Abstract
Quantitative imaging using CT, MRI, and PET modalities will play an increasingly important role in the design of oncology trials addressing molecularly targeted, personalized therapies. The advent of molecularly targeted therapies, exemplified by antiangiogenic drugs, creates new complexities in the assessment of response. The Quantitative Imaging Network (QIN) addresses the need for imaging modalities which can accurately and reproducibly measure not just change in tumor size, but changes in relevant metabolic parameters, modulation of relevant signaling pathways, drug delivery to tumor, and differentiation of apoptotic cell death from other changes in tumor volume. This article provides an overview of the applications of quantitative imaging to phase 0 through phase 3 oncology trials. We describe the use of a range of quantitative imaging modalities in specific tumor types including malignant gliomas, lung cancer, head and neck cancer, lymphoma, breast cancer, prostate cancer, and sarcoma. In the concluding section, we discuss potential constraints on clinical trials using quantitative imaging, including complexity of trial conduct, impact on subject recruitment, incremental costs, and institutional barriers. Strategies for overcoming these constraints are presented.
Keywords: MRI, CT, PET, quantitative imaging, clinical trials, cancer
INTRODUCTION
The conduct of oncology clinical trials in the 21st century must address the paradigm shifting recognition that effective cancer therapies will require individualized molecularly targeted drugs, either singly or in combination. Attempts to evaluate an array of molecularly targeted drugs with novel effects on virtually every aspect of tumor physiology have highlighted the limitations of response criteria based on anatomic imaging. Trials involving molecularly targeted drugs provide opportunities to develop equally novel functional imaging criteria for several important aspects of clinical design. These include non-invasive imaging approaches to pre-treatment tumor phenotyping; early, often quantitative, imaging criteria for treatment response; pharmacodynamic measurements of drug delivery and target pathway modulation; and differentiation of tumor treatment response from other physiologic effects of the experimental therapy.
The optimal application of quantitative imaging (QI) to oncology clinical trials requires systemic evaluation of matching of modalities to the most appropriate physiologic parameters, the ability to standardize modalities and algorithms across different platforms and among multiple institutions, awareness of incremental cost of the imaging techniques, and estimates of potential cost savings by enrichment of responders. The members of the Quantitative Imaging Network (QIN) contend that the investment of clinical trial funding resources by both industry and granting agencies into the innovation and development of validated quantitative imaging tools will be necessary for the development of molecularly targeted therapeutics that are therapeutically effective and delivered at minimal cost. This article provides an overview of applications of QI to phase 0 through phase 3 oncology trials. We describe the most common uses of QI for several specific tumor types, and identify challenges to standardization posed by the characteristics of these tumors. In the concluding section, we discuss potential constraints on QI, including complexity of trial design and conduct, impact on subject recruitment, incremental costs, and institutional barriers. Strategies for overcoming these constraints are presented.
CLINICAL TRIAL DESIGN AND QUANTITATIVE IMAGING
In phase 1 clinical trials of molecularly targeted agents, maximal tolerated dose may not be relevant, and biomarkers for indentifying the minimal dose required for biologically active drug delivery at the target site are needed. QI may be used to demonstrate actual drug delivery to tumor, or physiologic changes associated with target modulation. Phase 0 “proof of mechanism” trials to demonstrate activity of novel compounds may use QI endpoints to measure target modulation, or more downstream effects such as reduced proliferation or energy consumption.
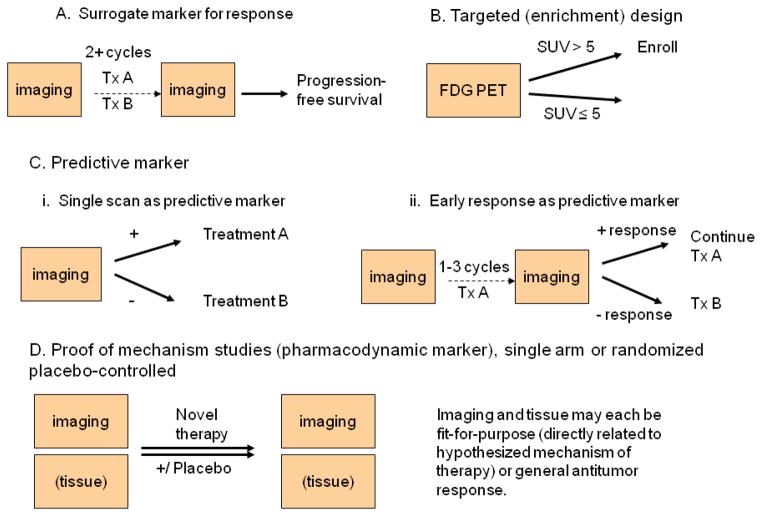
Phase 2 trials present a new array of challenges for imaging response. Many targeted agents can produce a cytostatic, rather than cytotoxic, result, where successful treatment will halt tumor growth but may not kill the tumor. Imaging modalities need to be individualized as “fit-for-purpose”, to measure the response correlates most relevant to the physiologic mechanism of the treatment strategy being tested [1], or to measure response in a manner that is clearly tied to clinically relevant outcomes. In a phase 2 trial examining activity of a new therapy or combination of therapies, it is often infeasible to wait for a “hard” clinical endpoint such as overall survival, while determination of progression free survival may be confounded by difficulty in differentiating between true progression and treatment related changes in the physiology of the tumor and its microenvironment. Very few biomarkers have been validated as surrogate endpoints which predict survival [2]. This was the context of the development of PERCIST 1.0 criteria (PET Response Criteria in Solid Tumors), which use percentage and absolute change in 18F-fluoro-2-D-glucose (FDG) PET measures to categorize metabolic response [3]. A typical design using surrogate measures for response is shown in Figure 1-A. Imaging occurs before treatment starts, and at one or more later time-points, usually after two or more cycles or at the completion of therapy. Assessment of treatment response using these serial images may be used to evaluate a single therapy, or to compare multiple therapies. Progression-free survival and overall survival are also assessed, to inform planning of future (i.e., Phase 3) trials.
Figure 1.
Study designs using quantitative imaging as a biomarker
Imaging can also improve the efficiency of clinical trials through patient selection. Imaging biomarkers may be used to screen patients for study enrollment in an enrichment design [4, 5], also called a “targeted” or “marker positive” design. Figure 1-B shows a study schema that uses FDG PET uptake as a study entry criterion, to identify thyroid cancer patients with aggressive disease for a phase 2 study of systemic therapy [6]. Imaging may also be used as a predictive marker. A single pre-treatment measurement could be used to direct therapy (Figure 1-Ci), or serial measurements over a short period of therapy could be used to inform whether a therapy is productive or futile (Figure 1-Cii). QI can also be used to help guide drug dosing; for example, patient specific dosimetry is commonly applied in radio-immunotherapy of non-Hodgkins lymphoma with I-131 tositumomab, and in treatments of aggressive metastatic thyroid cancers with I-131.
Serial imaging over a short period of time has particular promise in identifying a physiologic response to investigational therapy (Figure 1-D). Dramatic changes in tumor metabolism may be detected as changes in FDG PET standardized uptake value (SUV) in as few as 24 hours, as in the response of gastrointestinal stromal tumors to imatinib [7]. These changes in tumor metabolism frequently occur prior to changes in tumor size. While it is possible that these physiologic changes may not correlate with clinically relevant endpoints such as PFS or overall survival, the subsequent success of imatinib in randomized trials with sound clinical endpoints such as recurrence-free survival [8] encourages the exploration of functional imaging as a pharmacodynamic endpoint. Although serial biopsy is feasible in some tumor types, serial imaging may be seen as less invasive, and does not change the tumor as biopsy would.
QUANTITATIVE IMAGING TECHNOLOGIES
PET
PET imaging produces a quantitative, three-dimensional image of the distribution of PET radioisotopes in the body. The PET radiotracer chosen is designed to reflect a relevant biochemical processes in the body. A PET camera detects 511 KeV gamma photons emitted by positron annihilation events due to positron emission by radioisotopes (e.g. F-18), which are linked to a molecule of interest (e.g. 2-deoxy-2-[18F]fluoro-D-glucose, or FDG), which traces the early aspects of glucose metabolism in the body [9]. Therefore PET imaging depicts the biological activity of molecular events, and is particularly well suited for the assessment of early changes in disease pathophysiology at the molecular level. PET/CT can measure FDG uptake in tumors and normal tissues, FDG is FDA approved, and FDG PET is CMS funded and widely used to detect and manage cancers by assessing metabolic rate and measures of therapy response. FLT (18F-F-3′-fluoro-3′-deoxy-D-thymidine) is an investigational agent that estimates thymidine phosphorylation by thymidine kinase, and is substantially correlated with measures of cellular proliferation. Additional investigational PET tracers include markers for cell hypoxia and apoptosis. Recently, there has been progress on standardization of methods for image acquisition and interpretation resulting in more accurate use of PET/CT in multicenter clinical trials [10].
Quantitative methods using dynamic image acquisition and compartmental modeling have been used to assess tumor perfusion, tracer extraction and tissue metabolism [11]. A current challenge is to develop standardized, truncated procedures for conducting dynamic PET studies so that they can be integrated into routine patient clinical assessment. Some innovations toward this aim are obtaining the arterial input function through image-based methods (without blood withdrawals), and shortening dynamic scan times for the approximately 1 hour time slot allowed for patient uptake and scanning.
MRI
MRI technology is rapidly moving beyond anatomical or structural imaging to dynamic, functional and metabolic imaging. Rigorous investigation of multiple aspects of tumor biology and assessment of tumor response to treatment is now possible. The diversity of MRI sequences available allows for visualization of changes in very specific components of the tumor such as the tumor vascular network with perfusion or permeability imaging or the tumor microenvironment with diffusion weighted imaging (DWI) [12–14]. In less than an hour, we can non-invasively quantify response to new drugs and gain a better understanding of drug mechanism of action in humans without having to extrapolate what might be happening from pre-clinical models [15]. However, dynamic MRI techniques have generally been optimized for single institution studies and comparing data across MRI vendor platforms or institutions has been the largest barrier to the multicenter studies needed to validate these tools. A standardized way of acquiring MRI data (including dose of gadolinium for contrast-enhanced scans, specific acquisition parameters, post-processing analysis, etc.) needs to be established and good quality assurance has to be performed to ensure that data is collected in the most reliable way possible.
CT
X-Ray computed tomography (CT) imaging is the most mature of all 3D imaging techniques. The first patent application for CT imaging was filed in 1960 by Oldendorf, and CT imaging was first demonstrated by Allan Cormack in 1963. The first clinical CT scanner was developed by Houndsfield for EMI. In a CT image acquisition an X-Ray tube produces 120 – 140 KVp X-rays that then pass through a thin slice of patient and the attenuated X-rays are measured as they emerge from the patient. The slice of patient is broken into small rectangular prisms (voxels) and the CT reconstruction algorithms determine the apparent density (Houndsfield Unit) in each voxel. These slices are assembled to produce 3D images of patients. CT matured rapidly with the development of smaller and more efficient detectors and microprocessors. Sub millimeter resolution in all dimensions is now routine, with thoracic scans (75 – 80 cm in length) acquired in less than 5 seconds and the images reconstructed in a minute or less. As CT images are essentially a density map of a patient they are very useful in accurately determining boundaries where the densities change, such as lung tumors that are not adjacent to the chest wall or mediastinum.
Due to the prevalence of CT imaging for cancer staging and re-staging, and its use for measuring response in clinical trials [16], archives of CT images collected according to standardized (if not optimal) protocols exist for large clinical trials. These clinical trial archives will also include patient characteristics, treatment details, and clinical outcomes such as overall survival. With sufficient advances in data sharing procedures and informatics, these archives may be used as a cost-effective resource to develop and validate CT-based biomarkers, including automated and semi-automated volumetric measures.
APPLICATIONS OF QUANTITATIVE IMAGING TO ONCOLOGY CLINICAL TRIALS
Gliomas
Imaging has always played a critical role in assessing glioma response to treatment [17]. Repeated tumor biopsies are challenging in brain tumor patients and imaging has the advantage of non-invasively capturing the heterogeneity of gliomas that may be missed through sampling error in only partially resectable gliomas. Unfortunately, though, as opposed to tissue-based makers such as MGMT methylation or IDH1 mutation, no validated imaging biomarkers have been discovered and even seemingly straightforward assessments of glioma response using post-contrast T1 weighted images are challenging. The increasing importance of antiangiogenic therapy has led to further challenges for tumor response measurement. Changes in apparent tumor volume may result from changes in blood brain barrier dynamics secondary to antiangiogenic therapy (pseudoresponse), or transient radiation injury may occur after chemoradiation for newly diagnosed glioblastoma (pseudoprogression). New guidelines for response imaging in malignant glioma trials (RANO criteria) [18] attempt to address these challenges, largely by emphasizing the need for confirmation of response.
Particularly in the setting of antiangiogenic agents, inter-observer correlations of response are poor and contrast enhancement, historically considered the most appropriate surrogate marker for tumor burden, has been rendered unreliable [19]. Objective and quantitative measures are needed to differentiate true tumor cell killing from other physiologic changes. Preliminary findings suggest that histogram-based analysis [20] or parametric response maps, which co-register scans for voxelwise change [21, 22], may be more promising than summary apparent diffusion coefficient (ADC) values as DWI predictors of clinical outcomes.
FDG PET has demonstrated the ability to differentiate between high and low grade gliomas, and to differentiate radionecrosis from tumor recurrence [23]. However, high FDG uptake in normal cortex, thalamus and basal ganglia limit the utility of FDG PET to assess disease in many patients. More specific tracers have been used to assess glioma response, including 11C-methionine, FLT, and 18F-fluoromisonidazole (FMISO) [24–27]. FLT PET may distinguish pseudoprogression from glioblastoma progression after radiation therapy [28] and is being evaluated in an ongoing QIN trial (J Mountz, principal investigator).
Lung cancer
Imaging plays a major role in all aspects of lung cancer including screening, diagnosis, staging, and assessing response to therapy. The National Lung Cancer Screening Team concluded that screening with low-dose CT reduces mortality from lung cancer [29], and CT is usually used in determining extent of disease. FDG PET scanning can help to differentiate malignant from non-malignant solitary pulmonary nodules larger than 1 cm, and for smaller solitary nodules with a high FDG PET SUV. Evaluation of lymph nodes in the mediastinum is also aided by FDG PET scanning. A meta-analysis of evaluation of mediastinal lymph nodes as benign or malignant found sensitivity and specificity of 85% and 90% respectively for PET, compared to a median sensitivity and specificity for CT of 61% and 79% [30].
Quantitative FDG PET is well-established as a measure of response to lung cancer treatment, including neoadjuvant chemoradiation. For example, FDG PET was prospectively evaluated in predicting response to chemotherapy in 57 patients with lung cancer who underwent platinum-based chemotherapy. A 20% or greater reduction in FDG PET SUV was associated with longer survival [31]. Challenges to accurate quantitative measurement of FDG response to lung cancer treatment include respiratory motion of the chest during scanning, and varying background uptake in lung versus common invasive sites such as mediastinum and liver [32].
Volumetric, quantitative CT has also been found to be useful in lung cancer. Unidimensional tumor response assessments do not fully characterize tumor growth dynamics. Volumetric measurement and response assessment allow better classification of patient tumors as sensitive or resistant to targeted therapy, and may have a role in improving tissue biomarker discovery for novel therapies [33].
Head and Neck
In squamous cell head and neck cancer PET/CT imaging has been used for staging, follow-up and surveillance. In staging, the usual problem is distinguishing reactive lymph nodes from metastatic disease. Quantitation has not been particularly useful in this setting, since the uptake in small nodes is markedly underestimated due to the partial volume effect. This suggests that partial volume correction may be useful in improving the accuracy of assessment of nodes for metastatic disease. The current approach is to assess the most likely distribution of nodal metastases and to dismiss as false positives mild uptake signals in anatomic sites that are not likely to be involved. This process is currently done using observer experience and intuition. More objective assessment of nodal status could combine partial volume correction with probabilities of nodal involvement for different sites.
Currently, most head and neck tumors are treated with a combination of surgery, chemotherapy and radiotherapy. The major clinical management questions thereafter are: Has the patient been cured? And if not, where is the residual or recurrent tumor located? PET/CT imaging is usually performed about 12 weeks after the end of radiotherapy to assess the success of therapy. PET/CT has an excellent negative predictive value, 99% at the primary site, as well as at nodal sites, but much lower positive predictive values of 32% at the primary site and 71% at nodal sites [34]. A number of different approaches using QI are being explored to improve these low positive predictive values. The approaches include: 1. Measuring tissue uptake at two different times after injection of FDG. This approach seems to have some success in lung cancer [35]. 2. Using lesion-to-normal tissue ratio instead of the usual standardized uptake ratio. This approach has been shown to be more accurate than SUV alone in some settings [36]. Current efforts include developing an automated method for determining average SUV in liver, aorta, and cerebellum, which should improve the reproducibility of the method. 3. Combining all available clinical data, along with PET and CT data to derive a probability-of-malignancy estimate. Ongoing QIN research has compiled an extensive database with more than 200 subjects, and will employ neural networks or non-linear clustering methods to improve post-therapy classification of the presence of residual disease.
Lymphoma
Once a diagnosis of malignant lymphoma has been established, imaging is used to evaluate the anatomic localization of disease and to assess the bulk of the tumor burden. These assessments may influence estimates of the patient’s prognosis, and choice of optimal treatment (usually radiation therapy and/or chemotherapy). FDG PET has great value for evaluating lymphomas, since FDG PET can differentiate between viable tumor and or fibrosis in the residual masses which are frequently present after treatment. Consensus criteria have been published with recommendations for standardizing the use of FDG PET to provide a qualitative assessment of response to therapy [37]. However, both false positive and negatives occur. FDG PET imaging may misinterpret the metabolic activity of non-neoplastic lesions as active lymphoma. These include thymic hyperplasia, infection, sarcoidosis and brown fat [38]. Some histologic subtypes of lymphoma, such as marginal zone lymphoma and peripheral t-cell lymphoma show a variable uptake and frequently areas that are FDG negative contain viable tumor [39].
The revised response criteria for malignant lymphoma [40] recommended that PET be performed in all patients with routinely FDG-avid lymphomas, including diffuse large B-cell lymphoma and Hodgkin’s lymphoma. In non-Hodgkins lymphomas, early FDG PET has been used to direct patients with a poor qualitative response at mid-treatment to more aggressive therapy, including stem cell transplant. This “risk-adapted” therapy greatly improved over an expected 20% rate of 2-year progression-free survival, to 75% for patients who received transplant [41]. The routine use of PET scanning as a surveillance tool to identify relapse is an active clinical trial question. MRI techniques are currently under investigation in lymphoma, in particular, whole body MRI and DWI [42].
Breast Cancer
Most studies using QI to evaluate breast cancer therapeutic response have been conducted in the context of neoadjuvant chemotherapy to treat locally advanced breast cancer. This setting is attractive because pathologic complete response at surgery is a clinically relevant standard for evaluating imaging biomarkers of response. FDG PET was first explored for monitoring metabolic response to chemotherapy by Wahl and colleagues [43]. Changes in FDG influx (Ki) discriminated between 8 pathologic responders and 3 non-responders as early as 8 days after treatment initiation. Subsequent studies explored timing of serial scans, region of interest definition, and methods of quantification [44], and established metabolic response as a predictor of disease-free and overall survival [45].
The current breast cancer metabolic response literature largely supports prospective application of PERCIST 1.0 criteria [3] to categorize metabolic response after one or two cycles of neoadjuvant chemotherapy, and possibly to guide treatment changes in non-responders. However, several biophysical constraints complicate FDG PET imaging of early treatment response. Breast cancer lesions may have lower FDG uptake than other cancers routinely imaged with FDG PET. Although such lesions may still be detected, since normal breast tissue has less than average uptake [46], detection of a 0.8 absolute decrease in lean body mass-adjusted SUV may be difficult to attain. First, 0.8 SUV units would be a very large percentage of a lesion with low baseline uptake. Second, the proportion of non-phosphorylated FDG, which does not reflect tumor metabolism, is higher in lesions with a low initial SUV. Lesions with low SUV at baseline are important to characterize accurately, as lesions with low initial SUV are unlikely to achieve pathologic complete response [47, 48]. Assessment of metabolic response for lesions with a low initial SUV may require dynamic imaging with compartmental modeling, in order to distinguish between phosphorylated and non-phosphorylated FDG [49, 50].
DWI and dynamic contrast enhanced (DCE) MRI are also under development for monitoring response to cancer therapies [51]. An increase in the apparent diffusion coefficient (ADC) indicates increased water motility, and may be associated with the breakdown of tumor tissue or changes in interstitial fluid pressure [52]. Decrease in the transfer of gadolinium contrast from blood vessels to tumor tissue, measured by Ktrans, is observed with cytotoxic therapy [52] and antiangiogenic therapies [53]. Current challenges include development of standardized algorithms for Ktrans measurement which can be validated across different platforms, and determination of cutoff values for response.
In addition to studies of FDG PET or DCE MRI to monitor treatment response (Figure 1-A), other QI modalities and other study designs are being developed for evaluating and guiding breast cancer therapies. 18F-Fluoroestradiol (FES) PET scanning to determine estrogen receptor activity has promise for guiding hormonal therapy in patients with advanced breast cancer [54, 55], using designs such as Figure 1-Ci. Proof-of-mechanism studies (Figure 1-D) are planned to use imaging as a pharmacodynamic endpoint in early trials of novel agents [56]. Finally, magnetic resonance spectroscopy (MRS) can measure changes in the level of choline, a component of cell membranes found in many tumor cells, and may provide an early measure of response to neoadjuvant chemotherapy [57].
Prostate cancer
Imaging plays a role in prostate cancer diagnosis, tumor localization, tumor staging, determination of extracapsular disease spread, and determination of loco-regional nodal and distant metastatic disease. Imaging also plays a role in detection of recurrent disease after a biochemical relapse is suggested, and is critical in guiding prostate gland biopsy (either with Trans Rectal Ultrasound (TRUS) guidance, or with MRI guidance for cases where there have been negative TRUS biopsies or positive but low-grade low-volume tumor with discordant biochemical kinetics).
The prostate cancer imaging landscape has changed radically in the last few years. Faster sequences used on high field strength MRI scanners provide the opportunity for development of functional QI measures. The major functional techniques currently used for prostate imaging are DWI, DCE MRI, and proton MRS imaging. These techniques have the potential to provide quantitative biological information that can be used to predict tumor aggressiveness. Recent studies have assessed DWI biomarkers for prostate cancer aggressiveness, with ADC values correlating negatively with Gleason score and with D’Amico risk score [58–61]. ADC has also been shown to be a significant predictor of adverse repeat biopsy and time to radical therapy in patients on an active surveillance strategy [62]. In clinical stage T2/3 prostate cancer patients, a combination of DCE MRI and MRS to individualize neoadjuvant hormone therapy prior to radiation therapy has been proposed, as it non-invasively verifies metabolic and dynamic angiogenic changes within prostate tumors during therapy [63].
The multiparametric MRI (mpMRI) approach using all major functional techniques is the optimal approach for prostate cancer imaging [64, 65]. However, further refinements are necessary for the acceptance of mpMRI imaging response criteria in prostate cancer clinical trials. Optimization and standardization of functional imaging measurement is necessary. (An example of this is DCE MRI where, for quantitative pharmacokinetic analysis, a pharmacokinetic model of the MRI enhancement time course needs to be decided upon, and measurement of the intrinsic tissue T1 value and arterial input function (AIF) to be used in the model are necessary.) Another challenge is the need for spatial registration of multidimensional mpMRI data sets obtained over time. This is necessary for precise, detailed lesion/gland comparison. The goal is a “summary statistical map” or a “probability biomap”, whereby pertinent patient metadata could also be incorporated and displayed, in addition to the imaging findings.
FDG PET has limited use in the diagnosis and staging of organ-confined prostate cancer, although it may play a role in assessment of the extent of progressive metastatic prostate cancer and metabolically active castration-resistant disease [66, 67] Although 11C-choline and 11C-acetate do not reliably differentiate between benign and malignant prostate disease, both are being investigated as techniques for staging disease and for detecting locally recurrent or metastatic disease in patients with biochemical failure [66, 68, 69]. With advances in the understanding of castrate-resistant prostate cancer and therapies directed against the androgen-receptor (AR) signaling axis, molecular imaging strategies that probe this AR signaling axis (such as 18F-FDHT and 89Zr-591) have been investigated. Their development and validation may help accelerate drug development in castrate-resistant prostate cancer [70].
Sarcoma
Sarcomas are uncommon, malignant neoplasms of connective tissue, and are categorized as either bone or soft tissue. These broad groupings contain numerous subtypes with diverse biology and behavior. While certain sarcomas, such as pediatric rhabdomyosarcoma and bone sarcomas, are associated with relatively high cure rates, effective systemic therapies are lacking for most sarcomas. Clinical trials are vital for improving outcome for these tumors, and the small number of cases mandate multicenter trials. The systematic evaluation of QI techniques as biomarkers of response and resistance to treatment is crucial in optimizing the efficiency of clinical trial design for sarcoma therapies.
Neoadjuvant therapy is used in a number of different clinical settings in sarcoma management, and provides the opportunity for quantitative assessment of in vivo treatment response. Response to neoadjuvant chemotherapy is an important prognostic factor in bone sarcomas, with the degree of tumor necrosis in the operative specimen correlating with clinical outcome in patients with osteosarcoma and Ewing’s sarcoma [71]. There are no reliable anatomic imaging modalities to detect response to neoadjuvant chemotherapy in bone sarcomas as the size of these bony tumors often does not decrease despite the development of tumor necrosis.
Several studies have evaluated the utility of FDG PET in assessing chemotherapy response in pediatric and adult bone sarcomas. These analyses have demonstrated a relationship between changes in SUV and favorable histologic response to chemotherapy in osteosarcoma and Ewing’s sarcoma [72–74]. However, FDG PET has been imperfect in its ability to distinguish favorable from non-favorable responses, with physiologic processes such as inflammatory changes potentially confounding these analyses [73]. FDG PET has been incorporated as an exploratory biomarker endpoint into clinical trials of new agents, such as a phase II study of the VEGF-R inhibitor sorafenib in osteosarcoma [75]. Similarly, DCE MRI has been investigated in predicting therapy response in both localized osteosarcoma and Ewing’s sarcoma [76]. DCE MRI parameters have been reported to be associated with histologic response and survival in patients receiving neoadjuvant chemotherapy [77].
Soft tissue sarcomas have unique characteristics including variable anatomic locations, large size, and heterogeneity of composition (fibrosis, necrosis, hemorrhage) that present potential challenges in QI analysis. The wide range of different histologic subtypes in can introduce heterogeneity into clinical trial interpretation and pose challenges for trial design. Several groups have reported FDG PET predicts response to neoadjuvant therapy and its advantage over anatomical imaging methods for early response assessment and long-term patient outcome [78–80]. However, one trial of neoadjuvant therapy in soft tissue sarcoma evaluating FLT PET/CT noted that reductions in tumor uptake did not reliably predict histopathological response [81]. DCE MRI pharmacokinetic modeling is being evaluated in response assessment of neoadjuvant chemotherapy and antiangiogenic agents in extremity soft tissue sarcomas [82].
Gastrointestinal stromal tumors are one sarcoma subtype in which QI has played a prominent role in treatment response assessment. The responsiveness of GIST to the molecularly targeted agent imatinib has presented a number of challenges for QI markers of response. Standard anatomic response criteria in this disease have been criticized as insensitive and potentially misleading in determining patient benefit to targeted therapy [83, 84]. Early clinical trials of the KIT tyrosine kinase inhibitor imatinib incorporated PET assessment and demonstrated that metabolic changes on PET were predictive of subsequent radiologic response [7]. SUV changes on PET precede anatomic size change on CT and can occur early in the course of treatment, sometimes as soon as 24 hours after the first dose [7]. Subsequent studies have confirmed the role of PET in assessment of GIST response and its advantage over anatomical CT measurements in this disease [85]. While PET has established a role in response assessment for GIST, there remains a lack of a standardized response criteria for this modality which can complicate trial design and comparison of results [86].
The need for improved therapies and the rarity of sarcomas encourage novel trial design and provide fertile ground for incorporating alternative imaging endpoints into clinical trials. Perfecting the discriminatory ability of our current QI modalities in determining response will be necessary prior to their use as primary endpoints in efficacy trials.
LESSONS LEARNED AND FUTURE DIRECTIONS
While QI has promise for contributions to the conduct of clinical trials and oncology practice, QIN investigators have encountered several challenges when integrating QI into clinical trials. This section discusses describes these challenges, and the strategies developed to overcome them.
Uncertainty about measurement characteristics of QI biomarkers is a barrier to the planning of large-scale multicenter trials involving QI. Randomized Phase 3 clinical trials may fail if they are underpowered or if earlier studies are poorly characterized [87]. QIN studies are underway that build on earlier work characterizing measurement error in imaging biomarkers, and translate those findings to the design of clinical trials. For example, standard sample size formulas can be used to show that for a randomized study designed to have 80% power and a two-sided Type I error rate (α) of 0.05 to detect a 20 percentage point difference in SUV change between a novel therapy and standard of care, the required sample size increases from 8 to 126 as PET precision worsens from 10% to 40% [88]. QIN investigators recognize that the development of imaging measures as quantitative biomarkers [89] includes characterization of measurement uncertainty introduced by scanners, patient preparation, image analysis, region of interest definition, and the synthesis of these findings into clinical trial design protocols. Measurement error can be minimized through strict adherence to existing quality control and standardization protocols [90, 91]. The Uniform Protocols for Imaging in Clinical Trials (UPICT) template have been helpful and continue to evolve. Similarly, the RSNA Quantitative Imaging Biomarkers Alliance (QIBA) has complemented the NCI Centers of Quantitative Excellence Program (CQIE) and American College of Radiology Imaging Network (ACRIN) efforts to achieve quality control standards for QI studies. For now, it remains critical that test and follow up studies be done on the same scanner platform, ideally the same scanner, using the same software in a given patient on a clinical trial. Similarly, analysis must be done in a consistent fashion, ideally using automated and validated software aids.
One setback encountered in recruitment to clinical trials involving QI is patient reluctance. Participation in a trial involving QI may be very time-consuming. Procedures such as FDG PET require preparation (fasting), and usually several hours on site. When a trial incorporates information from several imaging modalities (i.e., PET and MRI), it is sometimes not feasible to schedule scans on the same day. (Faster scanners or combined devices such as PET/MRI may allow quantitative multiparametric data to be acquired simultaneously, addressing this logistical hurdle.) Some PET scans require an arterial line, which is far more invasive and uncomfortable than an intravenous line. Patients also incur costs for transportation and housing when they travel a long distance to participate in a study. We believe it is both ethical and rational to provide monetary compensation, to defray additional costs and aggravation incurred by research study participation [92]. However, compensation should not be withheld until completion of the study, or given at different rates for different stages of the study that require the same time commitment. We have observed that QI trials integrated closely into novel therapy trials appear to accrue more rapidly than pure imaging trials (other than screening). Patients are highly motivated to have access to novel treatments, and QI may be accepted as an important element of the trial.
Patients often have exaggerated fears about the incremental risks of QI components of clinical trials. Sometimes these concerns can be addressed through patient education (providing a clear explanation of what to expect during a procedure) and through attention to patient comfort. Investigators must be especially forthcoming regarding risk of radiation exposure. Use of serial CT for screening, re-staging, and response classification (i.e., RECIST) does result in considerable exposure: a body survey results in as much as 15 mSv exposure [93], compared to an average ambient radiation dose of 3 mSv/year for United States residents [94]. PET imaging, involving the direct injection of radiopharmaceuticals, is a more overt source of concern for patients. Although the effective radiation dose from injection of FDG is about 4–6 mSv for most oncology applications [95], the use of PET/CT devices can result in exposure as great as 25 mSv [96]. To address these legitimate concerns, investigators can actively advocate for CT scanning protocols that minimize radiation dose [96, 97], including ultra-low dose CT for PET/CT attenuation correction [98]. Again, patient education is another important factor. Graphics such as radiation dose charts [99] can put risk factors into context, including exposure due to prior radiotherapy (often 1000 mSv or greater).
We have found that referring physicians frequently share patients’ concerns about inconvenience, potential treatment delays, and radiation exposure. While oncologist investigators may be well-informed and enthusiastic about therapeutic clinical trials involving QI, other referring physicians will not have much contact with the radiologist investigators and will inevitably not be as dedicated to a trial that involves a significant role for specialists in other departments. Study coordinators with expertise in trials involving QI are an essential investment in centers committing to QI research activity. Discussion of the imaging components of clinical trials during disease-specific tumor boards allows investigators and coordinators to identify potential participants and be familiar with and integrated into the treatment teams. A QI-specific coordinator should be available to explain study procedures to both patients and referring physicians, and to coordinate scheduling to avoid treatment delays.
The multiplicity of demands on radiologists needs to be recognized as an institutional barrier to QI study execution. Radiologists (including department chairs) may not automatically agree to scheduling flexibility for research scans, longer time in PET scanners for dynamic imaging, and intensive quality control processes required for QI. Research requirements for increased scanner time, interpretation time, technician oversight, and processing time may not be acknowledged in clinical trial protocols that designate as much imaging as possible as “standard of care” to minimize cost to the trial. QI trial components must include the faculty who will do the work in discussions about study design and remuneration. When possible, study design should include strategies to minimize the incremental effort required for interpretation. For example, informatics advances that facilitate comparisons to earlier scans [100] would benefit all radiologists, not only those involved in QI research. QI trials always require proactive discussion with the radiology faculty and staff who will be impacted by the trial demands. The optimal solution to this problem requires academic protected time for radiology faculty with the appropriate expertise to participate as named (and partially funded) collaborators. If oncologists open a clinical trial without formal “buy-in” from radiologists [101], it can hardly be expected that radiologists will be enthusiastic collaborators.
Institutional limitations can have adverse effects on clinical trials involving QI. Coordination of institutional review, scientific review, and budgeting is complicated for many clinical trials, and may lead to delays that jeopardize meeting accrual goals [102]. Additional hurdles for trials involving QI include radiation safety review, and investigational new drug (IND) applications for novel tracers. Within academic medical centers, clinical trials involving imaging are perceived to be expensive, and relevant only to research rather than guiding clinical practice. Whereas drugs are often provided by manufacturers even when the manufacturer is not fully sponsoring a trial, novel imaging modalities and the use of QI for guiding therapy are not subsidized in this manner. The imaging community can address this perception by generating measures of reduced morbidity and cost savings that can be attained when imaging is used to guide patients toward effective therapies and away from ineffective therapies.
Opportunities and aspirations
The pathways involved in driving and sustaining malignant transformation and tumor growth are being methodically elucidated. Systemic integration of functional imaging tumor phenotyping with the wealth of data from the assessment of the tumor genome (methylome, etc) will facilitate the identification of appropriate patient subsets for specific treatments. However, not all pathway directed treatments will be successful, and resistance can develop. Baseline FDG PET/CT studies paired with early treatment monitoring PET/CT at two to four weeks into treatment (for FDG avid tumors) may be a highly informative systematic path to assess tumor biology and personalize the identification of futile regimens [56].
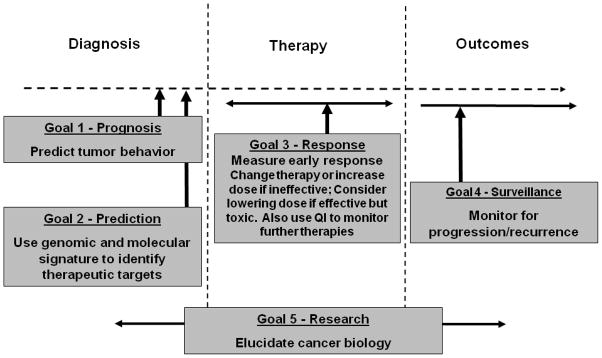
Resources linking the QI phenotype to the genotype and pathway directed therapeutics could be highly informative. A systematic catalogue of QI methods associated with a variety of tumor types and therapies could form a large library of response/tumor/treatment linkages with standard methods for quantitation, standard quantitative analysis methods, a standard lexicon, and standardized reporting exports. This resource would help inform both oncologic drug development and treatment choices for individuals. QI may become part of a comprehensive paradigm for individualized cancer treatment (Figure 2). It is also highly likely that combinations of QI methods, e.g. a hybrid multiparametric signal of PET/DCE MRI/DWI combined with a suitable biomarker from the blood or DNA, may be more predictive than any single imaging tool alone. Again, systematic prospective collection of such data in differing tumor types with varying therapies may be highly informative for future treatment choices in both clinical trials and in clinical practice.
Figure 2.
Paradigm for using quantitative imaging to guide targeted therapy
As more individualized, biomarker-driven therapies are developed, and with cost incentives for comparative effectiveness research to assess these individualized therapies, the multidisciplinary nature of oncology research must be accommodated. The QIN aims to aid the research community at large through these growing pains, through support of innovations in instrumentation, informatics, and clinical trial management, with ultimate linkage of our quantitative imaging results to patient care and improved patient outcomes.
Acknowledgments
This work is supported by the National Institutes of Health, Quantitative Imaging Network (U01-CA148131). The authors are grateful to Robert Nordstrom, Larry Clarke, Hannah Linden, David Mankoff, Mark Muzi, Savannah Partridge, Mia Levy, and Daniel Rubin for helpful discussions, and to Alicia DePastino for administrative support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dancey JE, Dobbin KK, Groshen S, Jessup JM, Hruszkewycz AH, Koehler M, Parchment R, Ratain MJ, Shankar LK, Stadler WM, True LD, Gravell A, Grever MR. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010;16:1745–55. doi: 10.1158/1078-0432.CCR-09-2167. [DOI] [PubMed] [Google Scholar]
- 2.Buyse M, Sargent DJ, Grothey A, Matheson A, de Gramont A. Biomarkers and surrogate end points--the challenge of statistical validation. Nat Rev Clin Oncol. 2010;7:309–17. doi: 10.1038/nrclinonc.2010.43. [DOI] [PubMed] [Google Scholar]
- 3.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 (Suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoering A, Leblanc M, Crowley JJ. Randomized phase III clinical trial designs for targeted agents. Clin Cancer Res. 2008;14:4358–67. doi: 10.1158/1078-0432.CCR-08-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon R. Clinical trial designs for evaluating the medical utility of prognostic and predictive biomarkers in oncology. Per Med. 2010;7:33–47. doi: 10.2217/pme.09.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16:5260–8. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CDM, Joensuu H. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumors. New England Journal of Medicine. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 8.Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 10.Boellaard R. Need for standardization of 18F-FDG PET/CT for treatment response assessments. J Nucl Med. 2011;52 (Suppl 2):93S–100S. doi: 10.2967/jnumed.110.085662. [DOI] [PubMed] [Google Scholar]
- 11.Muzi M, O’Sullivan F, Mankoff D, Doot R, Pierce L, Kurland B, Linden H, Kinahan P, QIN Quantitative assessment of dynamic PET imaging data in cancer imaging. Magnetic Resonance Imaging. 2012 doi: 10.1016/j.mri.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paldino MJ, Barboriak DP. Fundamentals of quantitative dynamic contrast-enhanced MR imaging. Magn Reson Imaging Clin N Am. 2009;17:277–89. doi: 10.1016/j.mric.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Gerstner ER, Sorensen AG. Diffusion and diffusion tensor imaging in brain cancer. Semin Radiat Oncol. 2011;21:141–6. doi: 10.1016/j.semradonc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Atri M. New technologies and directed agents for applications of cancer imaging. J Clin Oncol. 2006;24:3299–308. doi: 10.1200/JCO.2006.06.6159. [DOI] [PubMed] [Google Scholar]
- 15.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. Journal of Clinical Oncology. 1990;8:1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 18.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 19.Hygino da Cruz LC, Jr, Vieira IG, RCD Diffusion MR imaging: an important tool in the assessment of brain tumors. Neuroimaging Clin N Am. 2011:21. doi: 10.1016/j.nic.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Pope WB, Kim HJ, Huo J, Alger J, Brown MS, Gjertson D, Sai V, Young JR, Tekchandani L, Cloughesy T, Mischel PS, Lai A, Nghiemphu P, Rahmanuddin S, Goldin J. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252:182–9. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 21.Hamstra DA, Galbán CJ, Meyer CR, Johnson TD, Sundgren PC, Tsien C, Lawrence TS, Junck L, Ross DJ, Rehemtulla A, Ross BD, Chenevert TL. Functional Diffusion Map As an Early Imaging Biomarker for High-Grade Glioma: Correlation With Conventional Radiologic Response and Overall Survival. Journal of Clinical Oncology. 2008;26:3387–94. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galbán C, Chenevert T, Meyer C, Tsien C, Lawrence T, Hamstra D, Junck L, Sundgren P, Johnson T, Ross D, Rehemtulla A, Ross B. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat Med. 2009;15:572–76. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906–20. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 24.Herholz K, Kracht LW, Heiss WD. Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J Neuroimaging. 2003;13:269–71. [PubMed] [Google Scholar]
- 25.Wyss M, Hofer S, Bruehlmeier M, Hefti M, Uhlmann C, Bartschi E, Buettner UW, Roelcke U. Early metabolic responses in temozolomide treated low-grade glioma patients. J Neurooncol. 2009;95:87–93. doi: 10.1007/s11060-009-9896-2. [DOI] [PubMed] [Google Scholar]
- 26.Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Geist C, Dahlbom M, Silverman DH, Satyamurthy N, Phelps ME, Chen W. 3′-deoxy-3′-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med. 2012;53:29–36. doi: 10.2967/jnumed.111.092387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spence AM, Muzi M, Swanson KR, O’Sullivan F, Rockhill JK, Rajendran JG, Adamsen TC, Link JM, Swanson PE, Yagle KJ, Rostomily RC, Silbergeld DL, Krohn KA. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res. 2008;14:2623–30. doi: 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spence AM, Muzi M, Link JM, O’Sullivan F, Eary JF, Hoffman JM, Shankar LK, Krohn KA. NCI-sponsored trial for the evaluation of safety and preliminary efficacy of 3′-deoxy-3′-[18F]fluorothymidine (FLT) as a marker of proliferation in patients with recurrent gliomas: preliminary efficacy studies. Mol Imaging Biol. 2009;11:343–55. doi: 10.1007/s11307-009-0215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, Chan JK, Owens DK. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 31.Weber WA, Petersen V, Schmidt B, Tyndale-Hines L, Link T, Peschel C, Schwaiger M. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–7. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Hicks RJ. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J Nucl Med. 2009;50 (Suppl 1):31S–42S. doi: 10.2967/jnumed.108.057216. [DOI] [PubMed] [Google Scholar]
- 33.Zhao B, Oxnard GR, Moskowitz CS, Kris MG, Pao W, Guo P, Rusch VM, Ladanyi M, Rizvi NA, Schwartz LH. A pilot study of volume measurement as a method of tumor response evaluation to aid biomarker development. Clin Cancer Res. 2010;16:4647–53. doi: 10.1158/1078-0432.CCR-10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao M, Smith RB, Hoffman HT, Funk GF, Lu M, Menda Y, Graham MM, Buatti JM. Clinical significance of postradiotherapy [18F]-fluorodeoxyglucose positron emission tomography imaging in management of head-and-neck cancer-a long-term outcome report. International journal of radiation oncology, biology, physics. 2009;74:9–14. doi: 10.1016/j.ijrobp.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Alkhawaldeh K, Bural G, Kumar R, Alavi A. Impact of dual-time-point (18)F-FDG PET imaging and partial volume correction in the assessment of solitary pulmonary nodules. European journal of nuclear medicine and molecular imaging. 2008;35:246–52. doi: 10.1007/s00259-007-0584-1. [DOI] [PubMed] [Google Scholar]
- 36.Obrzut S, Pham RH, Vera DR, Badran K, Hoha CK. Comparison of lesion-to-cerebellum uptake ratios and standardized uptake values in the evaluation of lung nodules with 18F-FDG PET. Nuclear medicine communications. 2007;28:7–13. doi: 10.1097/MNM.0b013e328013dce7. [DOI] [PubMed] [Google Scholar]
- 37.Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L, Scheidhauer K, Buck A, Naumann R, Spaepen K, Hicks RJ, Weber WA, Reske SN, Schwaiger M, Schwartz LH, Zijlstra JM, Siegel BA, Cheson BD. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 38.Castellucci P, Nanni C, Farsad M, Alinari L, Zinzani P, Stefoni V, Battista G, Valentini D, Pettinato C, Marengo M, Boschi S, Canini R, Baccarani M, Monetti N, Franchi R, Rampin L, Fanti S, Rubello D. Potential pitfalls of 18F-FDG PET in a large series of patients treated for malignant lymphoma: prevalence and scan interpretation. Nuclear Medicine Communications. 2005:26. doi: 10.1097/01.mnm.0000171781.11027.bb. [DOI] [PubMed] [Google Scholar]
- 39.Elstrom R, Guan L, Baker G, Nakhoda K, Vergilio JA, Zhuang H, Pitsilos S, Bagg A, Downs L, Mehrotra A, Kim S, Alavi A, Schuster SJ. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood. 2003;101:3875–6. doi: 10.1182/blood-2002-09-2778. [DOI] [PubMed] [Google Scholar]
- 40.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 41.Kasamon Y, Wahl R, Ziessman H, Blackford A, Goodman S, Fidyk C, Rogers K, Bolanos-Meade J, Borowitz M, Ambinder R, Jones R, Swinnen L. Phase II study of risk-adapted therapy of newly diagnosed, aggressive non-Hodgkin lymphoma based on midtreatment FDG-PET scanning. Biology of Blood & Marrow Transplantation. 2009:15. doi: 10.1016/j.bbmt.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin C, Luciani A, Itti E, El-Gnaoui T, Vignaud A, Beaussart P, Lin SJ, Belhadj K, Brugieres P, Evangelista E, Haioun C, Meignan M, Rahmouni A. Whole-body diffusion-weighted magnetic resonance imaging with apparent diffusion coefficient mapping for staging patients with diffuse large B-cell lymphoma. Eur Radiol. 2010;20:2027–38. doi: 10.1007/s00330-010-1758-y. [DOI] [PubMed] [Google Scholar]
- 43.Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993;11:2101–11. doi: 10.1200/JCO.1993.11.11.2101. [DOI] [PubMed] [Google Scholar]
- 44.Krak NC, Hoekstra OS, Lammertsma AA. Measuring response to chemotherapy in locally advanced breast cancer: methodological considerations. Eur J Nucl Med Mol Imaging. 2004;31 (Suppl 1):S103–11. doi: 10.1007/s00259-004-1532-y. [DOI] [PubMed] [Google Scholar]
- 45.Dunnwald LK, Gralow JR, Ellis GK, Livingston RB, Linden HM, Specht JM, Doot RK, Lawton TJ, Barlow WE, Kurland BF, Schubert EK, Mankoff DA. Tumor metabolism and blood flow changes by positron emission tomography: relation to survival in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol. 2008;26:4449–57. doi: 10.1200/JCO.2007.15.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Standardized uptake values of normal breast tissue with 2-deoxy-2-[F-18]fluoro-D: -glucose positron emission tomography: variations with age, breast density, and menopausal status. Mol Imaging Biol. 2006;8:355–62. doi: 10.1007/s11307-006-0060-5. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz-Dose J, Untch M, Tiling R, Sassen S, Mahner S, Kahlert S, Harbeck N, Lebeau A, Brenner W, Schwaiger M, Jaenicke F, Avril N. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–41. doi: 10.1200/JCO.2008.17.2650. [DOI] [PubMed] [Google Scholar]
- 48.Specht JM, Kurland BF, Montgomery SK, Dunnwald LK, Doot RK, Gralow JR, Ellis GK, Linden HM, Livingston RB, Allison KH, Schubert EK, Mankoff DA. Tumor metabolism and blood flow as assessed by positron emission tomography varies by tumor subtype in locally advanced breast cancer. Clin Cancer Res. 2010;16:2803–10. doi: 10.1158/1078-0432.CCR-10-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med. 2005;46:983–95. [PubMed] [Google Scholar]
- 50.Dunnwald LK, Doot RK, Specht JM, Gralow JR, Ellis GK, Livingston RB, Linden HM, Gadi VK, Kurland BF, Schubert EK, Muzi M, Mankoff DA. PET tumor metabolism in locally advanced breast cancer patients undergoing neoadjuvant chemotherapy: value of static versus kinetic measures of fluorodeoxyglucose uptake. Clin Cancer Res. 2011;17:2400–9. doi: 10.1158/1078-0432.CCR-10-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLaughlin R, Hylton N. MRI in breast cancer therapy monitoring. NMR in Biomedicine. 2011;24:712–20. doi: 10.1002/nbm.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, Meszoely I, Mayer IA, Herman CR, McManus K, Price RR, Gore JC. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging. 2007;25:1–13. doi: 10.1016/j.mri.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007;96:189–95. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 55.Linden HM, Stekhova SA, Link JM, Gralow JR, Livingston RB, Ellis GK, Petra PH, Peterson LM, Schubert EK, Dunnwald LK, Krohn KA, Mankoff DA. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–9. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 56.Linden HM, Mankoff DA. Breast cancer and hormonal stimulation: is glycolysis the first sign of response? J Nucl Med. 2010;51:1663–4. doi: 10.2967/jnumed.110.078329. [DOI] [PubMed] [Google Scholar]
- 57.Meisamy S, Bolan PJ, Baker EH, Bliss RL, Gulbahce E, Everson LI, Nelson MT, Emory TH, Tuttle TM, Yee D, Garwood M. Neoadjuvant chemotherapy of locally advanced breast cancer: predicting response with in vivo (1)H MR spectroscopy--a pilot study at 4 T. Radiology. 2004;233:424–31. doi: 10.1148/radiol.2332031285. [DOI] [PubMed] [Google Scholar]
- 58.Woodfield CA, Tung GA, Grand DJ, Pezzullo JA, Machan JT, Renzulli JF., 2nd Diffusion-weighted MRI of peripheral zone prostate cancer: comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. AJR Am J Roentgenol. 2010;194:W316–22. doi: 10.2214/AJR.09.2651. [DOI] [PubMed] [Google Scholar]
- 59.Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, Scheenen T, Barentsz JO. Relationship between Apparent Diffusion Coefficients at 3.0-T MR Imaging and Gleason Grade in Peripheral Zone Prostate Cancer. Radiology. 2011 doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- 60.Verma S, Rajesh A, Morales H, Lemen L, Bills G, Delworth M, Gaitonde K, Ying J, Samartunga R, Lamba M. Assessment of aggressiveness of prostate cancer: correlation of apparent diffusion coefficient with histologic grade after radical prostatectomy. AJR Am J Roentgenol. 2011;196:374–81. doi: 10.2214/AJR.10.4441. [DOI] [PubMed] [Google Scholar]
- 61.Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, Locklin J, Baccala AA, Jr, Rastinehad AR, Merino MJ, Shih JH, Wood BJ, Pinto PA, Choyke PL. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488–95. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van As NJ, de Souza NM, Riches SF, Morgan VA, Sohaib SA, Dearnaley DP, Parker CC. A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur Urol. 2009;56:981–7. doi: 10.1016/j.eururo.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 63.Sciarra A, Panebianco V, Salciccia S, Lisi D, Alfarone A, Gentilucci A, Parente U, Cattarino S, Passariello R, Gentile V. Determination of the time for maximal response to neoadjuvant hormone therapy for prostate cancer using magnetic resonance with spectroscopy (MRSI) and dynamic contrast enhancement (DCEMR) Urol Oncol. 2011 doi: 10.1016/j.urolonc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Padhani AR. Integrating multiparametric prostate MRI into clinical practice. Cancer Imaging. 2011;11:S27–37. doi: 10.1102/1470-7330.2011.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitajima K, Kaji Y, Fukabori Y, Yoshida K, Suganuma N, Sugimura K. Prostate cancer detection with 3 T MRI: comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J Magn Reson Imaging. 2010;31:625–31. doi: 10.1002/jmri.22075. [DOI] [PubMed] [Google Scholar]
- 66.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–9. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris MJ, Akhurst T, Osman I, Nunez R, Macapinlac H, Siedlecki K, Verbel D, Schwartz L, Larson SM, Scher HI. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913–8. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 68.Yu EY, Muzi M, Hackenbracht JA, Rezvani BB, Link JM, Montgomery RB, Higano CS, Eary JF, Mankoff DA. C11-Acetate and F-18 FDG PET for Men With Prostate Cancer Bone Metastases: Relative Findings and Response to Therapy. Clinical Nuclear Medicine. 2011;36:192–8. doi: 10.1097/RLU.0b013e318208f140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mena E, Turkbey B, Mani H, Adler S, Valera VA, Bernardo M, Shah V, Pohida T, McKinney Y, Kwarteng G, Daar D, Lindenberg ML, Eclarinal P, Wade R, Linehan WM, Merino MJ, Pinto PA, Choyke PL, Kurdziel KA. 11C-Acetate PET/CT in Localized Prostate Cancer: A Study with MRI and Histopathologic Correlation. Journal of Nuclear Medicine. 2012;53:538–45. doi: 10.2967/jnumed.111.096032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fox JJ, Morris MJ, Larson SM, Schoder H, Scher HI. Developing imaging strategies for castration resistant prostate cancer. Acta Oncol. 2011;50 (Suppl 1):39–48. doi: 10.3109/0284186X.2011.572914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Picci P, Bohling T, Bacci G, Ferrari S, Sangiorgi L, Mercuri M, Ruggieri P, Manfrini M, Ferraro A, Casadei R, Benassi MS, Mancini AF, Rosito P, Cazzola A, Barbieri E, Tienghi A, Brach del Prever A, Comandone A, Bacchini P, Bertoni F. Chemotherapy-induced tumor necrosis as a prognostic factor in localized Ewing’s sarcoma of the extremities. J Clin Oncol. 1997;15:1553–9. doi: 10.1200/JCO.1997.15.4.1553. [DOI] [PubMed] [Google Scholar]
- 72.Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU, 3rd, Eary JF. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–34. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- 73.Hawkins DS, Rajendran JG, Conrad EU, 3rd, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002;94:3277–84. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- 74.Kim D, Kim S, Lee H, Song B, Kim D, Cho J, Lim J, Lee J. Assessment of Chemotherapy Response Using FDG-PET in Pediatric Bone Tumors: A Single Institution Experience. Cancer Res Treat. 2011:43. doi: 10.4143/crt.2011.43.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grignani G, Palmerini E, Dileo P, Asaftei SD, D’Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F, Casali PG, Ferrari S, Aglietta M. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23:508–16. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 76.Dyke JP, Panicek DM, Healey JH, Meyers PA, Huvos AG, Schwartz LH, Thaler HT, Tofts PS, Gorlick R, Koutcher JA, Ballon D. Osteogenic and Ewing sarcomas: estimation of necrotic fraction during induction chemotherapy with dynamic contrast-enhanced MR imaging. Radiology. 2003;228:271–8. doi: 10.1148/radiol.2281011651. [DOI] [PubMed] [Google Scholar]
- 77.Guo J, Reddick WE, Glass JO, Ji Q, Billups CA, Wu J, Hoffer FA, Kaste SC, Jenkins JJ, Ortega Flores XC, Quintana J, Villarroel M, NCD Dynamic contrast-enhanced magnetic resonance imaging as a prognostic factor in predicting event-free and overall survival in pediatric patients with osteosarcoma. Cancer. doi: 10.1002/cncr.26701. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benz MR, Czernin J, Allen-Auerbach MS, Tap WD, Dry SM, Elashoff D, Chow K, Evilevitch V, Eckardt JJ, Phelps ME, Weber WA, Eilber FC. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2009;15:2856–63. doi: 10.1158/1078-0432.CCR-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schuetze SM, Rubin BP, Vernon C, Hawkins DS, Bruckner JD, Conrad EU, 3rd, Eary JF. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer. 2005;103:339–48. doi: 10.1002/cncr.20769. [DOI] [PubMed] [Google Scholar]
- 80.Evilevitch V, Weber WA, Tap WD, Allen-Auerbach M, Chow K, Nelson SD, Eilber FR, Eckardt JJ, Elashoff RM, Phelps ME, Czernin J, Eilber FC. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2008;14:715–20. doi: 10.1158/1078-0432.CCR-07-1762. [DOI] [PubMed] [Google Scholar]
- 81.Benz MR, Czernin J, Allen-Auerbach MS, Dry SM, Sutthiruangwong P, Spick C, Radu C, Weber WA, Tap WD, Eilber FC. 3′-deoxy-3′-[(18) F]fluorothymidine positron emission tomography for response assessment in soft tissue sarcoma: A pilot study to correlate imaging findings with tissue thymidine kinase 1 and Ki-67 activity and histopathologic response. Cancer. 2011 doi: 10.1002/cncr.26630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meyer JM, Perlewitz KS, Hemmingson SL, Hayden JB, Hung A, Mansoor A, Holtorf ML, Woodward WJ, Springer CS, Huang W, Ryan CW. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) evaluation of preoperative therapy for extremity soft tissue sarcomas (STS) ASCO Meeting Abstracts. 2011;29:10098. [Google Scholar]
- 83.Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Charnsangavej C. We Should Desist Using RECIST, at Least in GIST. Journal of Clinical Oncology. 2007;25:1760–4. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 84.Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of Computed Tomography and Positron Emission Tomography in Patients With Metastatic Gastrointestinal Stromal Tumor Treated at a Single Institution With Imatinib Mesylate: Proposal of New Computed Tomography Response Criteria. Journal of Clinical Oncology. 2007;25:1753–9. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 85.Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, Podoloff D. The Role of 18F-FDG PET in Staging and Early Prediction of Response to Therapy of Recurrent Gastrointestinal Stromal Tumors. Journal of Nuclear Medicine. 2004;45:17–21. [PubMed] [Google Scholar]
- 86.Choi H. Imaging modalities of gastrointestinal stromal tumors. Journal of Surgical Oncology. 2011;104:907–14. doi: 10.1002/jso.21871. [DOI] [PubMed] [Google Scholar]
- 87.Hunsberger S, Zhao Y, Simon R. A comparison of phase II study strategies. Clin Cancer Res. 2009;15:5950–5. doi: 10.1158/1078-0432.CCR-08-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doot RK, Kurland BF, Kinahan PE, Mankoff DA. Design Considerations for using PET as a Response Measure in Single Site and Multicenter Clinical Trials. Acad Radiol. 2012;19:184–90. doi: 10.1016/j.acra.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sargent DJ, Rubinstein L, Schwartz L, Dancey JE, Gatsonis C, Dodd LE, Shankar LK. Validation of novel imaging methodologies for use as cancer clinical trial end-points. Eur J Cancer. 2009;45:290–9. doi: 10.1016/j.ejca.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, Larson S, Mankoff DA, Siegel BA, Van den Abbeele A, Yap J, Sullivan D. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–66. [PubMed] [Google Scholar]
- 91.Boellaard R, Oyen WJ, Hoekstra CJ, Hoekstra OS, Visser EP, Willemsen AT, Arends B, Verzijlbergen FJ, Zijlstra J, Paans AM, Comans EF, Pruim J. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging. 2008;35:2320–33. doi: 10.1007/s00259-008-0874-2. [DOI] [PubMed] [Google Scholar]
- 92.Emanuel EJ. Undue inducement: nonsense on stilts? Am J Bioeth. 2005;5:9–13. doi: 10.1080/15265160500244959. discussion W8-1, W7. [DOI] [PubMed] [Google Scholar]
- 93.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–63. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 94.National Research Council, Committee on the Biological effects of Ionizing Radiation. Health Effects of Exposure to Low Levels of Ionizing Radiation, BEIR V. Washington, DC: 1990. [Google Scholar]
- 95.Hays MT, Watson EE, Thomas SR, Stabin M. MIRD dose estimate report no. 19: radiation absorbed dose estimates from (18)F-FDG. J Nucl Med. 2002;43:210–4. [PubMed] [Google Scholar]
- 96.Brix G, Lechel U, Glatting G, Ziegler SI, Munzing W, Muller SP, Beyer T. Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med. 2005;46:608–13. [PubMed] [Google Scholar]
- 97.Hricak H, Brenner DJ, Adelstein SJ, Frush DP, Hall EJ, Howell RW, McCollough CH, Mettler FA, Pearce MS, Suleiman OH, Thrall JH, Wagner LK. Managing Radiation Use in Medical Imaging: A Multifaceted Challenge. Radiology. 2011;258:889–905. doi: 10.1148/radiol.10101157. [DOI] [PubMed] [Google Scholar]
- 98.Xia T, Alessio AM, De Man B, Manjeshwar R, Asma E, Kinahan PE. Ultra-low dose CT attenuation correction for PET/CT. Phys Med Biol. 2012;57:309–28. doi: 10.1088/0031-9155/57/2/309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. [Accessed 6/3/2012];Radiation Dose Chart. at http://xkcd.com/radiation/
- 100.Levy MA, Rubin DL. Current and future trends in imaging informatics for oncology. Cancer J. 2011;17:203–10. doi: 10.1097/PPO.0b013e3182272f04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jaffe TA, Wickersham NW, Sullivan DC. Quantitative imaging in oncology patients: Part 1, radiology practice patterns at major U.S. cancer centers. AJR Am J Roentgenol. 2010;195:101–6. doi: 10.2214/AJR.09.2850. [DOI] [PubMed] [Google Scholar]
- 102.Dilts DM, Sandler AB. Invisible barriers to clinical trials: the impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol. 2006;24:4545–52. doi: 10.1200/JCO.2005.05.0104. [DOI] [PubMed] [Google Scholar]