon co-stimulation with rsCD40L or rIL-6, they exclude pERK from the nucleus. To determine whether basal ERK phosphorylation was lost when tolerance was overcome in the 2-12H/MRL/lpr model, we compared basal pERK levels from B6, 2-12H, and 2-12H/MRL/lpr mice (Figure 5E). We found that like 2-12H/Vκ8 B cells, those from 2-12H mice exhibited heightened levels of pERK consistent with an anergic phenotype [45]. However, the levels of pERK in B cells from 2-12H/MRL/lpr mice were reduced to levels seen in B6 B cells.
To assess whether loss of tolerance in lupus-prone mice was associated with the entry of pERK into the nucleus, we stimulated B cells from 2-12H/MRL/lpr mice with LPS, LPS+rIL-6, or LPS+rsCD40L for 1 hour, 18 hours, or three days, then assessed the subcellular location of pERK by confocal microscopy. We found that at 1 hour, B cells from 2-12H/MRL/lpr mice stimulated with LPS, LPS+rIL-6, or LPS+rsCD40L failed to exclude pERK from the nucleus, a phenotype much like that seen with stimulated B6 B cells (Figure 5 F and G). Over time, lower levels of pERK were found in both the nucleus and cytoplasm of 2-12H/MRL/lpr B cells, with a punctate cytoplasmic staining pattern at 18 hours (supplemental Figure 4A and 4B), and a punctate nuclear stain by day 3 (supplemental Figure 4C and 4D). Surprisingly, at day 3, rIL-6 or rsCD40L treated cells from 2-12H/MRL/lpr mice once again trended toward excluding pERK from the nucleus (supplemental Figure 4D). Taken together, the data in Figures 4A/B and 5F show that repression of TLR4-induced Ig by rIL-6 or rsCD40L is coincident with exclusion of pERK from the nucleus. However when tolerance is overcome, TLR4 stimulation induces the translocation of pERK to the nucleus despite co-stimulation by rIL-6 or rsCD40L. This indicates that in autoreactive B cells, loss of susceptibility to IL-6 and sCD40L occurs coincident with loss of tolerogenic pERK, the failure to exclude pERK from the nucleus, and the inability to decrease the levels of Blimp-1 and XBP-1.
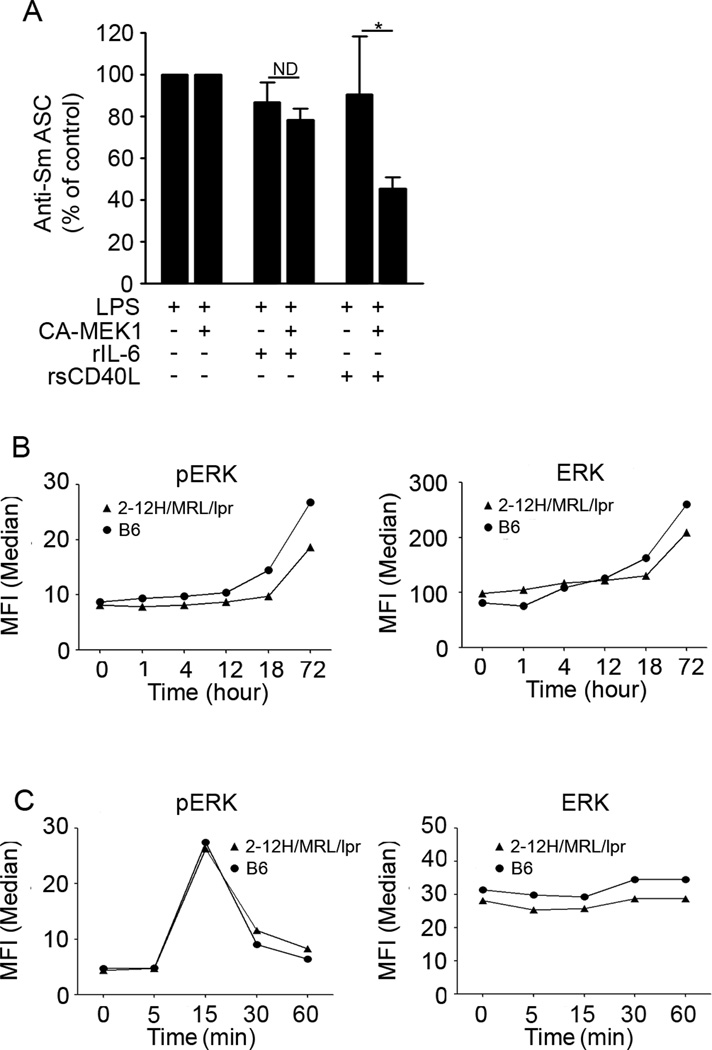
Inducing active MEK restores the ability of sCD40L to repress TLR4-induced Ig secretion
The data show that ERK activation is critical for sustaining anergy during TLR4-induced innate responses. Thus, we reasoned that if the loss of ERK phosphorylation caused the loss of susceptibility to IL-6 and sCD40L in lupus-prone B cells, restoring pERK through a gain-of-function approach might restore tolerance. To address this, we used retroviral gene transfer to express GFP-tagged constitutively active-MEK1 (CA-MEK1) in 2-12H/MRL/lpr B cells. We found that sorted CA-MEK1-expressing 2-12H/MRL/lpr B cells treated with rsCD40L repressed 54% of Ig secretion (Figure 6A); a level comparable to that found in 2-12H B cells treated with rsCD40L (Figure 5A). Surprisingly, Ig secretion from 2-12H/MRL/lpr B cells treated with rIL-6 was not repressed despite the expression of MEK (Figure 6A). This suggests that a second signaling effector is required for IL-6 to repress TLR4-induced Ig secretion. Ideally, defining the subcellular location of pERK in ex vivorsCD40L-treated B cells expressing CA-MEK1 would allow us to assess the role of spatial restriction. However, the inducible spatial restriction of pERK seen at 1 hour is not observed after the unavoidable delay (~18-24 hrs) needed to activate B cells and retrovirally infect them (see supplemental Figure 4A). Regardless, the data indicate that the regulation of TLR4-induced Ig secretion by rIL-6 requires pERK and another signaling effector(s), while pERK is sufficient for rsCD40L to repress autoantibody production.
Figure 6. Expression of constitutively active MEK1 restores the ability of sCD40L to repress TLR4-induced Ig secretion.
3 × 106 splenic B cells from 2-12H/MRL/lprmice were stimulated with LPS (30 µg/ml) in the absence or presence of rIL-6 (30 ng/ml)or rsCD40L (75 ng/ml) for 18 – 24 hr followed by CA-MEK1 gene transfer. On day 4, GFP+ B cells were sorted and ASCs were enumerated using ELISPOT assays. Data represent at least 3 experiments. (*P<0.05; ND not different). 1 × 106 splenic B cells from B6 or 2-12H/MRL/lpr mice were stimulated with LPS for various time periods followed by intracellular staining with pERK (B, left panel) and total ERK1/2 (B, right panel). 1 × 106 splenic B cells from B6, and 2-12H/MRL/lpr mice were stimulated with rsCD40L (75 ng/ml) for various time periods followed by FACS analysis of intracellular pERK (C, left panel)and total ERK staining (C, right panel).
High basal pERK is essential for CD40-mediated repression of Sm-specific B cells
Our findings point to a central role for ERK in CD40L and IL-6 mediated repression of Sm-specific B cells. However, ERK is activated by multiple receptors including BCR (basal tolerogenic pERK), TLR4, and CD40/IL-6 receptor. Which is responsible for repressive pERK is unknown. Our findings in Figure 5 indicate that IL-6 andsCD40L fail to repress activation of Sm-specific B cells from 2-12H/MRL/lpr mice, and they lack the high basal pERK characteristic of anergy. This implicates basal pERK coming from chronic BCR signaling as essential for sCD40L and IL-6 to repress TLR4-induced Ig secretion. To further test this possibility, we assessed whether ERK activation through the other two receptors (TLR4 and IL-6 receptor/CD40) was intact. As shown in Figure 6B,the levels of pERK in B6 and 2-12H/MRL/lpr B cells remained low until 18 hours following LPS stimulation, then increased between 18 and 72 hours (Figure 6B left panel). Although LPS-stimulated B cells from B6 mice achieved slightly higher levels of pERK compared to B cells from 2-12H/MRL/lpr micethe levels of total ERK were also slightly higher in B6 B cells(Figure 6B right panel). This indicates that following TLR4 stimulation, the magnitude and kinetics of pERK activation is not different between B6 and 2-12H/MRL/lpr B cells.
We hypothesized that sincesCD40L is solely dependent on pERK to regulate TLR4-induced Ig secretion, determining whether CD40-mediated signal transduction activates ERK might allow us to identify the source of pERK that is dysregulated in the 2-12H/MRL/lpr model. To assess this, we stimulated2-12H/MRL/lpr B cells with rsCD40L over a 1 hour time course. This timing was chosen because it represents the timeframe when pERK was excluded from the nucleus in the 2-12H/Vκ8 cells (Figure 4B).We found that rsCD40L induced phosphorylation of pERK in 2-12H/MRL/lprto a level that was comparable to that found in B6 (Figure 6C left panel).Since TLR4- and CD40L-induced ERK activation is normal in 2-12H/MRL/lpr B cells, the data suggest that loss of heightened basal pERK (tolerogenic pERK) from the BCR prevents sCD40L from repressing TLR4-induced autoantibody secretion in these autoreactive B cells. However, we cannot exclude the possibility that pERK induced by TLR4 and CD40 is also essential for CD40-mediated repression.
Discussion
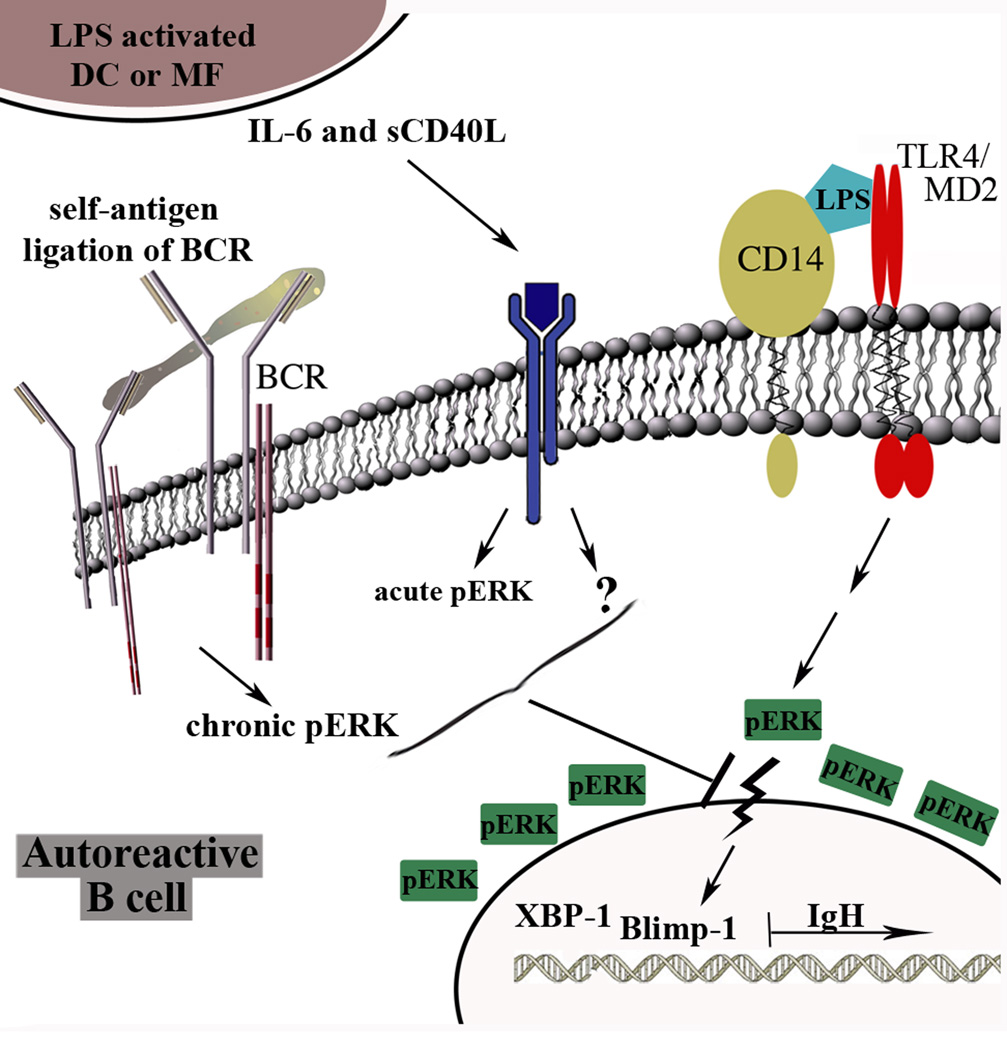
The ability of autoreactive B cells to regulate TLR-induced activation is essential in maintaining tolerance to self, as many self-antigens and pathogen-associated antigens bind TLR4, TLR7, or TLR9 [44]. Our study implicates a central role for BCR activation in directing the outcome of TLR4-induced Ig responses. We show that B cells expressing high affinity BCRs efficiently repress TLR4-induced Ig secretion through a B cell intrinsic mechanism, whereas cells expressing low affinity BCRs utilize a cell extrinsic mechanism. The cell extrinsic mechanism involves DC- and MF-secreted IL-6 and sCD40L, which blocks the nuclear translocation of pERK, prevents the expression of Blimp-1 and XBP-1, and there by terminates plasma cell differentiation (Figure 7). This regulatory mechanism fails in autoreactive B cells from autoimmune-prone MRL/lpr mice, explaining the susceptibility of these cells to activation during TLR4-induced innate immune responses.
Figure 7. Receptor cross-talk regulates autoreactive B cells during TLR4 stimulation.
We propose a model wherein the chronic ligation of self-antigen by the BCR distinguishes autoreactive B cells from antigenically naïve cells. Chronic BCR ligation induces chronically active ERK (Figure 3A). In addition to stimulating B cells, LPS induces DCs and MFs to secrete IL-6 and sCD40L, which in turn acutely stimulateIL-6 receptor or CD40 on B cells inducing an acute pERK signal (Figure 6B for CD40). The combined chronic and acute ERK signals exclude pERK from the nucleus, limiting expression of TLR4-induced Blimp-1 and XBP-1 and thereby repressing plasma cell differentiation. Stimulation through IL-6 receptor relies on pERK (Figure 3), but pERK is not sufficient (Figure 6). The identity of the second effector coupled to IL-6 receptor remains unknown, as denoted by the question mark.
Cross-regulation of receptor signaling is emerging as a critical mechanism for cells to achieve specific outcomes [45, 46]. For example, nucleic acid-containing immune complexes simultaneously stimulate B cells, MFs, and DCs through either FcγRs or BCR along with TLR7 or TLR9 [13, 15, 47]. Linked activation results in inflammatory cytokine production or antibody production. Another example is activation of ITAM-containing receptors and TLRs by ligands that are not physically linked. For example, DAP12 signaling via TREM-1 synergizes with TLR4 to increase cytokine production [48]. Our studies of TLR4 activation on autoreactive B cells are consistent with these examples and involve three receptors, the BCR, IL-6 receptor or CD40, and TLR4. The BCR is an ITAM-containing receptor that orchestrates TLR4 regulation. Like other autoreactive cells [21, 22], the chronic binding of self-antigen to Sm-specific BCR selevates the basal level of activated ERK likely through low-level Ca2+ oscillations and KSR2, a protein scaffold that links the Ca2+ pathway to the Ras/MAPK pathway [20–23]. However, high basal pERK levels, or tolerogenic ERK, are not sufficient to confer unresponsiveness by low affinity Sm-specific and ssDNA-specific (Ars/A1) B cells as it does for high affinity HEL-specific cells (Figure 1). This is consistent with our previous observations that in the absence of DCs and MFs, the affinity of the BCR for Sm directly correlates with impairment of TLR4-induced Ig secretion [49].
Our data indicate that Blimp-1 and XBP-1 are repressed when Sm-specific B cells are TLR4 stimulated in the presence of IL-6 or sCD40L (Figure 2) and this level of regulation is lost in autoreactive B cells from lupus-prone mice (Figure 5). Thus, the point where chronic pERK, and acute IL-6R/CD40 signaling intersect TLR4 could be at the level of Blimp-1 up-regulation. Blimp-1 expression is ERK-dependent, supporting this possibility[50]. Alternatively, the point of intersection may be upstream of Blimp-1. The unique spatiotemporal pattern of pERK in autoreactive B cells may impact immediate early gene (IEG) products such as c-Fos, Egr-1, Elk-1, and Myc, which in turn affect Blimp-1 transcription. In support of this idea, others have shown that transient ERK activation degrades IEG products, while sustained ERK activation induces their prolonged expression [28, 51, 52]. It is noteworthy that we find nuclear exclusion of pERK 1 hour after stimulation, but by 18hours post-stimulation pERK is present in the nucleus of Sm-specific B cells (Figure 4 and supplemental Figure 2). This is more consistent with an indirect effect of pERK on Blimp-1 expression.
TLR4 stimulation of B cells from B6 and 2-12H/Vκ8 mice translocates pERK to the nucleus; however, translocation is blocked in Sm-specific cells by an acute signal from IL-6R or CD40 (Figure 4). The importance of spatially regulating pERK in anergic B cells is evident from our analysis of autoimmune-prone MRL/lpr mice where TLR4 stimulation fails to exclude pERK from the nucleus and induces anti-Sm and anti-nucleosome secretion, regardless of acute IL-6 receptor and CD40 signaling. Coincident with these events, we find that the heightened basal pERK levels associated with B cell anergy are not evident. Moreover, CA-MEK restores the ability of at least sCD40L to block pERK nuclear translocation and antibody production in response to LPS. Thus, high basal pERK is central in repressing the TLR4-inducedactivation of low affinity Sm-specific B cells.
It is unclear which receptor(s) provides the signal to exclude pERK from the nucleus, since the BCR, IL-6R/CD40, and TLR4 activate ERK. Our analysis of 2-12H/MRL/lpr B cells provides some insight into this question. The high basal level of pERK associated with anergic B cells is absent in these cells (Figure 5), but the acute pERK activation induced by CD40 and LPS is not different between B cells from 2-12H/MRL/lpr and B6 mice (Figure 6). Since pERK is not excluded from the nucleus in autoreactive B cells from 2-12H/MRL/lpr mice (Figure 5), we propose that the high basal pERK generated by the BCR is essential for directing the outcome of acute CD40 signaling, although we do not exclude a role for pERK generated by other receptors. Whether IL-6 receptor signaling functions in the same way remains unclear since CA-MEK fails to restore IL-6 mediated repression in B cells from 2-12H/MRL/lpr mice. pERK appears to play a role in regulating TLR4 through IL-6 receptor since U0126 and PD98059 block IL-6 from repressing TLR4 activation in 2-12H/Vκ8 B cells; however, the effect by DN-MEK was not significant. Since these MEK/ERK inhibitors have few off-target effects at the concentrations used [53], we believe the difference could reflect the inability of DN-MEK to inhibit the activation of endogenous basal pERK although the exact reason remains unknown. Nonetheless, there is likely another effector molecule that is important for IL-6 to repress TLR4-induced Ig secretion. One possibility is STAT3, since recent studies have shown that both pERK and pSTAT3 are required for Blimp-1 expression [50] and both are activated by the IL-6 receptor [54]. In support of this possibility, we have found that pharmacologically inhibiting JAK2/STAT3 attenuates the ability of IL-6 to repress Ig secretion from Sm-specific B cells (unpublished observation). Our data support a model in which heightened basal pERK is essential for regulating TLR4 and suggest that, despite continued presence of self-antigen in MRL/lpr mice, the loss of tolerogenic pERK prevents the regulation of Sm-specific B cells during TLR4 stimulation.
One possible mechanism that might account for the loss of tolerogenic pERK in MRL/lpr mice is renewed signaling through the BCR. In anergic B cells, chronic antigen stimulation leads to the activation of inhibitory pathways that limit subsequent BCR responses. Studies show that complement-opsonized cross-reactive antigens can overcome B cell anergy and renew BCR signaling[55, 56].Another possibility is that higher avidity forms of self-antigen, such as those displayed on the surface of apoptotic debris [57, 58], might attenuate the chronic tolerogenic ERK signal and renew signal transduction [22].In support of this, we have found that 2-12H B cells exposed to Sm-containing apoptotic debris renew BCR-mediated signal transduction. Further, B cells from mice that fail to clear apoptotic debris (2-12H/Vκ8/MerTKkd) are not repressed by rIL-6 or rsCD40L during TLR4 stimulation, and they do not exhibit elevated basal pERK (unpublished observations).
In summary, we have identified cross-regulation of the BCR, TLR, and IL-6 receptor/CD40 pathways, possibly through nuclear exclusion of pERK, as a mechanism to regulateTLR4-induced plasma cell differentiation of low affinity autoreactive B cells. High basal pERK is required for IL-6 and sCD40L to induce nuclear exclusion of pERK. This is lost in B cells from autoimmune MRL/lpr mice providing an explanation for why sCD40L is unable to repress TLR4 activation and leads to the uncontrolled production of anti-nucleosome and anti-Sm. Thus, B cell tolerance is essential when either LPS or endogenous self-antigens ligate TLR4, and defects in the mechanism described here contributes to the autoimmune phenotype.
Supplementary Material
Acknowledgments
We thank Dr. Harvey Lodish for the CA-MEK1 and vector constructs, Dr. Chris Marshall for the DN-MEK1 construct, Dr. Larry Wysocki for Ars5BSA, Dr. Tom Tedder for anti-HEL IgMa, Dr. Robert Bagnell-Director of Microscopy Services Laboratory for his help with imaging, and members of the Vilen and Clarke labs for helpful discussions.
This work was supported by National Institutes of Health (NIAID) R01 grants AI070984 and AI053266. S.R.L. was supported by NIAMS postdoctoral training grant AR07416, J.A.R. and N.J.W. by NIAID predoctoral training grant AI07273, and M.A.K. by American Cancer Society postdoctoral fellowship grant PF04056. S.Z.J. was supported by a supplement to AI070984. We also thank the Flow Cytometry Core for their support (NCI Center Core Grant P30CA06086).
Abbreviations
- Ig
Immunoglobulin
- SLE
systemic lupus erythematosus
- ERK
extracellular signal regulated kinase
- BAFF
B cell-activating factor
- CA-MEK1
constitutively active MEK1
- DC
dendritic cell
- DN-MEK1
dominant negative-MEK1
- MF
macrophage
- BCR
B cell receptor
- IL-6
interleukin-6
- sCD40L
soluble CD40-ligand
- TNF
tumor necrosis factor
- IEG
immediate early gene
Footnotes
Authorship
Contributions: S.R.L., J.A.R., S.Z.J., A.J.M., J.R.R., K.N.K., N.J.W., M.A.K. performed experiments and analyzed data; S.A.K. and S.H.C. assisted in writing and contributed intellectually to the project; B.J.V. directed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
References
- 1.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luning Prak ET, Monestier M, Eisenberg RA. B cell receptor editing in tolerance and autoimmunity. Ann N Y Acad Sci. 2011;1217:96–121. doi: 10.1111/j.1749-6632.2010.05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pillai S, Mattoo H, Cariappa A. B cells and autoimmunity. Curr Opin Immunol. 2011;23:721–731. doi: 10.1016/j.coi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferry H, Leung JC, Lewis G, Nijnik A, Silver K, Lambe T, et al. B-cell tolerance. Transplantation. 2006;81:308–315. doi: 10.1097/01.tp.0000203830.79357.39. [DOI] [PubMed] [Google Scholar]
- 5.Kilmon MA, Rutan JA, Clarke SH, Vilen BJ. Low-affinity, Smith antigen-specific B cells are tolerized by dendritic cells and macrophages. J Immunol. 2005;175:37–41. doi: 10.4049/jimmunol.175.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilmon MA, Wagner NJ, Garland AL, Lin L, Aviszus K, Wysocki LJ, et al. Macrophages prevent the differentiation of autoreactive B cells by secreting CD40 ligand and interleukin-6. Blood. 2007;110:1595–1602. doi: 10.1182/blood-2006-12-061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Neill SK, Veselits ML, Zhang M, Labno C, Cao Y, Finnegan A, et al. Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic cells. Proc Natl Acad Sci U S A. 2009;106:6262–6267. doi: 10.1073/pnas.0812922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 9.Lartigue A, Colliou N, Calbo S, Francois A, Jacquot S, Arnoult C, et al. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J Immunol. 2009;183:6207–6216. doi: 10.4049/jimmunol.0803219. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Yang Y, Dai J, Medzhitov R, Freudenberg MA, Zhang PL, et al. TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J Immunol. 2006;177:6880–6888. doi: 10.4049/jimmunol.177.10.6880. [DOI] [PubMed] [Google Scholar]
- 11.Summers SA, Hoi A, Steinmetz OM, O'Sullivan KM, Ooi JD, Odobasic D, et al. TLR9 and TLR4 are required for the development of autoimmunity and lupus nephritis in pristane nephropathy. J Autoimmun. 2010;35:291–298. doi: 10.1016/j.jaut.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Hang L, Slack JH, Amundson C, Izui S, Theofilopoulos AN, Dixon FJ. Induction of murine autoimmune disease by chronic polyclonal B cell activation. J Exp Med. 1983;157:874–883. doi: 10.1084/jem.157.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 15.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, et al. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 18.Rakoff-Nahoum S, Medzhitov R. Role of toll-like receptors in tissue repair and tumorigenesis. Biochemistry (Mosc) 2008;73:555–561. doi: 10.1134/s0006297908050088. [DOI] [PubMed] [Google Scholar]
- 19.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 20.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 21.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 22.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 23.Dougherty MK, Ritt DA, Zhou M, Specht SI, Monson DM, Veenstra TD, et al. KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol Cell. 2009;34:652–662. doi: 10.1016/j.molcel.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreadi C, Noble C, Patel B, Jin H, Aguilar Hernandez MM, Balmanno K, et al. Regulation of MEK/ERK pathway output by subcellular localization of B-Raf. Biochem Soc Trans. 2012;40:67–72. doi: 10.1042/BST20110621. [DOI] [PubMed] [Google Scholar]
- 25.Balmanno K, Cook SJ. Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene. 1999;18:3085–3097. doi: 10.1038/sj.onc.1202647. [DOI] [PubMed] [Google Scholar]
- 26.Adams CL, Grierson AM, Mowat AM, Harnett MM, Garside P. Differences in the kinetics, amplitude, and localization of ERK activation in anergy and priming revealed at the level of individual primary T cells by laser scanning cytometry. J Immunol. 2004;173:1579–1586. doi: 10.4049/jimmunol.173.3.1579. [DOI] [PubMed] [Google Scholar]
- 27.Morton AM, McManus B, Garside P, Mowat AM, Harnett MM. Inverse Rap1 and phospho-ERK expression discriminate the maintenance phase of tolerance and priming of antigen-specific CD4+ T cells in vitro and in vivo. J Immunol. 2007;179:8026–8034. doi: 10.4049/jimmunol.179.12.8026. [DOI] [PubMed] [Google Scholar]
- 28.Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. NAT CELL BIOL. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 29.Borrero M, Clarke SH. Low-affinity anti-Smith antigen B cells are regulated by anergy as opposed to developmental arrest or differentiation to B-1. J Immunol. 2002;168:13–21. doi: 10.4049/jimmunol.168.1.13. [DOI] [PubMed] [Google Scholar]
- 30.Santulli-Marotto S, Retter MW, Gee R, Mamula MJ, Clarke SH. Autoreactive B cell regulation: peripheral induction of developmental arrest by lupus-associated autoantigens. Immunity. 1998;8:209–219. doi: 10.1016/s1074-7613(00)80473-2. [DOI] [PubMed] [Google Scholar]
- 31.Arnold LW, Pennell CA, McCray SK, Clarke SH. Development of B-1 cells: segregation of phosphatidyl choline-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J Exp Med. 1994;179:1585–1595. doi: 10.1084/jem.179.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santulli-Marotto S, Qian Y, Ferguson S, Clarke SH. Anti-Sm B cell differentiation in Ig transgenic MRL/Mp-lpr/lpr mice: altered differentiation and an accelerated response. J Immunol. 2001;166:5292–5299. doi: 10.4049/jimmunol.166.8.5292. [DOI] [PubMed] [Google Scholar]
- 33.Kastner B, Luhrmann R. Purification of U small nuclear ribonucleoprotein particles. Methods Mol Biol. 1999;118:289–298. doi: 10.1385/1-59259-676-2:289. [DOI] [PubMed] [Google Scholar]
- 34.Inaoki M, Sato S, Weintraub BC, Goodnow CC, Tedder TF. CD19-regulated signaling thresholds control peripheral tolerance and autoantibody production in B lymphocytes. J Exp Med. 1997;186:1923–1931. doi: 10.1084/jem.186.11.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Culton DA, O'Conner BP, Conway KL, Diz R, Rutan J, Vilen BJ, et al. Early preplasma cells define a tolerance checkpoint for autoreactive B cells. J Immunol. 2006;176:790–802. doi: 10.4049/jimmunol.176.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marino JH, Cook P, Miller KS. Accurate and statistically verified quantification of relative mRNA abundances using SYBR Green I and real-time RT-PCR. J Immunol Methods. 2003;283:291–306. doi: 10.1016/s0022-1759(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu JL, Chiles TC, Sen RJ, Rothstein TL. Inducible nuclear expression of NF-kappa B in primary B cells stimulated through the surface Ig receptor. J Immunol. 1991;146:1685–1691. [PubMed] [Google Scholar]
- 40.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 41.Rui L, Healy JI, Blasioli J, Goodnow CC. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4-driven plasma cell differentiation. J Immunol. 2006;177:5337–5346. doi: 10.4049/jimmunol.177.8.5337. [DOI] [PubMed] [Google Scholar]
- 42.Whitehurst A, Cobb MH, White MA. Stimulus-coupled spatial restriction of extracellular signal-regulated kinase 1/2 activity contributes to the specificity of signal-response pathways. MOL CELL BIOL. 2004;24:10145–10150. doi: 10.1128/MCB.24.23.10145-10150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Nicholas MW, Conway KL, Sen P, Diz R, Tisch RM, et al. EBV latent membrane protein 2A induces autoreactive B cell activation and TLR hypersensitivity. J Immunol. 2006;177:2793–2802. doi: 10.4049/jimmunol.177.5.2793. [DOI] [PubMed] [Google Scholar]
- 44.Richez C, Blanco P, Rifkin I, Moreau JF, Schaeverbeke T. Role for toll-like receptors in autoimmune disease: the example of systemic lupus erythematosus. Joint Bone Spine. 2011;78:124–130. doi: 10.1016/j.jbspin.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Bezbradica JS, Medzhitov R. Integration of cytokine and heterologous receptor signaling pathways. Nat Immunol. 2009;10:333–339. doi: 10.1038/ni.1713. [DOI] [PubMed] [Google Scholar]
- 46.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turnbull IR, McDunn JE, Takai T, Townsend RR, Cobb JP, Colonna M. DAP12 (KARAP) amplifies inflammation and increases mortality from endotoxemia and septic peritonitis. J Exp Med. 2005;202:363–369. doi: 10.1084/jem.20050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diz R, McCray SK, Clarke SH. B cell receptor affinity and B cell subset identity integrate to define the effectiveness, affinity threshold, and mechanism of anergy. J Immunol. 2008;181:3834–3840. doi: 10.4049/jimmunol.181.6.3834. [DOI] [PubMed] [Google Scholar]
- 50.Yasuda T, Kometani K, Takahashi N, Imai Y, Aiba Y, Kurosaki T. ERKs induce expression of the transcriptional repressor Blimp-1 and subsequent plasma cell differentiation. Sci Signal. 2011;4:ra25. doi: 10.1126/scisignal.2001592. [DOI] [PubMed] [Google Scholar]
- 51.Murphy LO, MacKeigan JP, Blenis J. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. MOL CELL BIOL. 2004;24:144–153. doi: 10.1128/MCB.24.1.144-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 53.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Lyubchenko T, dal Porto J, Cambier JC, Holers VM. Coligation of the B cell receptor with complement receptor type 2 (CR2/CD21) using its natural ligand C3dg: activation without engagement of an inhibitory signaling pathway. J Immunol. 2005;174:3264–3272. doi: 10.4049/jimmunol.174.6.3264. [DOI] [PubMed] [Google Scholar]
- 56.Lyubchenko T, Dal Porto JM, Holers VM, Cambier JC. Cutting edge: Complement (C3d)-linked antigens break B cell anergy. J Immunol. 2007;179:2695–2699. doi: 10.4049/jimmunol.179.5.2695. [DOI] [PubMed] [Google Scholar]
- 57.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-f1 B cells. J Immunol. 2004;172:625–635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 58.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–6700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.