Abstract
In order to determine the efficacy of curcumin in ameliorating symptoms of neurodegeneration in the mouse model of Niemann–Pick C1, a variety of formulations and dosages of curcumin, one comparable to one previously reported as efficacious, were provided orally to Npc1−/−mice. Plasma levels of curcumin, survival, tests of motor performance, and memory (in some cases) were performed. We found variable, but mild, increases in survival (1.5% to 18%). The greatest increased survival occurred with the highest dose (which was unformulated) while the control for the lipidated formulation (containing phosphatidylcholine and stearic acid) had an equivalent impact and other formulations, while not significantly increased, are also not statistically different in effect from the highest dose.
We conclude that curcumin is not a highly efficacious treatment for neurodegeneration in Npc1−/− mice. Phosphatidylcholine and stearic acid should be studied further.
Keywords: Neurodegeneration, Memory, Motor skills, Curcumin, Niemann–Pick C
1. Introduction
There has been wide interest in the use of curcumin, the active ingredient of turmeric, for treatment of several chronic diseases which are believed to have a low incidence in populations such as heterogenous Indian populations who ingest from 60 to 200 mg of curcumin daily (Aggarwal et al., 2007).
In animal models, curcumin exerts anti-Alzheimer’s effects. Curcumin, if absorbed unglucuronidated, readily penetrates the blood–brain barrier (Begum et al., 2008; Garcia-Alloza et al., 2007) and decreases amyloid plaques (Begum et al., 2008; Frautschy et al., 2001; Garcia-Alloza et al., 2007; Lim et al., 2001; Yang et al., 2005). Plaque reducing effects of curcumin require the dienone ketone bridge, since reduction of the double bonds that occurs by metabolism to tetrahydrocurcumin leads to loss of plaque reducing effects but not loss of iNOS and other anti-inflammatory effects (Begum et al., 2008). Bisdemethoxycurcumin also has the dienone bond and immunomodulatory effects on mRNA expression of toll like receptors and MGAT-III in ex vivo human lymphocytes, but the effects on plaques have not been investigated (Fiala et al., 2007). Turmeric extracts typically have 26 fold lower bisdemethoxycurcumin than curcumin.
Niemann–Pick C1, a neurodegenerative disease of the young, has been called “juvenile Alzheimer’s” because of child onset of dementia and accumulation of neurofibrillary tangles (Vincent et al., 2003). Tau is abnormally phosphorylated (Hallows et al., 2006) and curcumin reduces the accumulation of phospho-Tau in a triple transgenic Alzheimer’s disease mouse model (Ma et al., 2009). There are several experimental therapies for Neimann–Pick C1 but none which are strongly efficacious (reviewed in Perez-Poyato and Pineda, 2011). Recently, Lloyd-Evans et al. (2008) reported a beneficial effect of curcumin on elevation of cytosolic calcium (in vitro) and of survival in the mouse model of Npc1−/−. We have studied the effects on survival and determined plasma therapeutic ranges using a variety of dosages and preparations, including one similar to the one used by Lloyd-Evans et al. (2008). Only slight effects were found on survival and motor coordination. Surprisingly a lipidated vehicle was as effective as the best curcumin preparation.
2. Methods
2.1. Mice
Npc1−/− mice were obtained through breeding heterozygous Npc1+/− mice which are maintained on the BALB/cJ background. At 15 days animals were genotyped by PCR as described by Loftus et al. (1997). At weaning, Npc1−/− mice were caged together and fed one of the specific diets. A minimum of 6 mice (of roughly equal sexes) were tested on each diet, and the survival curves were obtained for each set. Powder diets were fed in plastic cups that were maintained full at all times. All animals were permitted constant access to water. Mice were maintained on a 12-hour light/dark cycle, with behavioral and memory testing performed during the light phase. All experiments were approved by the University of Arizona I.A.C.U.C.
2.2. Diets
We tested a total of five diets consisting of three concentrations of curcumin and controls: 1) Chromadex, 2% curcumin (Billerey-Larmonier et al., 2008) mixed in NIH 7013 chow (Harlan Teklad, Madison, WI); 2) Acros, 0.17% curcumin (Acros Organics, Fisher catalog #AC 1858–0100) mixed in 5P14 base chow (PMI Nutrition International, Brentwood, MO); 3) NIH 7013, chow alone; 4) Lipidated curcumin Verdure Sciences, 0.05%curcumin (at 20% in a stearic acid/phosphatidyl choline mix; lot 024908G2711CLEG, Verdure Sciences, Noblesville, IN) delivered at 0.25% in NIH 7013 chow and 5) Lipidated vehicle, (Verdure lapidated control, containing stearic acid and phosphatidylcholine, Verdure Sciences, Noblesville, IN) at 0.25% in 5P14 chow (Table 1).
Table 1.
Curcumin table.
| Name | Description | % Curcumin in chow | Curcumin intake (mg/kg day)a | Plasma curcumin concentration (μM) | Survival (days) |
|---|---|---|---|---|---|
| Chromadex | 99% pure curcumin from turmeric powder mixed at 20,000 ppm with NIH 7013 base chow | 2% | 3500 | <0.1b,f | 84.3±2.0** |
| Acros | 85% pure curcumin from turmeric powder mixed at 2000 ppm with 5P14 base chowe. Harlan Teklad TD.09288. | 0.17% | 298 | 0.305±0.164c | 83.8±3.8 |
| NIH 7013 | Modified 6% mouse/rat sterilizable diet | 0% | 0 | NDd | 72.6±2.6 |
| Lipidated Curcumin Verdure Sciences | Lot 024908G2711CLEG 0.25% proprietary mixture of curcumin with stearic acid and phosphatidyl choline mixed with NIH 7013 base chow | 0.05% | 88 | 0.044±0.017c | 77.6±1.4 |
| Lipidated vehicle | Proprietary mixture of stearic acid and phosphatidyl choline (Verdure Sciences) made in 5P14 base chowe. Harlan Teklad TD.09288 | 0% | 0 | 0c | 86.2±1.9** |
p<0.01 against NIH 7013.
Curcumin intake was calculated using weights of 20 g per mouse, which for Npc1−/− mice is their peak weight.
Laboratory 1.
Laboratory 2.
Not determined.
5P14 similar to 7013 except has slightly higher protein and less fat than NIH 7013.
No blood from group. Separate group of animals fed same dose. Subtle differences in curcumin chow preparation may have affected absorption.
2.3. Motor behavior
In order to evaluate balance and motor coordination, mice were tested using two different tests, the balance beam and the coat hanger. The balance beam consists of a 1 m rod with marked sections every 10 cm attached to two support columns 50 cm above a padded surface. Mice were tested on a weekly basis, and were given 2 trials every testing session. For each trial, animals were placed at the center of the beam and released. They were allowed 180 s to freely move on the beam. The time they stayed on the beam was recorded for both trials as well as the number of sections crossed, (the higher the number the better the performance). The coat hanger consists of a wire hanger suspended 35 cm above a padded surface in the beam apparatus. Initially animals are permitted to grasp the wire with their forepaws and then released. Mice were allowed two trials of 30 s each. A rating system was used to assess their performance. The ratings are as follows: 0 = falls in less than 10 s; 1 = stays on hanger with one limb; 2 = stays on hanger with two limbs; 3 = stays on hanger with three limbs; 4 = stays on hanger with four limbs; 5 = reaches the end of the hanger and escapes onto beam.
2.4. Memory test
We used the passive avoidance shock chamber test in order to test memory (Ader et al., 1972). Mice were 4 to 5 weeks old when trained. The training period consisted of 3 days followed by weekly testing. On day 0, mice were deprived of food overnight. On day 1, mice were kept in a dark area for at least 2 h; two pellets of food were placed in the dark side of the chamber; mice were placed head first into the left corner of the light compartment; they were allowed 300 s to explore the chamber and the latency time to enter the dark compartment was recorded. On day 2, mice were kept in a dark area for at least 2 h; mice were then placed head first into the left corner of the light compartment; they were allowed 300 s to explore the chamber; 5 s after the mouse entered the dark compartment, a five second shock at 85 V to the grid in series with a 224 kΩ current limiting resistor was administered (about 0.6 mA, similar to a static electricity shock); if the mouse remained in the dark compartment, a second shock was administered 30 s after the initial shock; every time the mouse re-entered the dark compartment, the shock was administered; the latency time to enter the dark compartment was recorded. On day 4 or subsequent testing days, mice were kept in a dark area for at least 2 h; mice were then placed head first into the left corner of light compartment; they were allowed 300 s to explore the chamber; the latency time to enter the dark compartment was recorded, and they were considered to have failed the test at this point if they entered the dark compartment.
2.5. Plasma and tissue sample preparation for HPLC and LC/MS/MS
Mouse plasma samples were extracted in 95% ethyl acetate / 5% methanol and analyzed by HPLC with detection at 262 (curcumin) and 282 (tetrahydrocurcumin, TC) nm, using 17β-estradiol as an internal standard as described previously (Heath et al., 2003, 2005). The extraction efficiency from plasma for curcumin and TC was 54% (laboratory 1) and 88–97% (laboratory 2). Using the HPLC method, the lower limits of detection for curcumin extracted from plasma were 36.8 ng/ml (100 nM) in laboratory 1. Using the LC/MS/MSmethod the lower limit of detection for curcumin was 3 ng/ml (8.15 nM) in laboratory 2. For LC/MS/MS mouse plasma samples were extracted in 95% ethyl acetate/5% methanol using tetramethoxycurcumin as an internal standard as described previously (Begum et al., 2008) with the following modification. We used Varian International, Lake Forest, CA, Agilent Technologies system, consisting of the proStar 410 autosampler and pumps and a 310mass spectrometerwith ESI (−), which can optimize each ion automatically while generating a MS/MS breakdown curve showing ion intensity andmost favorable fragment ions (product ion of the compound). Curcumin and internal std (tetramethoxycurcumin) gave strong signals for the [M-H] ion in negative ion electrospray mass spectrometrywhen infused in solutions ofwater/acetonitrile containing 10 mMammoniumacetate. The MS/MS of curcumin produced a number of fragment ions, the most intense fragment ionswere selected for quantitation by MS/MS-multiple reaction monitoring (MRM). Then the unknown dried plasma extracts samples with the internal standard were redissolved in acetonitrile: water (50/50, by volume) in 10mM ammonium acetate, and an aliquot of the solution (typically 50 μl) was injected onto a reverse phase HPLC column (Atlantis T3, 150×2.1 mm; Waters Corporation, Milford, MA). The m/z values of the specific transitions were for curcumin, 367.0→148.7, 172.4, 216.3, and for tetramethoxycurcumin, 396.4→149.5,297.1, 337.6 (the internal standard). Instrument manufacturer-supplied LC/MS/MS Varian software (Varian MS Workstation, Version 6.9.1) was used to calculate each ion according to its qualifier ion. The retention time of curcumin and internal standard was 8.40, and 10.02min, respectively.
2.6. Statistics
For the statistical analysis of the plasma levels of curcumin from 3 groups of mice from different diets, we used ANOVA. For the analysis of survival of all mice in this study, we used Kaplan–Meier survival curves, and a Log-rank test to statistically compare the survival from each diet to the survival of the control diets (NIH 7013 or Lipidated Vehicle).
3. Results
3.1. Survival
Except for one outlier, all of the treated and control Npc1−/− mice survived ≤20 days longer than the mean survival for mice on the control NIH 7013 diet (Table 1, Fig. 1). Chromadex, the diet with the highest curcumin dose (2% in NIH 7013 chow) and purity, and the lipidated vehicle, control diet (containing stearic acid and phosphatidyl choline) for Verdure Science’s proprietary mixtures (0.25% in 5P14 chow) were the most efficacious with a high statistical significance. The Acros diet (0.17% curcumin), most comparable to the one used by Lloyd-Evans et al. (2008), showed only a 10 day increase in survival. However, this diet and the lapidated diet are not statistically different from the Chromadex and lipidated vehicle either. The two control chows are only slightly different (they differ in protein [5P14 is 25% higher protein] and fat [NIH 7013 is 25% higher fat]) suggesting that the diet the formulations were delivered in (excluding the stearic acid and phosphatidyl choline supplemented control was not the critical variable.
Fig. 1.
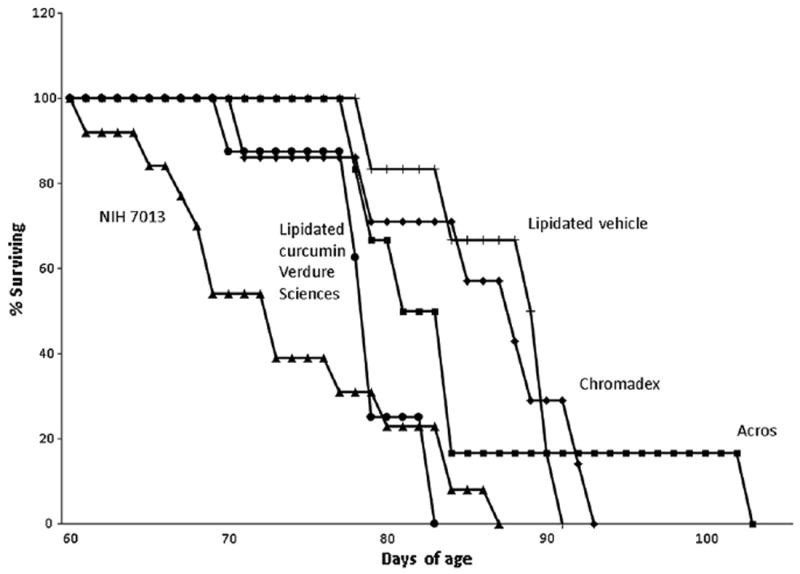
Kaplan–Meier survival analysis.
3.2. Blood levels of curcumin
We determined the concentration of curcumin in blood plasma from 3 groups of mice from different diets (Table 1). Since we were unable to collect blood from the highest dose group (Chromadex) we analyzed blood from a separate group fed the same preparation for 1 month, but levels were under limits of detection of the HPLC assay used (<0.1 μM). Using a more sensitive method, the LC/MS/MS method, the Acros diet, themiddle dose of curcumin (0.17%), resulted in a plasma concentration of 0.305±0.164 μM (n=6); lipidated curcumin from Verdure Sciences, the lowest dose curcumin diet (0.05%) resulted in a 0.044±0.017 μM concentration (n=5) and the lipidated vehicle diet resulted in the expected concentration of 0 (n=6) (Table 1).
3.3. Motor behavior
The results from the balance beam show that Npc1+/+, wild-type control mice and Npc1−/− mice on the 2% curcumin (Chromadex) diet performed quite similarly (Fig. 2). This is important since mice might show improvements in motor behavior and/or memory and yet not have greatly increased survival. The time spent on the beam was not different (not shown). The Npc1−/− untreated and the Npc1−/− mice on the Acros diet were similar and had lower scores. On the other hand, the results from the coat hanger test show a decline in score (performance rating) over age for Npc1−/− mice untreated or treated with either diet, as opposed to a constant high score for Npc1+/+ control mice (Fig. 3). The high dose (Chromadex) that improved balance beam performance, showed a slight, non-significant improvement by 9 weeks which again could be important for morbidity.
Fig. 2.
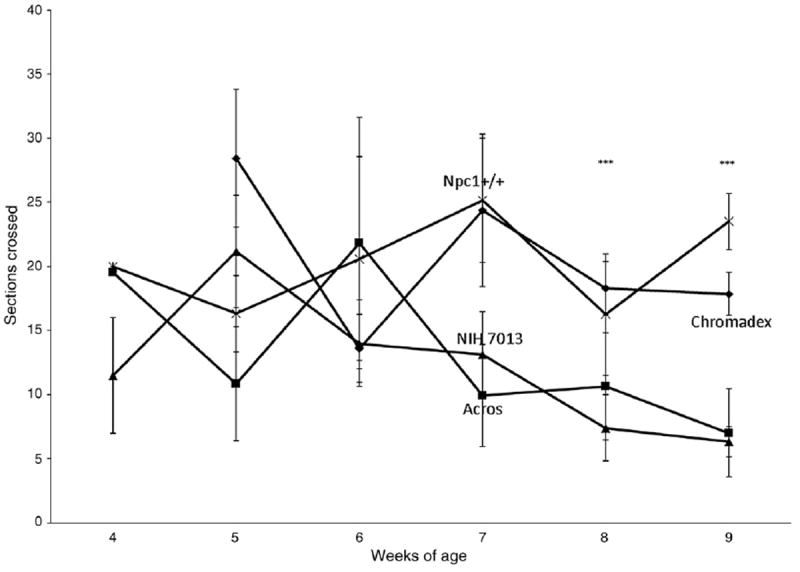
Balance beam.
Fig. 3.
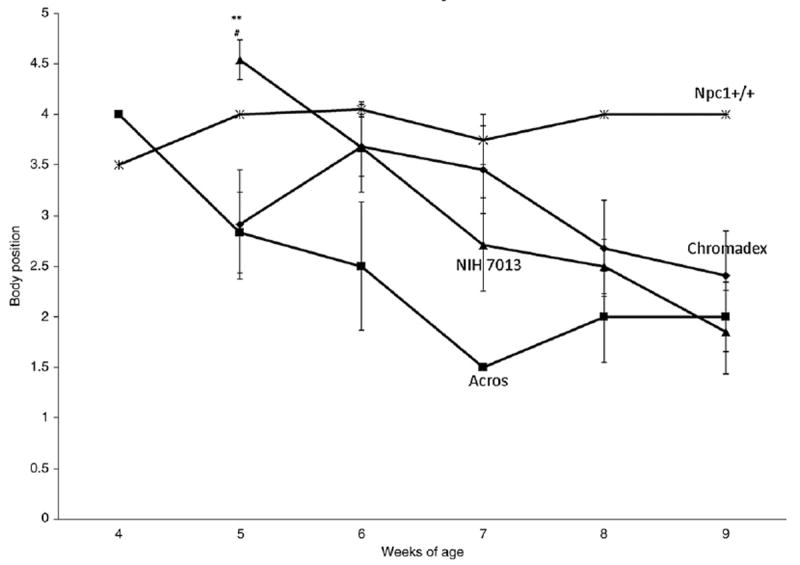
Coat hanger.
3.4. Memory test
Although the memory test results show a slight increase in memory retention for Npc1−/− mice on the lipidated curcumin diet from Verdure Sciences (0.05% curcumin), compared to the Npc1−/− mice on the NIH 7013 diet, this difference is not significant (χ2 Npc1−/−vs Npc1−/− on 0.05% lipidated curcumin diet at 1 week, p=0.459) (Fig. 4). Due to the poor performance of the Npc1−/− mice, this test was not repeated for other diets.
Fig. 4.
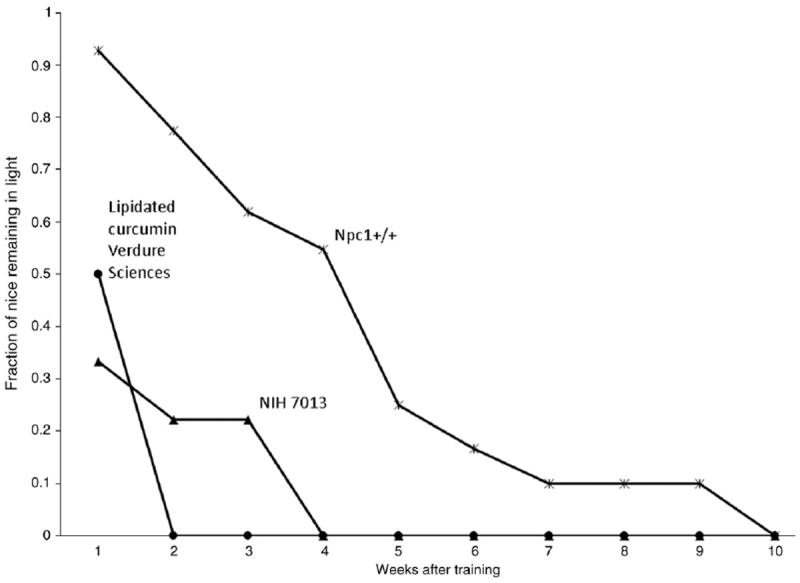
Passive avoidance.
4. Discussion
We studied the effects of curcumin on the neurodegeneration of Npc1−/− mice since Lloyd-Evans et al. (2008) had found an efficacious effect on elevating cytosolic calcium (in vitro) and prolonging survival. They found a 35% increase in survival using a dose of 150 mg/kg/day of “Sigma” curcumin (65–70% pure, batch #046 K0933; Frances Platt, personal communication) with appropriate improvements in weight gain, activity and reduced tremor. Assuming that their treated Npc1−/− mice weigh about 20 g at their maximum, and assuming 4 g of chow consumed a day, this corresponds to a diet containing about 0.050% curcumin. This is a very small dose and not greatly dissimilar from the 0.17% dose of the Acros preparation we tested (given the variables of purity and absorption). We have used mice on the same genetic background (which can influence the pharmacological effects of curcumin [Billerey-Larmonier et al., 2008]) and a similar control diet (they used the SDS #3 diet which has a very similar composition to the NIH 7031 diet, including levels of zinc, which complexes with curcumin and enhances efficacy [Mei et al., 2011]). Plasma levels of curcumin ormetabolites were not determined in the Lloyd-Evans et al. (2008) study.
While Lloyd-Evans et al. (2008) found an extension of survival from about 72 days to about 96 days, our resultsweremoremodest; the best diets increased survival from 73±2 days to 86±2 days. At intermediate (0.17%) and low doses of curcumin (0.05%), plasma levels of curcumin were detected (44–305 nM) within the range known to reduce plaques (100–400 nM) (Begum et al., 2008). Based on dose escalation studies and the fact that the low dose yielded 44 nM and the moderate dose 305 nM, we would expect that total blood levels of the high dose were greater than 305 nM. However, we only found that the levels were below 100 nM in the high dose group (the diet was being consumed—the mice did not lose weight [Billerey-Larmonier et al., 2008]). Circulating levels of curcumin are highly dependent on bioavailability and tissue distribution. Thus, this discrepancy could be due to more sequestration into the red blood cell fraction of this curcumin preparation (Frautschy and Cole, 2010). In some situations, plasma levels can be non-detectablewith high brain levels (Begumet al., 2008).
Phosphatidylcholine complexes of curcumin without lipids have been reported to increase absorption in animals (Maiti et al., 2007; Marczylo et al., 2007), but have not been developed extensively clinically. In this study we used unformulated curcumin from two different sources and one dose of a proprietary formulation demonstrated to increase absorption of free curcumin in the plasma and brain of animals (Begum et al., 2008) and plasma of humans (Gota et al., 2010). We observed a mild prolongation of survival with 2% curcumin (Fig. 1) which is a dose which does not cause a decrease in weight (Billerey-Larmonier et al., 2008). We also established a range of plasma levels that may be helpful for optimizing dosing, arguing that greater than 305 nM plasma levels might be needed for optimal efficacy. Further work needs to be done to determine the range of efficacy.
Another important and also unexpected outcome of this study is that the lipidated vehicle (without curcumin) was as efficacious as the high dose of unformulated curcumin in extending the lifespan of Npc1−/− mice (Fig. 1). Whether stearic acid or phosphatidylcholine in the vehicle is causing this significant, although modest, effect of vehicle is unknown. It has been reported by Burgess et al. (2003) that phosphatidylinositol promotes cholesterol transport and excretion. Alternatively, stearic acid may also increase cholesterol excretion, but this requires higher doses than used here (Rasmussen et al., 2006). However, a positive role for stearic acid seems unlikely since stearic acid may exacerbate tau pathogenesis through its reported effects in increasing tau hyperphosphorylation in cortical cultures (Patil and Chan, 2005).
Motor behavior, as measured by balance beam performance, was significantly enhanced only in the mice fed the high curcumin dose (2%, Chromadex) but not by the other experimental diets studied (0.17% Acros, Fig. 2). Neither the 2% nor 0.17% doses enhanced the coat hanger test score (Fig. 3), although there was a slight improvement in performance with the high curcumin dose 2% (Chromadex) at 7 and 9 weeks. We do not have an explanation for the discrepancy between balance beam and coat hanger tests for the Chromadex group but the balance beam includes an “exploratory component” whereas the coat hanger has an “escape” component. Passive avoidance memory deficits were not corrected by the low dose curcumin (Fig. 4).
Many cell pathways relevant to NPC1 neuropathology are inhibited by curcumin (reviewed by Frautschy and Cole, 2010). Curcumin can reduce tau hyperphosphorylation and is also a tau aggregation inhibitor, which is relevant to NPC and the Npc1−/− model (Hallows et al., 2006). It is important that future experiments examine tau and other pathology in response to treatment.
Lloyd-Evans et al. (2008) examined the impact of curcumin in calcium metabolism. They hypothesized that the earliest step in Npc1−/−pathophysiology is the accumulation of sphingosine, causing a decrease in lysosomal calcium, which then cannot be released to the intracellular pool for signaling, thereby disrupting vesicular transport, including that of cholesterol. Most research on NPC1 disease has confirmed a primary defect in cholesterol trafficking (Langmade et al., 2006; Berger et al., 2007) supported by the finding that the very similar disease, Niemann–Pick C2, is due to a defect in a small lysosomal cholesterol binding protein (Naureckiene et al., 2000). However, NPC2 does not bind sphingosine (Liou et al., 2006) nor oxysterols which NPC1 binds (Infante et al., 2008). Thus, the importance of the sphingosine/calcium role in the pathophysiology of NPC1 disease remains moot. While curcumin is a low affinity inhibitor of the SERCA calcium pump (Bilmen et al., 2001), the concentrations required to inhibit SERCA are many times those which have been achieved in vivo.
Curcumin has been demonstrated to attenuate inflammatory bowel disease, both in animal models (Salh et al., 2003; Sugimoto et al., 2002; Larmonier et al., 2008; Billerey-Larmonier et al., 2008) and humans (Hanai et al., 2006), but the precise molecular mechanisms are unclear. Some beneficial effectsmay reflect curcumin’s immunomodulatory role (Jobin et al., 1999; Kim et al., 2003, 2005). In addition, curcumin is an anti-oxidant with free radical-scavenging and nitric acid synthase inhibitory properties (Frautschy et al., 2001; Begum et al., 2008; Pugazhenthi et al., 2007; Chan et al., 1998; Sreejayan and Rao, 1996; Zhao et al., 1989). Curcumin also induces the cellular defense response (Kato et al., 1998) and Nrf2 (Balogun et al., 2003; Yang et al., 2009) and FoxO (Weisberg et al., 2008) mediated defense pathways. Curcumin inhibits signaling by the mammalian target of rapomycin (mTOR; Beevers et al., 2009; Yu et al., 2008) and inhibiting mTOR increases longevity in mice (Harrison et al., 2009). Curcumin antagonizes the NFKappa pathway, exerting pro-apoptotic effects on cancer cells and anti-inflammatory activities through modulation of the redox status of the cell (reviewed by Sandur et al., 2007). Curcumin also inhibits the expression of NPC1L1, the ezetimide-inhibitable intestinal cholesterol uptake protein (Feng et al., 2010; Kumar et al., 2011), but this can not be the mechanism in mice as there is no cholesterol in their diet and they synthesize all of their own cholesterol.
Although curcumin is brain permeant and stable in lipophilic compartments, its therapeutic uses may be limited by instability in blood (its non-enzymatic rapid hydrolysis to vanillin and ferulic acid in aqueous solution and the enzymatic reduction to tetrahydrocurcumin and hexahydrocurcumin). Also therapeutic efficacy may be limited by rapid metabolism in the intestinal mucosa (particularly in humans) and high first pass metabolism (Ireson et al., 2002; Baum et al., 2008; Begum et al., 2008). Measuring curcumin in blood is problematic because plasma values are typically at least 10 fold lower than brain values, sometimes yielding non-detectable blood levels but high brain levels (Begum et al., 2008). In addition to the rapid distribution to lipophilic tissues like the brain, it is also important to consider residual curcumin in the buffy coat and red blood cell fractions, ex vivo reduction to tetrahydrocurcumin during processing of samples (Frautschy and Cole, 2010).
Curcumin is very poorly absorbed in humans because of extensive glucuronidation. However in rodents a diet containing 0.2% curcumin yielded plasma concentrations of 0.465 μM (Begum et al., 2008) similar to the concentration we achieved with the Acros diet. This argues that Npc1−/− mice absorb curcumin similarly to the AD mice. The dose used by Lloyd-Evans et al. (2008) was approximately 3 fold lower, and plasma concentrations would be expected to be that much lower. However, 30 μM curcumin was used in vitro to inhibit the SERCA pump (Lloyd-Evans et al., 2008). Rapid hydrolysis of curcumin in aqueous culture buffers may yield actual levels far lower than 30 μM, so the mechanisms of action of curcumin in Npc1−/− remain unclear.
In summary, we confirmed that curcumin has a modest impact on prolongation of survival in Npc1−/− mice. We demonstrated that a very high concentration of curcumin (2%) was necessary to achieve this result. This preparation was 99.9% pure, demonstrating that other bioactive curcuminoids, like bisdemethoxycurcumin, are not mediating efficacy. Although the mechanisms remain unclear, the plasma levels from the low and middle dose argue that therapeutic plasma levels of curcumin are likely to require levels above 305 nM. In addition, an unexpected result was the survival promoting mechanisms of phosphatidylcholine and/or stearic acid, the major components of the lipidated vehicle, an exciting finding requiring further investigation of cholesterol transport effects and dose optimization.
5. Conclusion
Although a very low dose of curcumin was reported to extend survival by about 35%, we only achieved a 19% extension of life with one, from a variety of formulations and dosages, preparation of curcumin. Plasma levels of curcumin were enhanced when consumed in a mixture of stearic acid and phosphatidylcholine. Only one of the preparations enhanced motor performance and memory was not improved with a preparation that gave detectable plasma levels. Unexpectedly, a lipidated control containing phosphatydilcholine had an impact equivalent to the high dose of curcumin, arguing for further investigations of its role in cholesterol export in bile.
Acknowledgments
We thank Blake Ebersole and Verdure Scientific, Noblesville IN for the Verdure’s Curcumin diet, and Aynun Begum for technical assistance.
Footnotes
Support: The Addi and Cassi Fund, NIH 5RO1 ED000343-5 (T. Trouard, P.I.), NIH AG-U01028583 (S. Frautschy, P.I.), NIH 2R01DK067286 (P. Kiela, P.I.), and the Holsclaw Family Professorship of Human Genetics and Inherited Diseases (RPE).
References
- Ader R, Weijnen JAWM, Moleman P. Retention of a passive avoidance response as a function of the intensity and duration of electric shock. Psychon Sci. 1972;26:125–8. [Google Scholar]
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin:the Incian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–95. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum L, Lam CWK, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients With Alzheimer Disease. J Clin Psychopharmacol. 2008;28:110–3. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- Beevers CS, Chen L, Liu L, Luo Y, Webster NJG, Huang S. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. 2009;69:1000–8. doi: 10.1158/0008-5472.CAN-08-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AC, Salazar G, Styers ML, Newell-Litwa KA, Werner E, Maue RA, et al. The subcellular localization of the Niemann-Pick Type C proteins depends on the adaptor complex AP-3. J Cell Sci. 2007;120:3640–52. doi: 10.1242/jcs.03487. [DOI] [PubMed] [Google Scholar]
- Billerey-Larmonier C, Uno JK, Larmonier N, Midura AJ, Timmermann bb, Ghishan FK, et al. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis. 2008;14:780–93. doi: 10.1002/ibd.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilmen JG, Khan SZ, Javed MH, Michelangeli F. Inhibition of the SERCA Ca2+ pumps by curcumin. Curcumin putatively stabilizes the interaction between the nucleotide-binding and phosphorylation domains in the absence of ATP. Eur J Biochem. 2001;268:6318–27. doi: 10.1046/j.0014-2956.2001.02589.x. [DOI] [PubMed] [Google Scholar]
- Burgess JW, Boucher J, Neville TAM, Rouillard P, Stamler C, Zachariah S, et al. Phosphatidylinositol promotes cholesterol transport and excretion. J Lipid Res. 2003;44:1355–63. doi: 10.1194/jlr.M300062-JLR200. [DOI] [PubMed] [Google Scholar]
- Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955–62. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]
- Feng D, Ohlsson L, Duan R-D. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1l1 expression. Lipids Health Dis. 2010;9:40–4. doi: 10.1186/1476-511X-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–54. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Cole GM. Beneficial effects of curcumin and DHA on human health:Relevance to chronic diseases of aging. In: Farooqui AA, Farooqui T, editors. Phyto-chemical and Human Health:Pharmacological and Molecular Aspects. Chapter 14. Hauppauge NY: Nova Science Publishers Inc; 2010. pp. 369–387. [Google Scholar]
- Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, et al. Phenolic anti-inflammatory antioxidant reversal of Aβ-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Backsai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer’s mouse model. J Neurochem. 2007;102:1095–104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. 2010;58:2095–9. doi: 10.1021/jf9024807. [DOI] [PubMed] [Google Scholar]
- Hallows JL, Iosif RE, Biasell RD, Vincent I. p35/p25 is not essential for tau and cytoskeletal pathology or neuronal loss in Niemann-Pick type C disease. J Neurosci. 2006;26:2738–44. doi: 10.1523/JNEUROSCI.4834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, et al. Curcumin maintenance therapy for ulcerative colitis:randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–6. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath DD, Pruitt MA, Brenner DE, Rock CL. Curcumin in plasma and urine:quantitation by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;783:287–95. doi: 10.1016/s1570-0232(02)00714-6. [DOI] [PubMed] [Google Scholar]
- Heath DD, Pruitt MA, Brenner DE, Begum AN, Frautschy SA, Rock CL. Tetrahydrocurcumin in plasma and urine:quantitation by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;824:206–12. doi: 10.1016/j.jchromb.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. Binding of cholesterols and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008;283:1052–63. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- Ireson CR, Jones DJL, Orr S, Coughtrie MWH, Boocock DJ, Williams ML, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–11. [PubMed] [Google Scholar]
- Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, et al. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474–83. [PubMed] [Google Scholar]
- Kato K, Ito H, Kamei K, Iwamoto I. Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chaperones. 1998;3:152–60. doi: 10.1379/1466-1268(1998)003<0152:sotsie>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Park EJ, Joe EH, Jou I. Curcumin supresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–9. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, et al. Curcumin inhibits immunostimulatory function of dendritic cells:MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174:8116–24. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- Kumar P, Malhotra P, Ma K, Singla A, Hedroug O, Sakesena S, et al. SREB2 mediates the modulation of intestinal NPC1L1 expression by curcumin. Am J Physiol Gastrointest Liver Physiol. 2011;301:G148–55. doi: 10.1152/ajpgi.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, et al. Pregnane X receptor (PXR) activation:a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc Natl Acad Sci U S A. 2006;103:13807–12. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larmonier CB, Uno JK, Lee KM, Karrasch T, Laubitz D, Thurston RD, et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10 dependent mechanism of protection. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G1079–91. doi: 10.1152/ajpgi.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–7. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou HL, Dixit SS, Xu S, Tint GS, Stock AM, Lobel P. NPC2, the protein deficient in Niemann-Pick C2 disease, consists of multiple glycoforms that bind a variety of sterols. J Biol Chem. 2006;281:36710–23. doi: 10.1074/jbc.M608743200. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14:1247–55. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, et al. Murine model of Niemann-Pick C disease:mutation in a cholesterol homeostasis gene. Science. 1997;277:232–5. doi: 10.1126/science.277.5323.232. [DOI] [PubMed] [Google Scholar]
- Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling:suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009;29:9078–89. doi: 10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–63. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Stetward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–7. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- Mei X, Xu D, Xu S, Zhen Y, Xu S. Gastroprotective and antidepressant effects of a new zinc (II)-curcumin complex in rodent models of gastric ulcer and depression induced by stresses. Pharmacol Biochem Behav. 2011;99:66–74. doi: 10.1016/j.pbb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, et al. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- Patil S, Chan C. Palmitic and stearic fatty acids induce Alzheimer-like hyperphosphorylation of tau in primary rat cortical neurons. Neurosci Lett. 2005;384:288–93. doi: 10.1016/j.neulet.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Perez-Poyato MS, Pineda M. New agents and approaches to treatment in Niemann-Pick type C disease. Curr Pharm Biotech. 2011;12:897–901. doi: 10.2174/138920111795542697. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Akhov L, Selvaraj G, Wang M, Alam J. Regulation of heme oxygenase-1 expression by demethoxycurcuminoids through Nrf2 by a PI3-kinase/Akt-mediated pathway pathway in mouse beta cells. Am J Physiol Endocrinol Metab. 2007;293:E645–55. doi: 10.1152/ajpendo.00111.2007. [DOI] [PubMed] [Google Scholar]
- Rasmussen HE, Guderian DM, Jr, Wray CA, Dussault PH, Schlegel VL, Carr TP. Reduction in cholesterol absorption is enhanced by stearate-enriched plant sterol esters in hamsters. J Nutr. 2006;136:2722–7. doi: 10.1093/jn/136.11.2722. [DOI] [PubMed] [Google Scholar]
- Salh B, Assi K, Templeman V, Parhar K, Owen D, Gomez-Munoz A, et al. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235–43. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–73. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- Sreejayan N, Rao MN. Free radical scavenging activity of curcuminoids. Arzneimittel-forschung. 1996;46:169–71. [PubMed] [Google Scholar]
- Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912–22. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- Vincent I, Bu B, Erickson RP. Understanding Niemann-Pick type C disease:a fat problem. Curr Opin Neurol. 2003;16:155–61. doi: 10.1097/01.wco.0000063764.15877.1c. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–58. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–41. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Yu S, Shen G, Khor TO, Kim JH, Kong ANT. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. 2008;7:2609–20. doi: 10.1158/1535-7163.MCT-07-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BL, Li XJ, He RG, Cheng SJ, Xin WJ. Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys. 1989;14:175–85. doi: 10.1007/BF02797132. [DOI] [PubMed] [Google Scholar]


