Abstract
Rapidly sinking fecal pellets are an important component of the vertical flux of particulate organic matter (POM) from the surface to the ocean's interior; however, few studies have examined the role fish play in this export. We determined abundance, size, prey composition, particulate organic carbon/nitrogen (POC/PON), and sinking rates of fecal pellets produced by a forage fish, likely the northern anchovy, in the Santa Barbara Channel. Pellet abundance ranged from 0.1–5.9 pellets m−3. POC and PON contents averaged 21.7 µg C pellet−1 and 2.7 µg N pellet−1. The sinking rate averaged 787 m d−1; thus pellets produced at the surface would reach the benthos (~500 m) in <1 day. Estimated downward flux of fish fecal POC reached a maximum of 251 mg C m−2 d−1. This is equal to or exceeds previous measurements of sediment trap POM flux, and thus may transport significant amounts of repackaged surface material to depth.
Rapidly sinking fecal pellets are a major contributor to the ‘biological pump’, the vertical transport of biologically generated particulate organic matter (POM) from the surface to the ocean's interior. Sinking rates of small or low mass fecal pellets of some zooplankton (i.e., copepods, euphausiids, doliolids, appendicularians, heteropods), as well as phytodetritus and marine snow, range from <10 to hundreds of meters per day1,2,3, while very large or high mass fecal pellets of other zooplankton (i.e., salps, pteropods, chaetognaths) tend to sink faster (tens to thousands of meters per day)4,5,6. Sinking rates of fecal pellets produced by fish, of which only a few have been reported in the literature, reach well over thousands of meters per day7,8,9. Fast-sinking fish feces could therefore contribute substantially to export of organic matter; fecal matter of anchovies in the Peru upwelling system (Engraulis ringens) contained high amounts of organic carbon and nitrogen, and represented up to 17% of total carbon flux in sediment traps8.
The northern anchovy and Pacific sardine are commercially important forage fishes that can dominate pelagic systems in many coastal upwelling regions10,11. They are omnivorous planktivores and, when abundant, are capable of consuming a significant fraction of primary and secondary production12,13,14. In a study conducted in the Southern California Bight, the northern anchovy (Engraulis mordax Girard), whose diet is dominated by crustacean zooplankton, consumed 35–50% of the total zooplankton biomass and 95–100% of large crustacean zooplankton biomass13. Similar to fecal flux previously measured for the Peruvian anchovy of the same genus (E. ringens)8, repackaged material from the similarly-sized E. mordax likely sinks rapidly, serving as a dominant source of carbon and nitrogen export out of the surface ocean and of nutrition to the deep sea. However, measurements of fish fecal pellet sinking rates and the vertical flux of particulate carbon are very limited, and no previous study has measured the abundance of fish fecal pellets in situ.
In this study, we determined abundance, sinking rates, particulate organic carbon and nitrogen contents, and phytoplankton and zooplankton composition of fish fecal pellets, likely produced by the northern anchovy, in a coastal, seasonal upwelling region – the Santa Barbara Channel, California – and discuss their importance in carbon and nitrogen export in this region.
Results
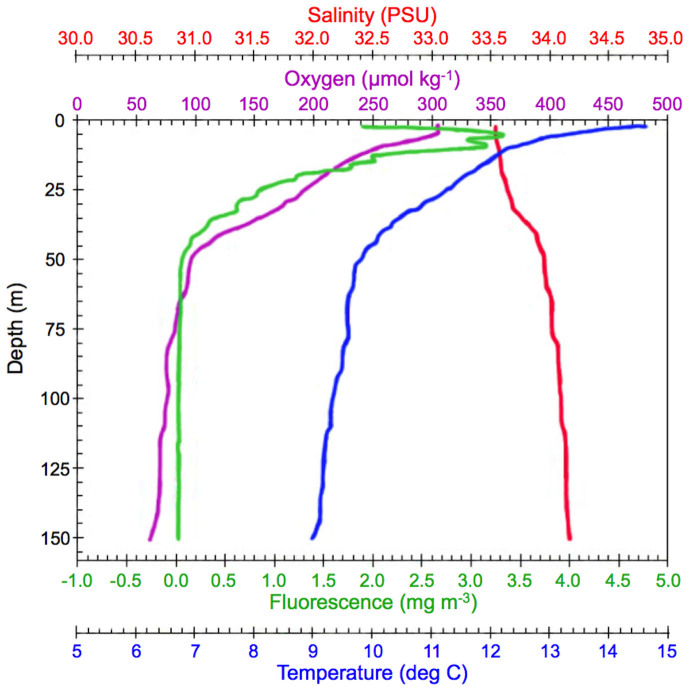
During our occupation of the study site, the water column (512 m depth) was linearly stratified with little to no upper mixed layer, and was characterized by a sea-surface temperature of 14.5°C, low concentrations of sea-surface nutrients (2.4 µM nitrate, 0.05 µM ammonium, 0.1 µM phosphate), and low chlorophyll a biomass (4.2 µg chl L−1) (Fig. 1). Conditions remained relatively uniform during our 3-day study.
Figure 1. Vertical CTD profile of water column at location and the start of our study.
Based on four additional casts made during the three-day study, conditions remained relatively uniform during our occupation of the study site.
The sampled feces were likely produced by the northern anchovy. We observed large schools of small (~13 cm) silver fish near the surface during our sampling, and although we did not have the appropriate sampling equipment to obtain a positive identification or determine their abundance, data compiled by California Fish and Game indicate that northern anchovy landings (in kilogram, kg) dominated total fish landings in the Santa Barbara area during the time of our study (Table 1). Northern anchovy contributed nearly 86% to total commercial fish landings during the time of our study, whereas the similarly sized Pacific sardine, the other potential producer of fish fecal pellets in our study, only contributed 6% to total fish landings (Table 2). Feces produced by the fish in our study were long cylinders, that likely broke across the length axis into pellet fragments of varying length; we collectively refer to these pellets and their fragments as “pellets” hereafter. We compared our fecal pellets with pellets produced by adult anchovy in large aquaria at the Monterey Bay Aquarium, and they were of similar appearance and size (Table 2).
Table 1. Landings (in kilograms, kg) and percent contribution to total commercial fish landings (%) of Northern Anchovy and Pacific Sardines in the Santa Barbara area during April 2006 (the time of our study), the entire year of 2006, and the period 2000–2010. Table prepared using commercial catch data from California Department of Fish and Game (http://www.dfg.ca.gov/marine/fishing.asp#Commercial).
| Northern Anchovy Landings (kg) | Northern Anchovy (%) | Pacific Sardine Landings (kg) | Pacific Sardine (%) | |
|---|---|---|---|---|
| 2006 (April only) | 307,214 | 85.6 | 22,916 | 6.4 |
| 2006 (Entire year) | 4,181,130 | 60.5 | 1,935,210 | 28.0 |
| 2000–2010 | 21,142,843 | 37.8 | 28,207,943 | 50.4 |
Table 2. Comparison of fish fecal pellet abundance, size, dry weight, particulate organic carbon (POC) and nitrogen (PON), C:N, and sinking rate from present study, northern anchovy pellets from the Monterey Bay Aquarium (MBA), and Peruvian anchovy pellets from Staresinic et al.8.
| Abundance (# pellets m−3) | Length (mm) | Width (mm) | Volume (mm3) | Dry Weight (µg pellet−1) | POC (µg C pellet−1) | PON (µg N pellet−1) | C:N (molar) | Sinking Rate (m day−1) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 1.8 (1.9) | 2.1 (1.0) | 1.0 (0.2) | 1.8 (1.1) | 314 (208) | 21.7 (5.0) | 2.7 (1.1) | 10.1 (1.8) | 787 (201) | Present study |
| Range | 0.1–5.9 | 1.0–6.0 | 0.6–1.5 | 0.2–7.9 | 100–771 | 14.5–31.0 | 1.5–5.6 | 6.5–12.5 | 485–1370 | |
| Mean (SD) | 3.2 (1.1) | 1.0 (0.2) | 2.9 (1.4) | MBA anchovy pellets | ||||||
| Range | 1.5–5.6 | 0.8–1.4 | 0.8–5.7 | |||||||
| Mean | 1.1 | 1.0 | 0.9 | 286 | 18.4 | 1.9 | 11.3 | 1100 | Staresinic et al.8 | |
| Range | 223–347 | 14.4–21.1 | 1.2–2.4 | 10.1–15.0 | 691–1987 |
Fish fecal pellets were abundant in our study location, ranging from 0.1 to 5.9 pellets m−3 (Table 2). Abundance of pellets was highest (mean = 2.9 pellets m−3) during Day 1 of our study, decreased on Day 2 (mean = 1.8 pellets m−3), and was lowest on Day 3 of our study (mean = 0.2 pellets m−3).
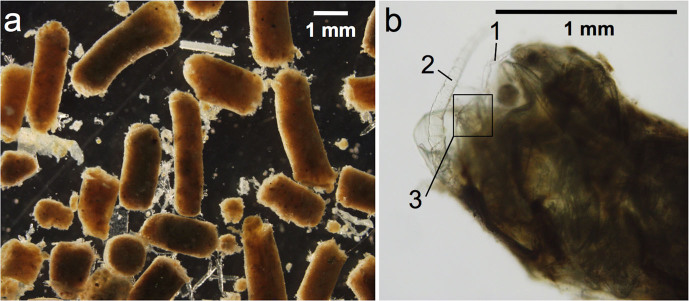
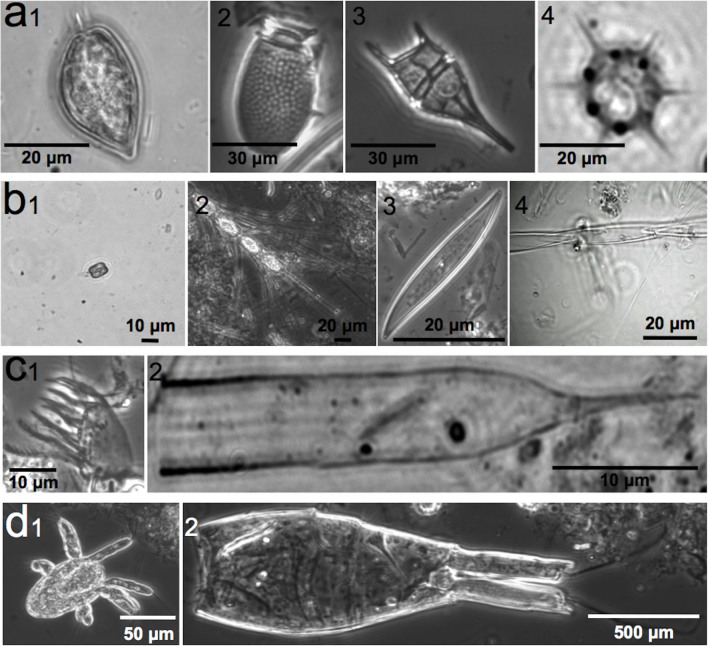
Fecal pellets were similar in width, averaging 1.0 ± 0.2 mm (range: 0.6–1.5 mm), but were variable in length (average: 2.1 ± 1.0 mm, range: 1.0–6.0 mm) (Table 2; Fig. 2). Qualitative examination of prey items found in feces suggests the dinoflagellate Prorocentrum micans was very common in the fish diet (Fig. 3, panel a1). The dinoflagellates Dinophysis sp. and Ceratium sp., various diatoms, silicoflagellates, ciliates, and copepods were also common components of fecal pellets (Fig. 3).
Figure 2. Example fish fecal pellets collected in Santa Barbara Channel and used for analyses.
Scale bar shown on individual panels. Copepod body parts are visible within the fish fecal pellet in the panel b: 1, swimming leg; 2, antenna; 3, furcal rami.
Figure 3. Dominant prey items found within fish fecal pellets.
Panel a: 1–3 are dinoflagellates including 1) Prorocentrum micans, 2) Dinophysis sp., and 3) Ceratium sp.; 4) silicoflagellate. Panel b (diatoms): 1–2 are centric diatoms including 1) Thalassiosira sp. and 2) Chaetoceros sp.; 3–4 are pennate diatoms including 3) Pleurosigma sp. and 4) Pseudo-nitzschia sp. Panel c (ciliates): 1) aloricate ciliate and 2) tintinnid ciliate. Panel d (copepods): 1) nauplius and 2) adult copepod. Prey items were identified using epifluorescence microscopy.
Fecal pellet dry weight, POC, and PON content averaged 314 µg pellet−1, 21.7 µg C pellet−1, and 2.7 µg N pellet−1, respectively (Table 2). The mean molar C:N ratio was 10.1, and ranged from 6.5 to 12.5 (Table 2).
Sinking rates of fecal pellets varied from 0.5 to 1.6 cm s−1 (458–1370 m d−1) and averaged about 0.9 cm s−1 (787 m d−1) (Table 2), and in general, pellets of larger volume sank faster. Our measurements suggest that pellets produced near the surface sinking at constant rate would reach the benthos (512 m) in < 1 day.
Discussion
Fecal matter produced by the Peruvian anchovy is hypothesized to play an important role in carbon and nitrogen flux in highly productive, upwelling systems8. Our study demonstrates that fecal matter likely produced by the northern anchovy may also export significant amounts of repackaged surface material to depth in the California coastal system.
The size, C and N content, and sinking rates of fish fecal pellets in our study were similar to those measured for Peruvian anchovy by Staresinic et al.8 (Table 2). Pellets were considerably larger than those sampled for Euphausia pacifica, a common species of krill found in the Santa Barbara Channel, in previous studies15,16, and the cylindrical shape differed from the flattened ribbon-shaped pellets produced by bloom-forming gelatinous salps17. Fecal pellets were ~1 mm in diameter and varied in length (1.0–6.0 mm). Staresinic et al.8 reported an average Peruvian anchovy pellet fragment length of 1.1 mm but observed pellets in situ up to 1 cm in length. The reported mean POC content of fish fecal pellets normalized to pellet volume (0.012 mg C mm−3) was relatively low compared to values previously reported for Acartia tonsa copepods feeding on Thalassiosira weissflogii diatoms18 (0.17 – 2.5 mg C mm−3) but similar to those measured for large copepods feeding omnivorously19 and salps feeding on natural seston17. This variability in fecal pellet carbon per volume is due to a combination of differences between organisms in their nutrient requirements, physiology, and diet19,20,21, as well as differences in food quality18 and food concentration18,19. Pellet C:N ratios (5.6–10.7 g g−1) measured in the present study were within range of those previously measured for copepods (3.2–25.0), euphausiids (5.7–10.0), and salps (7.3–12.8) (reviewed in Steinberg and Saba 200822). Sinking rates of fish fecal matter were, in general, higher than those reported for euphausiids (16–862 m d−1)2,23,24 but within range of those reported for salps (300–2470 m d−1)4,17,25 and several species of midwater fish (147–2739 m d−1)7.
Estimated POC and PON fluxes (calculated by multiplying pellet POC and PON content [mg pellet−1], abundance within surface 50 m [pellet m−3], and sinking rate [m d−1]) measured in our study while upwelling was absent averaged 30.7 mg C m−2 d−1 and 3.8 mg N m−2 d−1, and reached a maximum pellet POC flux of 251 mg C m−2 d−1, which is higher than the maximum reported for anchovy in a coastal Peru upwelling system8 (92 mg C m−2 d−1; calculated from free-drifting sediment traps). Additionally, fish fecal pellet POC flux was equal to, and sometimes exceeded, total POC flux measured previously by bottom-moored sediment traps deployed in the Santa Barbara Channel (20–200 mg C m−2 d−1 [540 m; Aug. 1993-Sept. 199626], 50–300 mg C m−2 d−1 [470 m; June 1995-July 199927], and 7–108 mg m−2 d−1 [100 m; July 1999-Aug. 200228]). Our fish fecal POC flux estimates are out of the surface 50 m; the sediment trap flux measurements were made deeper and thus reflect POC attenuation due to remineralization29,30. However, the rapid sinking rates of these cohesive fish feces would suggest little opportunity for remineralization as previously shown for salps25, thus comparison of the flux measurements between these different depths is reasonable and questions, 1) how much material in sediment traps in this region are fish-feces derived? and 2) are fish feces adequately sampled by sediment traps (see below)? Future studies in the region including microscopic examination of sediment trap material would help address these issues.
Measurements of in situ abundance of fish fecal pellets or their flux are lacking, likely due to the difficulty of adequately sampling these particles as a result of lateral advection of pellets, breakage of pellets in traps rendering them indistinguishable from other particles, and the high mobility and schooling of fish leading to spatial heterogeneity or “patchiness” in fecal pellet production in surface waters. To our knowledge, this is the first study to present estimates of fish fecal pellet abundance, which ranged from 0.1 to 5.9 pellets m−3 (Table 2). Our reported abundances, and consequently estimated POC and PON fluxes, likely represent near maximum values associated with schools of these fish and, because of the patchy distribution of these schools, cannot be considered average abundances of pellets and fluxes of POM. Abundances of fish pellets in this study are orders of magnitude lower than those previously determined for euphausiid fecal pellets in the Santa Barbara Channel16 (500 – 98,000 m−3) determined using a camera system; however, because of the relatively higher C content and sinking rates of fish fecal matter, the estimated POC flux of fish pellets (2–251 mg C m−2 d−1) was within range of those estimated for euphausiids in the Santa Barbara Channel16 (46–3708 mg C m−2 d−1). POC flux of fish fecal matter were similar to those reported for salps, which range from 0.01–137 mg C m−2 d−1 (25,31,32,33,34).
Caution must be taken when calculating flux using laboratory measurements of particle sinking rates1,16, and rates calculated in the present study likely represent the maximum potential fecal pellet sinking rates. This is because residence times of fecal matter in the upper mixed layer may be increased due to biological processes such as microbial degradation3,25,35,36 or fragmentation of pellets by zooplankton feeding or swimming35,37,38, physical processes such as horizontal advection1,39, accumulation at density discontinuities and entrainment into the mixed layer during mixing events16,40, or turbulent mixing16,41. Net tows contained mainly structurally intact fecal pellets and little to no fecal “fluff” (crushed or degraded particles that are unrecognizable as fecal pellets yet may have been fecal in origin42). As mentioned above, the cohesive nature of these fecal pellets would render them less susceptible to bacterial decomposition during rapid decent to the benthos, as has been suggested for temperate and tropical reef fish fecal pellets by Geesey et al. 198443. Similarly, microbial degradation and protozoan activity on large, intact fecal pellets of salps had little affect on sinking rates25.
Upwelling conditions were not present during our occupation of the study area; thus, resuspension of accumulated fish fecal matter above density layers was unlikely. Turbulent mixing also did not play a significant role in the resuspension of fecal matter during the present study. To test whether vertical sinking or turbulent diffusion dominated the transport of fecal matter in our study, we applied the calculations described by Alldredge et al.16. We compared the characteristic time for settling, ts = z/ws, where z is mixed layer depth (mean = 18 m) and ws is sinking rate (mean = 787 m d−1), to the characteristic time for turbulent diffusion, td = z2/2kz, where kz is the coefficient of vertical diffusivity44. We calculated kz (62 cm2 s−1) according to Alldredge et al.16, kz = c2U3/π2tm, where constant c equals 1.3 when length and time are given in meters and seconds, respectively, tm is the time of mixed layer mixing (1 hour or 3600 seconds, Alldredge et al.16), and U is average wind speed (5.06 m s−1). When td/tsvalues are < 1, the movement of fecal matter is controlled by turbulent diffusion. Our td/ts ratio was > 1 (mean = 114), suggesting that vertical sinking dominated the transport of fish fecal matter.
Dinoflagellates, diatoms, silicoflagellates, ciliates, and copepods were dominant prey items found in fish feces in our study (Fig. 3). Algae associated with red-tide formation (dinoflagellates Prorocentrum micans, Ceratium sp.) or toxin production (dinoflagellate Dinophysis sp., diatom Pseudo-nitzschia sp.) were frequently encountered. While these fish may exert control over potentially harmful algal blooms, they may also subsequently act as vectors for toxin transfer to higher trophic levels such as mammals and sea birds45,46,47. Additionally, fish fecal pellets likely accumulate toxins46,48, which may be fed upon by other pelagic or benthic organisms. Nonetheless, fish likely play an important role in the top-down control of certain phytoplankton and zooplankton groups, and act as a link between pelagic and benthic realms through the downward flux of repackaged particulate matter.
Northern anchovy contributed a large proportion to total commercial fish landings in the Santa Barbara Channel during the last decade (Table 1); thus, their fecal matter likely contributes a significant component of the vertical flux of organic carbon and nitrogen. Our estimates suggest that anchovy pellets produced near the surface would reach the benthos (512 m) in < 1 day. Additionally, anchovies may transport particulate carbon spatially along the coast as they exhibit both vertical and onshore-offshore diel migrations to mimic prey movements49,50. It is unknown if all bony fish form similar cohesive, rapidly sinking fecal pellets or if some fish form loose, porous pellets which will break up and degrade easily in the upper water column as reported for marine mammals such as whales51. However, all reports on fish fecal pellets thus far (northern anchovy [present study], seven midwater fish species7, the Peruvian anchovy8, and the blacksmith reef fish9) have demonstrated the formation of cohesive, rapidly sinking pellets. Finally, recent studies revealed that fish contribute up to 15% of total oceanic carbonate production (inorganic C) via the formation and excretion of various forms of precipitated (non-skeletal) calcium carbonate from their guts52,53. Thus, the downward transport of particulate matter produced by fish could be a significant, but underappreciated, component of both organic and inorganic carbon flux in coastal environments.
Methods
Sampling was conducted aboard the R/V Point Sur in the Santa Barbara Channel (California, USA) (34°17′N, 119°55′W) from 20 April 2006 to 22 April 2006. Abundance, size, plankton prey composition, particulate organic carbon and nitrogen content (POC and PON), and sinking rates of fecal pellets were determined from pellets collected from eight net tows over the course of three consecutive days. Tows were conducted vertically from the surface to 50 m at a rate of 20 m min−1 using a 1 m diameter, 500 µm mesh net equipped with a flow meter and attached to a non-filtering cod end. All fecal pellets were counted in each tow. Pellets were gently picked into well plates using a wide-bore pipette, and length and width were measured immediately under a dissecting scope (Olympus SZX12) using an ocular ruler at 230x magnification (n = 90). Volumes were calculated applying the formula for a cylinder shape. Subsamples of fecal pellets preserved in 0.22 µm filtered seawater and 37% buffered formaldehyde were gently broken apart with forceps and qualitatively analyzed for composition using an Olympus IX71 epifluorescence microscope and digital camera (RETIGA EXi) under dark- and light-field illumination. The phytoplankton and zooplankton prey that were most commonly present in fecal pellets were identified and photographed. Repeated attempts to enumerate individual prey taxa for quantitative estimates of composition were unsuccessful due to the inability to completely break apart individual fecal pellets via manual dissection (similar to methods described for zooplankton gut contents in Wilson and Steinberg54) or centrifugation8.
Fecal pellets were pipetted onto pre-weighed, combusted GF/F filters and frozen until POC/PON analysis (a total of 976 fecal pellets; 13 filters each containing between 14 and 198 pellets). Filters were dried at 55°C, fumigated with 6 N HCl to remove inorganic C prior to analysis55, weighed, and analyzed for POC and PON on a CHN elemental analyzer (EA1108).
Sinking rates were determined for 24 individual fecal pellets aboard ship under calm sea state using a tall, clear 8 L Nalgene bottle (diameter = 19.5 cm, height = 30 cm) filled with surface seawater of 14.6°C, 33.56‰ salinity. Pellets were released just under the water surface and timed while falling a distance of 20.8 cm. The water temperature in the bottle did not change over the course of the sinking rate experiment (as pellets sank rapidly, thus the experiment was completed quickly). The pellets used to determine sinking rates were randomly selected and ranged in volume from 1.2 to 7.9 mm3, representing the range of sizes collected in situ. Fecal pellets were durable and cohesive, and thus no fragmentation occurred during handling, sampling for size and chemical composition, or throughout the duration of the sinking rate experiments.
Author Contributions
Grace Saba and Deborah Steinberg conducted the experiments reported in this study. Grace Saba analyzed the data and wrote the manuscript. Both authors reviewed and edited the manuscript and were involved in the development of the figures and tables.
Acknowledgments
We thank Deborah Bronk, and the captain and crew of the R.V. Point Sur for field assistance, Evan Tyler at Monterey Bay Aquarium for collecting anchovy fecal pellets, Kevin Hill at National Marine Fisheries Service for sharing anchovy data, and Walker Smith for the use of his Elemental Combustion Analyzer to measure carbon and nitrogen content. The research was supported by the Biocomplexity Program of the U. S. National Science Foundation (OCE-0221825) and the United States Environmental Protection Agency (EPA) under the Science to Achieve Results (STAR) Graduate Fellowship Program. EPA has not officially endorsed this publication and the views expressed herein may not reflect the views of the EPA. This manuscript is Contribution No. 3247 of the Virginia Institute of Marine Science, The College of William and Mary.
References
- Alldredge A. L. & Gotschalk C. C. In situ settling behavior of marine snow. Limnol. Oceanogr. 33, 339–351 (1988). [Google Scholar]
- Yoon W. D., Kim S. K. & Han K. N. Morphology and sinking velocities of fecal pellets of copepod, molluscan, euphausiid, and salp taxa in the northeastern tropical Atlantic. Mar. Biol. 139, 923–928 (2001). [Google Scholar]
- Turner J. T. Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat. Microb. Ecol. 27, 57–102 (2002). [Google Scholar]
- Bruland K. W. & Silver M. W. Sinking rates of fecal pellets from gelatinous zooplankton (salps, pteropods, doliolids). Mar. Biol. 63, 295–300 (1981). [Google Scholar]
- Dilling L. & Alldredge A. L. Can chaetognath fecal pellets contribute significantly to carbon fiux? Mar. Ecol. Prog. Ser. 92, 51–58 (1993). [Google Scholar]
- Phillips B., Kremer P. & Madin L. P. Defecation by Salpa thompsoni and its contribution to vertical flux in the Southern Ocean. Mar. Biol. 156, 455–467 (2009). [Google Scholar]
- Robison B. H. & Bailey T. G. Sinking rates and dissolution of midwater fish fecal matter. Mar. Biol. 65, 135–142 (1981). [Google Scholar]
- Staresinic N., Farrington J., Gagosian R. B., Clifford C. H. & Hulburt E. M. Downward transport of particulate matter in the Peru coastal upwelling: Role of the anchoveta, Engraulis ringens. In: Suess, E. & Theide, J. (Eds.) Coastal Upwelling: Its Sediment Record. Part A. Responses of the Sedimentary Regime to Present Coastal Upwelling. Plenum, New York, pp. 225–240 (1983). [Google Scholar]
- Bray R. N., Miller A. C. & Geesey G. G. The fish connection: A trophic link between planktonic and rocky reef communities? Science 214, 204–205 (1981). [DOI] [PubMed] [Google Scholar]
- Crawford R. J. M. Food and population variability in five regions supporting large stocks of anchovy, sardines, and horse mackerel. S. Afr. J. Mar. Sci. 5, 735–757 (1987). [Google Scholar]
- Cury P. et al. Small pelagics in upwelling systems: patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 57, 603–618 (2000). [Google Scholar]
- Loukashkin A. S. On the diet and feeding behavior of the Northern Anchovy, Engraulis mordax (Girard). Proc. Cal. Acad. Sci., 4th Ser. 37, 419–458 (1970). [Google Scholar]
- Koslow J. A. Feeding selectivity of schools of northern anchovy Engraulis mordax, in the Southern California Bight. Fish. Bull. US 79, 131–142 (1981). [Google Scholar]
- Blaxter J. H. S. & Hunter J. R. The biology of the clupeoid fishes. In: Blaxter, J. H. S., Russell, F. S. & Yonge, R. (Eds.) Advances in Marine Biology, Vol 20 Academic Press, New York, pp. 1–223 (1982). [Google Scholar]
- Osterberg C., Carey A. G. & Curl J. Acceleration of sinking rates of radionuclides in the ocean. Nature 200, 1276–1277 (1963). [Google Scholar]
- Alldredge A. L., Gotschalk C. C. & MacIntyre S. Evidence for sustained residence of macrocrustacean fecal pellets in surface waters off southern California. Deep Sea Res. 34, 1641–1652 (1987). [Google Scholar]
- Madin L. P. Production, composition, and sedimentation of salp fecal pellets in oceanic waters. Mar. Biol. 67, 39–45 (1982). [Google Scholar]
- Butler M. & Dam H. G. Production rates and characteristics of fecal pellets of the copepod Acartia tonsa under simulated phytoplankton bloom conditions: implications for vertical fluxes. Mar. Ecol. Prog. Ser. 114, 81–91 (1994). [Google Scholar]
- Urban-Rich J., Hansell D. A. & Roman M. R. Analysis of copepod fecal pellet carbon using a high temperature combustion method. Mar. Ecol. Prog. Ser. 171, 199–208 (1998). [Google Scholar]
- Komar P. D., Morse A. P., Small L. F. & Fowler S. W. An analysis of sinking rates of natural copepod and euphausiid fecal pellets. Limnol. Oceanogr. 26, 172–180 (1981). [Google Scholar]
- Feinberg L. R. & Dam H. G. Effects of diet on dimensions, density and sinking rates of fecal pellets of the copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 175, 87–96 (1998). [Google Scholar]
- Steinberg D. K. & Saba G. K. Nitrogen consumption and metabolism in marine zooplankton. In: Capone, D. G., Bronk, D. A., Mulholland, M. R. & Carpenter, E. J. (Eds.) Nitrogen in the Marine Environment, 2nd Edition Academic Press, Boston, pp. 1135–1196 (2008). [Google Scholar]
- Fowler S. W. & Small L. F. Sinking rates of euphausiid fecal pellets. Limnol. Oceanogr. 17, 293–296 (1972). [Google Scholar]
- Youngbluth M. J. et al. Fecal pellet production and diel migratory behavior by the euphausiid Meganyctiphanes norvegica effect benthic-pelagic coupling. Deep-Sea Res. 36, 1491–1501 (1989). [Google Scholar]
- Caron D. A., Madin L. P. & Cole J. J. Composition and degradation of salp fecal pellets: Implications for vertical flux in oceanic environments. J. Mar. Res. 47, 829–850 (1989). [Google Scholar]
- Thunell R. C. Particle fluxes in a coastal upwelling zone: sediment trap results from Santa Barbara Basin, CA. Deep Sea Res. II 45, 1863–1884 (1998). [Google Scholar]
- Shipe R. F. et al. Effects of the 1997–1998 El Niño on seasonal variations in the suspended and sinking particles in the Santa Barbara Basin. Prog. Oceanogr. 54, 105–127 (2002). [Google Scholar]
- Goldthwait S. A. & Alldredge A. L. An investigation of diel synchronicity between water column marine snow concentration and the flux of organic matter in the Santa Barbara Channel, California. Deep Sea Res. I 53, 485–505 (2006). [Google Scholar]
- Martin J. H., Knauer G. A., Karl D. M. & Broenkow W. W. VERTEX: carbon cycling in the NE Pacific. Deep Sea Res. 34, 267–285 (1987). [Google Scholar]
- Buesseler K. O. et al. Revisiting carbon flux through the ocean's twilight zone. Science 316, 567–570 (2007). [DOI] [PubMed] [Google Scholar]
- Wiebe P. H., Madin L. P., Haury L. R., Harbison G. R. & Philbin L. M. Diel vertical migration by Salpa aspera and its potential for large-scale particulate organic matter transport to the deep-sea. Mar. Biol. 53, 249–255 (1979). [Google Scholar]
- Iseki K. Particulate organic matter transport to the deep sea by salp fecal pellets. Mar. Ecol. Prog. Ser. 5, 55–60 (1981). [Google Scholar]
- Matsueda H., Handa N., Inoue I. & Takano H. Ecological significance of salp fecal pellets collected by sediment traps in the eastern North Pacific. Mar. Biol. 91, 421–431 (1986). [Google Scholar]
- Phillips B., Kremer P. & Madin L. P. Defecation by Salpa thompsoni and its contribution to vertical flux in the Southern Ocean. Mar. Biol. 156, 455–467 (2009). [Google Scholar]
- Noji T. T., Estep K. W., MacIntyre F. & Norrbin F. Image analysis of faecal material grazed upon by three species of copepods: evidence for coprohexy, coprophagy, and coprochaly. J. Mar. Biol. Assoc. UK 71, 465–480 (1991). [Google Scholar]
- Urban-Rich J., Nordby E., Andreassen I. J. & Wassmann P. Contribution by mesozooplankton fecal pellets to the carbon flux on Nordvestbanken, north Norwegian shelf in 1994. Sarsia 84, 253–264 (1999). [Google Scholar]
- Lampitt R. S., Noji T. T. & von Bodungen B. What happens to zooplankton faecal pellets? Implications for material flux. Mar. Biol. 104, 15–23 (1990). [Google Scholar]
- Dilling L. & Alldredge A. L. Fragmentation of marine snow by swimming macrozooplankton: a new process impacting carbon cycling in the sea. Deep Sea Res. I 47, 1227–1245 (2000). [Google Scholar]
- Asper V. L., Honjo S. & Orsi T. H. Distribution and transport of marine snow aggregates in the Panama basin. Deep Sea Res. 39, 939–952 (1992). [Google Scholar]
- MacIntyre S., Alldredge A. L. & Gotschalk C. C. Accumulation of marine snow at density discontinuities in the water column. Limnol. Oceanogr. 40, 449–468 (1995). [Google Scholar]
- Denman K. L. & Gargett A. E. Time and space scales of vertical mixing and advection of phytoplankton in the upper ocean. Limnol. Oceanogr. 28, 801–815 (1983). [Google Scholar]
- Wilson S. E., Steinberg D. K. & Buesseler K. O. Changes in fecal pellet characteristics with depth as indicators of zooplankton repackaging of particles in the mesopelagic zone of the subtropical and subarctic North Pacific Ocean. Deep Sea Res. II 55, 1636–1647 (2008). [Google Scholar]
- Geesey G. G., Alexander G. V., Bray R. N. & Miller A. C. Fish fecal pellets are a source of minerals for inshore reef communities. Mar. Ecol. Prog. Ser. 15, 19–25 (1984). [Google Scholar]
- Lick W. Entrainment, deposition, and transport of fine-grained sediments in lakes. Hydrobiol. 91, 31–40 (1982). [Google Scholar]
- Work T. M. et al. Domoic acid intoxication of brown pelicans and cormorants in Santa Cruz, California. In: Smayda, T. J. & Shimizu, Y. (Eds.) Toxic Phytoplankton Blooms. Elsevier, Amsterdam, pp. 643–649. (1993). [Google Scholar]
- Lefebvre K. A. et al. Detection of domoic acid in northern anchovies and California sea lions associated with an unusual mortality event. Nat. Toxins 7, 85–92 (1999). [DOI] [PubMed] [Google Scholar]
- Scholin C. A. et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 403, 80–84 (2000). [DOI] [PubMed] [Google Scholar]
- Hardy R. W. et al. Domoic acid in rainbow trout (Oncorhynchus mykiss) feeds. Aquaculture 131, 253–260 (1995). [Google Scholar]
- Allen L. G. & DeMartini E. E. Temporal and spatial patterns of nearshore distribution and abundance of the pelagic fishes off San Onofre-Oceanside, California. Fish. Bull 81, 569–586 (1983). [Google Scholar]
- Robinson C. J., Arenas F. V. & Gomez G. J. Diel vertical and offshore-inshore movements of anchovies off the central Baja California coast. J. Fish Biol. 47, 877–892 (1995). [Google Scholar]
- Roman J. & McCarthy J. J. The whale pump: Marine mammals enhance primary productivity in a coastal basin. PLoS ONE 5, e13255 (2010). 10.1371/journal.pone.00132552010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. W. et al. Contribution of fish to the marine inorganic carbon cycle. Science 3232, 359–362 (2009). [DOI] [PubMed] [Google Scholar]
- Perry C. T. et al. Fish as major carbonate mud producers and missing components of the tropical carbonate factory. Proc. Natl. Acad. Sci. USA 10.1073/pnas.1015895108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. E. & Steinberg D. K. Autrotrophic picoplankton in mesozooplankton guts: evidence of aggregate feeding in the mesopelagic zone and export of small phytoplankton. Mar. Ecol. Prog. Ser. 412, 11–27 (2010). [Google Scholar]
- Harris D., Horwáth W. R. & van Kessel C. Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon-13 isotopic analysis. Soil Sci. Soc. Am. J. 65, 1853–1856 (2001). [Google Scholar]