Abstract
Background:
The consumption of a high carbohydrate diet may be associated with an increased risk of type 2 diabetes and obesity. Previous studies in vitro have revealed that grape seed extract (GSE) inhibited the intestinal α-glucosidases and α-pancreatic amylase that may delay carbohydrate digestion and absorption, resulting in the suppression of postprandial glycemia. The objective of the study was to assess whether consumption of GSE together with high carbohydrate meal affects postprandial glycemia in healthy participants.
Materials and Methods:
The study used acute, randomized, controlled crossover design in which eight healthy subjects (four female and four male, mean aged 21.25 ± 3.69 years; body mass index =20.28 ± 1.40 kg/m2) received high carbohydrate (HC) meal (73.6 %) together with or without 100 and 300 mg GSE.
Results:
Results showed that postprandial plasma glucose concentrations at 15 min and 30 min after ingestion HC meal together with 100 mg GSE (5.33 ± 0.41 mmol/L and 5.62 ± 0.47 mmol/L, respectively) and 300 mg GSE (5.27 ± 0.29 mmol/L; 5.75 ± 0.44 mmol/L, respectively) were significantly lower than that of HC meal (P<0.05). There was statistically significant difference in the 2 h area under the glucose response curve between HC meal and HC meal plus GSE.
Conclusions:
GSE reduces postprandial plasma glucose in healthy participants. The delayed and attenuated hyperglycemia may have a useful strategy to prevent development of diabetes in the healthy population.
Keywords: Grape seed extract, postprandial plasma glucose, polyphenols
INTRODUCTION
Diabetes mellitus is a growing and significant health problem strongly associated with obesity, and dyslipidemia. The increased energy intake and decreased physical activity are causing overweight and obesity, leading to an epidemic increase in the risk of type 2 diabetes. An increase in the consumption of high-sugar diets correlates positively with the development of diabetes.[1] Scientific evidence clearly indicates that the consumption of edible plants enriched in polyphenolic compounds has been associated with a reduced risk of developing type 2 diabetes.[2–4] Especially, several studies have revealed that polyphenols may delay carbohydrate digestion and absorption by inhibiting α-glucosidase and α-amylase, consequently, reducing postprandial hyperglycemia which may prevent development of diabetes mellitus.[5–9]
Grape seed (Vitis vinifera Linn.) is an excellent source of various vitamins, minerals, and polyphenols including flavonoids, proanthocyanidins, and procyanidins.[10] Numerous studies have shown that grape seed extract (GSE) have anti-platelet aggregation,[11] antioxidant,[12] cardioprotective activity,[13] improvement of endothelial function,[14] reduction of postprandial hypertriglyceridemia and hypercholesterolemia,[15] and prevention of insulin resistance.[16,17] Moreover, GSE exhibits favorable inhibitory effect against intestinal α-glucosidases, pancreatic α-amylase activities.[18] Another research has shown that GSE significantly improves markers of inflammation and glycemia and reduces plasma fructosamine in obese type 2 diabetic subjects.[19] However, to date, there have been no studies examining the effects of GSE on postprandial plasma glucose. The present study was therefore conducted to determine the effect of GSE on postprandial plasma glucose in healthy subjects after a high carbohydrate load meal.
MATERIALS AND METHODS
Participants
A total of nine healthy participants (five female and four male) were recruited from the staff and student populations at Faculty of Allied Health Science, Chulalongkorn University. One female subject was dropped out of the study because of her personal reason. At the screening visit, participant's health status was checked by routine blood chemistry test (blood glucose, total cholesterol, Low Density Lipoprotein [LDL]-cholesterol). A structured interview on habitual diet, previous and current diseases, medications use, alcohol consumption, physical activity, and dietary supplements use was implemented by dietitian. Participants were excluded from the study if they had any of the following: abnormal blood chemistry, history of metabolic disorder, taking medication or dietary supplement which would interfere with normal gastrointestinal function. The mean age was 21.25 ± 3.69 (female: 20.25 ± 0.96, male: 22.25 ± 5.32) years, BMI 20.28 ± 1.40 (female: 19.38 ± 0.91, male: 21.19 ± 1.25) kg/m2, and percent body fat 22.48 ± 6.24 (female: 24.97 ± 1.86, male: 19.98 ± 8.41). The randomized, controlled, and crossover trial was carried out. This study was conducted under approval of the Ethical Review Committee for Research Involving Human Research Participants, Health Science Group, Chulalongkorn University. Written informed consent was obtained from all study participants.
Study design
One tablet GSE (100 mg) containing 95% proanthocyanidin was purchased from Natural Source™. Eight participants were randomly assigned into one of the three experiment tests including: high carbohydrate (HC) meal, HC meal + 100 mg GSE, and HC meal + 300 mg GSE (HC+ 300 mg GSE). The experiment began in the morning following an overnight fast. The fasting blood samples (3 ml) were collected in tubes EDTA-containing solution before an intake of test meal. Thereafter, the subjects were advised to consume the test meal within 5 min. The first bite in the mouth was set as time 0. Blood samples were taken at 15, 30, 45, 60, 90, and 120 min for laboratory analysis. Blood samples were immediately centrifuged (2,500 × g) and the plasma was separated and frozen at –20°C until analyzed for glucose concentration. Plasma glucose concentration was determined by glucose oxidase method by using an autoanalyzer at Service Unit, Faculty of Allied Health Sciences, Chulalongkorn University. Each test was conducted on a separate day and at least 2 week apart. Participants were requested to keep their lifestyles and body weight unchanged and to follow their habitual diet throughout the study.
Test meal
A high carbohydrate meal consists of three slices of white bread, two table spoon of condense milk, and a cup of low fat milk (a total of 92 g carbohydrate, 8 g fat, and 14 g protein). This meal provided approximately 73.6 % carbohydrate.
Statistical analysis
Data are expressed as means ± S.E.M. The incremental plasma glucose concentration curves were plotted as the change in incremental plasma glucose over time. The incremental changes in plasma glucose concentrations were calculated relative to fasting at all postprandial times. AUC was calculated according to the trapezoidal rule. Plasma glucose, incremental glucose concentration, and AUC of the different tests were compared using analysis of variance with Bonferroni correction for multiple comparisons. A P-value < 0.05 was considered statistically significant.
RESULTS
The mean body weight, BMI, and percent body fat of all participants did not differ between the study population groups throughout the study. Figure 1 shows the effect of difference GSE doses (100 and 300 mg) on plasma glucose concentration. The peak plasma glucose concentration was reached at 30 min in HC meal (6.99 ± 0.55 mmol/L); HC meal + 100mg GSE: 5.63 ± 0.43 mmol/L, and HC meal + 300 mg GSE:5.75 ± 0.44 mmol/L). Results showed that postprandial plasma glucose concentrations at 15 min and 30 min in both HC + 100 mg GSE (5.33 ± 0.41 mmol/L and 5.62 ± 0.47 mmol/L, respectively) and HC + 300 mg GSE (5.27 ± 0.29 mmol/L, 5.75 ± 0.44 mmol/L, respectively) were significantly lower than those in HC meal. As shown in Figure 2, the incremental postprandial plasma glucose concentration was also significantly decreased at 15 min after ingestion of 100 mg GSE (1.02 ± 0.37 mmol/L) and 300 mg GSE (0.59 ± 0.27 mmol/L) when compared to HC meal (2.13 ± 0.36 mmol/L). Intakes of HC meal plus GSE (100 and 300 mg) markedly reduced the AUC of glucose concentration by 46% and 75% (P<0.05), respectively (AUC for HC meal = 134.09 ± 19.17 mmol/L. min; AUC for HC meal + GSE (100 mg) = 72.01 ± 19.2 mmol/L. min; AUC for HC meal + GSE (300 mg) = 33.8 8± 20.4 mmol/L.min) [Figure 3].
Figure 1.
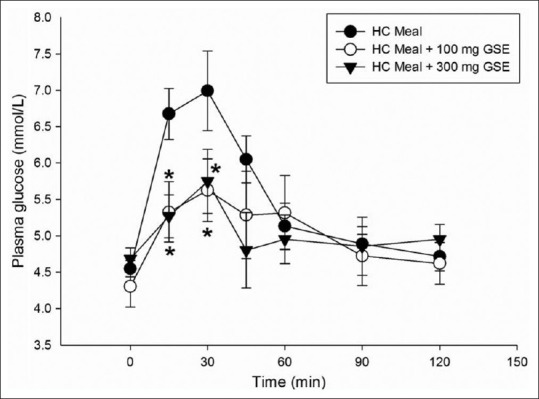
Plasma glucose concentration of high carbohydrate meal (•), HC meal + 100 mg GSE (∘), HC meal + 300 mg GSE (▼) in healthy participants (n=8). Values are means with standard error of the means represented by vertical bars. Mean value was significantly different from that of a HC meal: *P < 0.05
Figure 2.
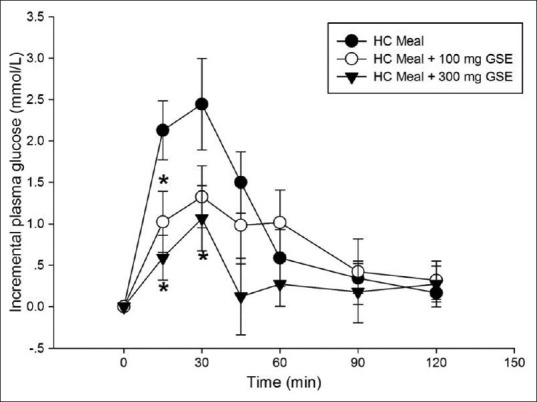
Incremental plasma glucose concentration of high carbohydrate meal (•), a HC meal + 100 mg GSE (∘), a HC meal + 300 mg GSE (▼) in healthy participants (n=8). Values are means with standard error of the means represented by vertical bars. Mean value was significantly different from that of a HC meal. *P < 0.05
Figure 3.
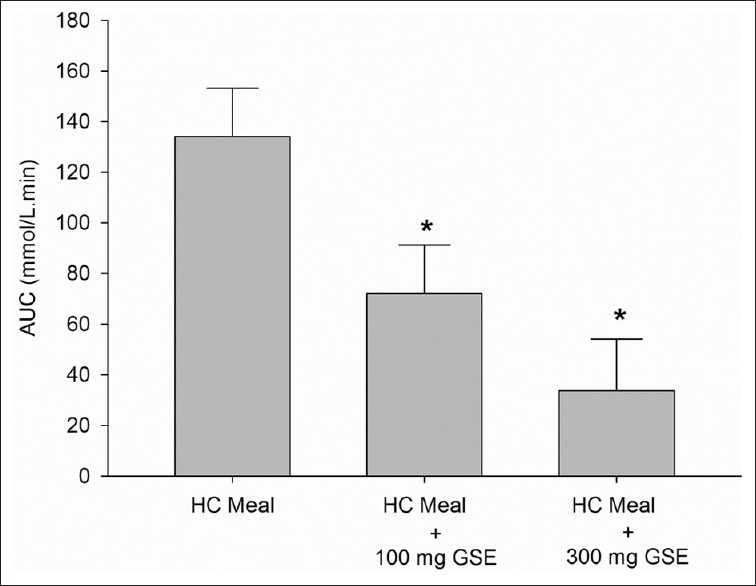
Area Under the Curve (AUC) of plasma glucose concentration (mmol/L) of high carbohydrate (HC) meal and HC meal + GSE (100 and 300 mg) in healthy participants (n=8). Value was significantly different from that of a HC meal: *P < 0.05
DISCUSSION
This study is the first to demonstrate the effect of GSE on postprandial hyperglycemia in healthy participants. The primary outcome in this study was the effect of grape seed extract on postprandial glucose levels. We found that the consumption of high carbohydrate meal together with GSE (100 and 300 mg) reduces postprandial glucose in healthy subjects after 15 min administration. In addition, only GSE (300 mg) can suppress postprandial glucose level after at 0 min of consumption. The secondary outcome in this study was measured plasma glucose AUC (0-120 min) after intake of high carbohydrate meal. Our findings indicate that the AUCs were markedly lowered after ingestion of GSE (100 and 300 mg) together HC meal than after ingestion of HC meal. One of the limitations of this study was the narrow age range of the subjects. While the small sample size of the study limit the generalizability of the results, a larger trial involving a greater number of patients would be needed to validate the findings of this small study.
Previous research by our group has demonstrated that GSE markedly inhibits the intestinal α-glucosidases, pancreatic α-amylase activities in vitro. Interestingly, proanthocyanidins, a major component in GSE, have shown potent intestinal α-glucosidase and pancreatic α-amylase inhibitory activities.[18] It is possible that the reduced postprandial glycemia observed in the present study can be explained by the inhibitory activity of GSE proanthocyanidins against α-glucosidase and pancreatic α-amylase.
Much research has been focused on the control of postprandial glucose by the inhibition of pancreatic-amylase and the intestinal-glucosidases, the key enzymes of dietary carbohydrate digestion.[20] The slowing carbohydrate digestion and/or absorption is the most probable mechanisms underlying potential the attenuated postprandial hyperglycemia, as this condition is associated with the prevention of impaired glucose tolerance (pre-diabetes) and a significant reduction in risk of developing type 2 diabetes.[21,22] Recently, it has been reported that the treatment with an α-glucosidase inhibitor (acarbose) specifically delays postprandial hyperglycemia, reduced the risk of type 2 diabetes.[23] It is possible that consumption of GSE may prevent or delay developing type 2 diabetes in healthy people. The earlier evidence reports that suppression of postprandial glucose may contribute to decreasing the level of HbA1c resulting in a significant reduction in the incidence of chronic vascular complication such as macro- and micro vascular diseases.[24] Similarly, although this study included only healthy subjects, it may nevertheless apply to individuals with diabetes, as hyperglycemia is an independent predictor of future cardiovascular events in both healthy and diabetic individuals. Therefore, an intake of GSE might help people with type 2 diabetes mellitus control the postprandial hyperglycemia as thereby prevent the progression of diabetic complications.
Kar et al reported that after four weeks administration of GSE (600 mg/day) to diabetic patients, fructosamine was significantly decreased when compared to the basal level.[19] The fructosamine levels are used to access glycemic control since they can indicate the accumulation of early (Amadori) glycation products in diabetic patients. The reduction of fructosamine level can prevent the progression of diabetic complications. The recently published showed that GSE (200 mg/day) did not reduce plasma glucose and HbA1C level in diabetic patients after two months of supplementation.[25] However, the effect of GSE on postprandial glucose and HbA1C level in diabetic patients remains controversial. Further studies would be needed to determine the effect of GSE in diabetic subjects focusing on examining postprandial glucose and HbA1C level which could yield important new insights into the preventive of diabetic complications.
CONCLUSION
GSE markedly reduces postprandial plasma glucose in healthy participants after consuming a high carbohydrate meal which suggests it may be a useful addition to other strategies aimed to prevent development of diabetes in the healthy population.
ACKNOWLEDGEMENT
This research work was financially supported by Faculty of Allied Health Science, Chulalongkorn University. The authors would like to thank the Medical Food Research and Development Center, and Special Task Force for Activating Research (STAR) which has been financially and institutionally supported by Chulalongkorn University. There are no conflicts of interests. The authors’ contributions were as follows: Suwimol Sapwarobol (40%), Sirichai Adisakwattana (40%), Sawitree Changpeng (5%), Wilwan Ratanawachirin (5%), Kanokporn Tanruttanawong (5%), and Waridtha Boonyarit (5%).
Footnotes
Source of Support: Supported by Faculty of Allied Health Science, Chulalongkorn University. The authors would like to thank the Medical Food Research and Development Center, and Special Task Force for Activating Research (STAR) which has been financially and institutionally supported by Chulalongkorn University
Conflict of Interest: No.
REFERENCES
- 1.Gerrits PM, Tsalikian E. Diabetes and fructose metabolism. Am J Clin Nutr. 1993;58(5 Suppl):S796–9. doi: 10.1093/ajcn/58.5.796S. [DOI] [PubMed] [Google Scholar]
- 2.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliövaara M, Reunanen A, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–8. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 3.Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J Am Coll Nutr. 2005;24:376–84. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- 4.van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: A prospective cohort study in younger and middle-aged US women. Diabetes Care. 2006;29:398–403. doi: 10.2337/diacare.29.02.06.dc05-1512. [DOI] [PubMed] [Google Scholar]
- 5.Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of α-glucosidase and α-amylase by flavonoids. J Nutr Sci Vitaminol (Tokyo) 2006;52:149–53. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- 6.Akkarachiyasit S, Charoenlertkul P, Yibchok-Anun S, Adisakwattana S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int J Mol Sci. 2010;11:3387–96. doi: 10.3390/ijms11093387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akkarachiyasit S, Yibchok-Anun S, Wacharasindhu S, Adisakwattana S. In vitro Inhibitory effects of cyandin-3-rutinoside on pancreatic α-amylase and its combined effect with acarbose. Molecules. 2010;16:2075–83. doi: 10.3390/molecules16032075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanamura T, Hagiwara T, Kawagishi H. Structural and functional characterization of polyphenols isolated from acerola (Malpighia emarginata DC) fruit. Biosci Biotechnol Biochem. 2005;69:280–6. doi: 10.1271/bbb.69.280. [DOI] [PubMed] [Google Scholar]
- 9.Cermak R, Landgraf S, Wolffram S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br J Nutr. 2004;91:849–55. doi: 10.1079/BJN20041128. [DOI] [PubMed] [Google Scholar]
- 10.Weber HA, Hodges AE, Guthrie JR, O’Brien BM, Robaugh D, Clark AP, et al. Comparison of proanthocyanidins in commercial antioxidants: Grape seed and pine bark extracts. J Agric Food Chem. 2007;55:148–56. doi: 10.1021/jf063150n. [DOI] [PubMed] [Google Scholar]
- 11.Vitseva O, Varghese S, Chakrabarti S, Folts JD, Freedman JE. Grape seed and skin extracts inhibit platelet function and release of reactive oxygen intermediates. Cardiovasc Pharmacol. 2005;46:445–51. doi: 10.1097/01.fjc.0000176727.67066.1c. [DOI] [PubMed] [Google Scholar]
- 12.Terra X, Fernández-Larrea J, Pujadas G, Ardèvol A, Bladé C, Salvadó J, et al. Inhibitory effects of grape seed procyanidins on foam cell formation in vitro. J Agric Food Chem. 2009;57:2588–94. doi: 10.1021/jf803450a. [DOI] [PubMed] [Google Scholar]
- 13.Cheng M, Gao HQ, Xu L, Li BY, Zhang H, Li XH. Cardioprotective effects of grape seed proanthocyanidins extracts in streptozocin induced diabetic rats. J Cardiovasc Pharmacol. 2007;50:503–9. doi: 10.1097/FJC.0b013e3181379ef6. [DOI] [PubMed] [Google Scholar]
- 14.Zhang FL, Gao HQ, Shen L. Inhibitory effect of GSPE on RAGE expression induced by advanced glycation end products in endothelial cells. J Cardiovasc Pharmacol. 2007;50:434–40. doi: 10.1097/FJC.0b013e3181342bfa. [DOI] [PubMed] [Google Scholar]
- 15.Adisakwattana S, Moonrat J, Srichairat S, Chanasit C, Tirapongporn H, Chanathong B, et al. Lipid-Lowering mechanisms of grape seed extract (Vitis vinifera L) and its antihyperlipidemic activity. J Med Plant Res. 2010;4:2113–20. [Google Scholar]
- 16.Suwannaphet W, Meeprom A, Yibchok-Anun S, Adisakwattana S. Preventive effect of grape seed extract against high-fructose diet-induced insulin resistance and oxidative stress in rats. Food Chem Toxicol. 2010;48:1853–7. doi: 10.1016/j.fct.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Meeprom A, Sompong W, Suwannaphet W, Yibchok-Anun S, Adisakwattana S. Grape seed extract supplementation prevents high-fructose diet-induced insulin resistance in rats by improving insulin and adiponectin signalling pathways. Br J Nutr. 2011;106:1173–81. doi: 10.1017/S0007114511001589. [DOI] [PubMed] [Google Scholar]
- 18.Adisakwattana S, Jiphimai P, Prutanopajai P, Chanathong B, Sapwarobol S, Ariyapitipan T. Evaluation of α-glucosidase, α-amylase and protein glycation inhibitory activities of edible plants. Int J Food Sci Nutr. 2010;61:295–305. doi: 10.3109/09637480903455963. [DOI] [PubMed] [Google Scholar]
- 19.Kar P, Laight D, Rooprai HK, Shaw KM, Cummings M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med. 2009;26:526–31. doi: 10.1111/j.1464-5491.2009.02727.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Park MH, Heo SJ, Kang SM, Ko SC, Han JS, et al. Dieckol isolated from Ecklonia cava inhibits alpha-glucosidase and alpha-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem Toxicol. 2010;48:2633–7. doi: 10.1016/j.fct.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 21.Ceriello A, Hanefeld M, Leiter L. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164:2090–5. doi: 10.1001/archinte.164.19.2090. [DOI] [PubMed] [Google Scholar]
- 22.Dickinson S, Brand-Miller J. Glycemic index, postprandial glycemia and cardiovascular disease. Curr Opin Lipidol. 2005;16:69–75. doi: 10.1097/00041433-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet. 2002;359:2072–7. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 24.Baron AD. Postprandial hyperglycaemia and alpha-glucosidase inhibitors. Diabetes Res Clin Pract. 1998;40(Suppl):S51–5. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 25.Gargari BP, Abedini S, Babaei H. Effect of supplementation with grape seed (Vitis vinifera) extract on antioxidant status and lipid peroxidation in patient with type II diabetes. J Med Plants Res. 2011;5:2039–34. [Google Scholar]


