Abstract
Background:
Curcumin is a phenolic natural product isolated from the rhizome of Curcuma longa (turmeric) and has effects on bone health and fat formation. The bone marrow mesenchymal stem cells (MSCs) are multipotent cells capable of differentiating into osteoblasts and adipocytes. Osteoblast differentiation of MSCs can be a result of upregulation of heme oxygenase (HO)-1 expression. Curcumin can potently induce HO-1 expression.
Objective:
The present study describes the effects of curcumin on rat MSC (rMSCs) differentiation into osteoblasts and adipocytes.
Materials and Methods:
Rat bone marrow MSCs were isolated and treated with or without curcumin. Osteoblast differentiation was confirmed and determined by alkaline phosphatase (ALP) activity, mineralized nodule formation, the expression of Runx2 (runt-related transcription factor 2) and osteocalcin. Adipocyte differentiation was determined by Oil red O staining and the expression of peroxisome proliferator-activated receptor-γ 2 (PPARγ2) and CCAAT/enhancer-binding protein (C/EBP) α.
Results:
Curcumin increased ALP activity and osteoblast-specific mRNA expression of Runx2 and osteocalcin when rMSCs were cultured in osteogenic medium. In contrast, curcumin decreased adipocyte differentiation and inhibited adipocyte-specific mRNA expression of PPARγ2 and C/EBPα when rMSCs were cultured in adipogenic medium. HO-1 expression was increased during osteogenic differentiation of rMSCs.
Conclusions:
These findings demonstrate that curcumin can promote osteogenic differentiation of rMSCs and inhibit adipocyte formation. The effect of curcumin on osteogenic differentiation of rMSCs is correlated with HO-1 expression.
Keywords: Adipogenesis, curcumin, mesenchymal stem cell, osteogenesis
INTRODUCTION
The bone marrow contains multipotential mesenchymal stem cells (MSCs) that have recently received widespread attention because of their potential use in bone engineering.[1,2] MSCs can differentiate into osteoblasts and adipocytes.[3] There is a reciprocal relationship between adipogenesis and osteogenesis of MSCs.[4,5] Factors that increase fat accumulation always lead to enhanced bone loss.[6] In order to treat osteoporosis and other bone diseases, it is of importance to understand the role of these factors that regulate the differentiation of MSCs.
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a phenolic natural product isolated from the rhizome of Curcuma longa (turmeric). It has been shown that curcumin exhibits anti-inflammatory and antimutagenic properties in addition to anticarcinogenic activity.[7,8] A growing body of evidence also suggests that curcumin affects both bone health and fat formation. On the one hand, curcumin can induce apoptosis in osteoclasts, inhibit osteoclastogenesis in RAW 264.7 cells, and prevent osteoclast formation by decreasing the level of expression of RANKL induced by IL-1 α in BMSCs.[9,10] On the other hand, curcumin can improve obesity-associated inflammation and diabetes in obese mice.[11] Curcumin directly interacts with adipocytes and suppresses adipocyte differentiation.[12] Due to these effects, curcumin is now considered to take part in the regulation of bone remodeling. Therefore, the effect of curcumin on osteogenesis of MSCs warranted further evaluation.
Previous studies have revealed that low concentrations of curcumin potently induce heme oxygenase (HO)- 1 expression in endothelial cells, leading to increased resistance to oxidative stress-mediated damage.[13] HO is the rate-limiting enzyme that catalyzes the degradation of heme to biliverdin, carbon monoxide, and free iron.[14] The expression of HO-1 is induced by a variety of stimuli including oxidative stress, hypoxia, and pro-inflammatory cytokines.[15–17] HO-1 plays an important role during bone marrow stem cell differentiation.[18,19] It has been reported that increased HO-1 expression and HO activity are essential for MSC growth to the osteoblast lineage and is consistent with the role of HO-1 in hematopoietic stem cell differentiation.[20]
The purpose of this study is to investigate the possible role of curcumin in the differentiation of MSCs. We demonstrated here that curcumin could significantly enhance the osteoblastic differentiation of rMSCs and suppress adipocyte differentiation. HO-1 is involved in the effect of curcumin on osteoblastic differentiation of rMSCs. These findings suggest that curcumin could be used in the treatment of secondary bone lesions associated with breast cancer and multiple myeloma and those associated with nonmalignant diseases like postmenopausal osteoporosis.
MATERIALS AND METHODS
Reagents
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] (Sigma-Aldrich, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) and stored at -20°C. For experiments, curcumin was added to the culture media at indicated concentrations. DMSO was used in control experiments at 0.1%.
Rat mesenchymal stem cells isolation
Sprague--Dawley rats (female, 5 weeks old, weighing 150 g) were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences, and maintained under pathogen-free conditions. All animal experiments complied with the animal protocols approved by the Institutional Review Board of the Institute of Soochow University. Bilateral femora and tibias were dissected from rats. The bone marrow cells were flushed out of the femora and tibias with α-MEM (HyClone, Logan, UT) by a 5 ml syringe. The marrow cells were seeded into 10 cm petri dish at a density of 5 × 105/cm2, containing α-MEM supplemented with 10% fetal calf serum (FCS) and 1% penicillin-streptomycin. The cells were then incubated at 37°C in 5% CO2. The medium was changed at every 3 days. When the cells were grown to 80% confluency, cells were trypsinized and expanded into dishes as passage 1. The medium was changed every 3 to 4 days, and the cells were passaged every 12--14 days. All cells used for the experiments were passage 3.
Induction of osteoblastic and adipogenic differentiation in rat mesenchymal stem cells
To induce osteoblastic differentiation, 1 × 104 rat MSCs were seeded in 24-well plates in α-MEM supplemented with 10% FCS, 1% penicillin-streptomycin, 10-8 Mdexamethasone (Sigma), 10 mM β-glycerophosphate (Sigma) and 50 μg/ml ascorbic acid (Sigma). To induce adipogenic differentiation, 1 × 104 rat MSCs were seeded in 24-well plates for 10 days in α-MEM supplemented with 10% FCS, 1% penicillin-streptomycin, 10-8 M dexamethasone (Sigma), and 5 μg/ml insulin (Sigma).
Alkaline phosphatase activity assay
After 7 days of treatment, the cells grown under osteogenic media were harvested and resuspended in 250 μl of culture supernatants, followed by cell breaking with an ultrasound breaker. After centrifugation, the Alkaline phosphatase (ALP) activities in the cell supernatants were quantified by an (ALP) Detection Kit (Nanjing Jiancheng Biotechnology Institute, Nanjing) and a spectrophotometer (Bio-Rad, Hercules, CA) at a wavelength of 520 nm. Each value was normalized to the protein concentration.
Von Kossa staining
Von Kossa staining was performed as previously described[21] with minor modification. After 21 days of treatment, the cells grown under osteogenic media were washed three times with PBS, and fixed in 4% formaldehyde for 30 min. The cells were then washed three times with distilled water and incubated in 5% silver nitrate (Sigma) for 1 h under UV light. The silver nitrate solution was removed and the cells were washed three times with distilled water. Five percent sodium thiosulfate was added for 5 min and the plates were then rinsed with water. The mineralized nodules/well was counted.
Oil red O staining
Oil red O staining was performed as previously described.[22] 0.5% Oil Red O solution (Sigma) was used. Briefly, adipocytes were fixed in 1% formaldehyde, washed in Oil red O for 20 min, rinsed with 85% propylene glycol (Sigma) for 3 min, washed in distilled water and mounted with aqueous mounting medium.
RNA isolation and real-time PCR
Total RNA was isolated from the cells using RNAiso Reagent (Takara Bio Inc., Kyoto), according to the manufacturer's instructions. cDNA was prepared by using PrimeScript II 1st Strand cDNA Synthesis Kit (Takara). For real-time PCR quantification, the GAPDH gene was used as an endogenous control to normalize for differences in the amount of total RNA in each sample. Real-time PCR was carried out in a 25 μl volume with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Reactions were carried out and fluorescence was detected on an ABI 7500 system (PE Applied Biosystems). Primer sequences for real-time PCR were listed as follows: GAPDH: 5′-GACATGCCGCCTGGAGAAAC-3′ (forward) 5′-AGCCCA
GGATGCCCTTTAGT-3′ (reverse); Runx2: 5′-GCCGGGAATGATGAGAACTA-3′ (forward) and 5′-GGACCGTCCACTGTCACTTT-3′ (reverse); ALP: 5′-ACGGTGAACGGGAGAAC-3′ (forward) and 5′-CTCAGAACAGGGTGCGTAG-3′ (reverse); PPARγ2: 5′-CTGATGCACTGC
CTATGAGC-3′ (forward) and 5′-TGGACTCCATAGTGGAAGCC-3′ (reverse); C/ EBPα: 5′-AGAACAGCAACGAGTACCGG-3′ (forward) and 5′-TTGACCAAGGAGCTCTCAGG-3′ (reverse); LPL: 5′-ACTGCCACTTCAACCACAGC-3′ (forward) and 5′-AATACTTCGACCAG
GCGACC-3′ (reverse); αp2: 5′-CGTCTCCAGTGAGAACTTCG-3′ (forward) and 5′-TCATGA CACATTCCACCACC-3′ (reverse).
Western blot analysis
Protein extracts were resolved on SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membrane, blocked with 5% nonfat milk, and probed with Abs specific for HO-1 (Stressgen Biotechnologies, Victoria) and β-actin (Sigma). Signals were detected with horseradish peroxidase-labeled secondary Abs using chemiluminescence labeling.
Statistical analysis
All of the results were expressed as means ± SEM. Differences among groups were analyzed by one-way ANOVA. In the case of two groups, a Student's t-test was used. P values less than 0.05 were considered significant.
RESULTS
The morphology of rMSCs in culture
The primary rMSCs showed either a fibroblast-like or a monocyte-like morphology. By day 7, the fibroblast-like cells were predominant, and the colonies were seen to arise from single cells [Figure 1a]. By day 13, the colonies were grown to confluency [Figure 1b]. The P3 rMSCs were then cultured in osteoblast differentiation medium, and showed a change in their morphology from fibroblast-shape to cuboid in as little as 4 days and were more apparent by days [Figure 1c]. In adipogenic differentiation, the rMSCs cultured in adipogenic medium changed from a typical fibroblast-shape to polygonal cells with perinuclear accumulations of fat droplets in the cytoplasm [Figure 1d].
Figure 1.

The morphology of rMSCs. The MSCs were derived from bone marrow of rats: (a) 7 days after plating, (b) 13 days after plating, (c) 9 days after cultured in osteogenic medium, (d) 10 days after cultured in adipogenic medium. Magnification × 100 (at column width)
Curcumin increases osteoblast differentiation of rMSCs
To determine the effect of curcumin on the onset of osteoblast differentiation of rMSCs, we analyzed ALP activity in rMSCs that were treated with different concentrations of curcumin for 9 days. The effect of increasing concentrations of curcumin on ALP activity is shown in Figure 2a. Curcumin at 10 μM and 15 μM significantly increased ALP activity in rMSCs as compared with control group (P < 0.01). We further examined the effect of curcumin on osteoblast maturation by detecting the mineralization of rMSCs. The results showed that incubation of rMSCs with curcumin at 10 μM and 15 μM had no effect on mineralization as determined by von Kossa staining [Figure 2b]. These results suggest that curcumin could increase the osteoblast differentiation of rMSCs only at an earlier stage.
Figure 2.

The effect of curcumin on osteoblast differentiation of rMSCs. Cells were cultured in 24-well plates and treated for indicated days in osteogenic medium with or without curcumin. (a) ALP activity was measured at day 9 in rMSCs. Values are expressed as means ± SEM. **P < 0.01. (b) Von Kossa staining was performed at day 21 as described in the Materials and Methods section. Magnification × 100 (at column width)
Curcumin reduces adipocyte differentiation of rMSCs
Curcumin can affect both bone health and fat formation. It has been reported that curcumin could suppress adipocyte differentiation.[12] To determine the effect of curcumin on adipocyte differentiation of rMSCs, the cells were cultured in adipogenic medium for 10 days, and stained with Oil Red O. Red areas in Figure 3 indicate lipid droplets produced by adipocyte differentiation. Treatment with curcumin reduced Oil Red O staining at 10 and 15 μM (P < 0.05).
Figure 3.
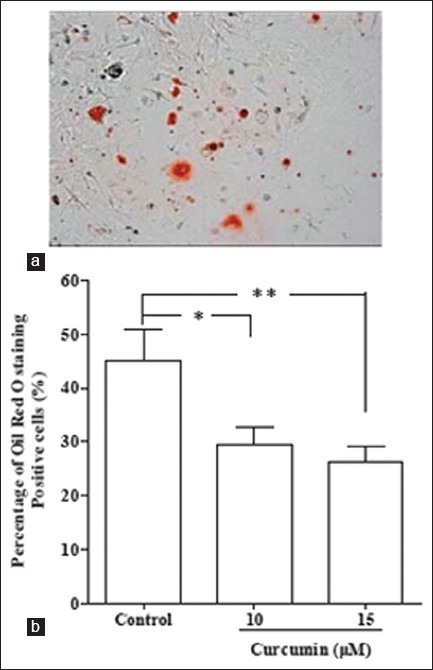
The effect of curcumin on adipocyte differentiation of rMSCs. Cells were cultured in 24-well plates and treated for 10 days in adipogenic medium with or without curcumin. (a) Oil red O staining was performed as described in the Materials and Methods section. Magnification × 100. (b) The number of adipocyte was counted and reported as a percentage of total stem cells. Values are expressed as means ± SEM. *P < 0.05, **P < 0.01 (at column width)
Curcumin reduces adipocyte gene expression and increases osteoblast gene expression of rMSCs
The differentiation of MSCs into osteoblasts is critically dependent on Runx2.[23] To provide further evidence that the effect of curcumin on ALP activity was due to changes in osteoblast differentiation, we detected the effects of curcumin on the expression of Runx2 and osteocalcin in osteogenic cultures. Curcumin at 10 μM and 15 μM increased Runx2 and osteocalcin expression as compared with control group (P < 0.01) [Figure 4a]. We next detected the expression of adipocyte-specific genes such as PPARγ2, LPL, αP2, and C/EBPα in adipogenic cultures. Curcumin at 10 μM and 15 μM significantly decreased the expression of PPARγ2 and C/EBPα (P < 0.01) [Figure 4b]. However, curcumin had no significant effects on LPL and αP2 expression (data not shown).
Figure 4.

The effect of curcumin on osteogenic and adipogenic genes in rMSCs. (a) Cells were cultured for 21 days in osteogenic medium with or without curcumin. Total RNA was isolated and analyzed by real-time PCR as described in the Materials and Methods section. (b) Cells were cultured for 10 days in adipogenic medium with or with out curcumin. Total RNA was isolated and analyzed by real-time PCR as described in the Materials and Methods section. Values are expressed as means ± SEM. **P < 0.01 (at column width)
Curcumin increases HO-1 expression
It has been reported that curcumin dose-dependently induces HO-1 expression. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. To investigate the mechanism that curcumin increases osteoblasts but decreases adipocyte lineage, we examined the HO-1 expression after treatment with curcumin. Curcumin at the concentration of 10 μM significantly increased the expressions of HO-1 mRNA and protein in rMSCs after 15 days of osteoblast differentiation [Figure 5a and 5b]. These results suggest that curcumin may increase the osteoblast differentiation of rMSCs through upregulation of HO-1 expression.
Figure 5.

Curcumin increases the expression of HO-1. Cells were cultured for 15 days in osteogenic medium with or without curcumin. (a) Total RNA was isolated and analyzed by real-time PCR as described in the Materials and Methods section. (b) The expression of HO-1 was analyzed using western blot. Values are expressed as means ± SEM. **P < 0.01 (at column width)
DISCUSSION
In the present study we show, for the first time, that curcumin increases osteoblast differentiation of rMSCs with reduction in adipocytes. The increased osteoblast differentiation is determined by an increase in ALP activity and the expressions of Runx2 and osteocalcin mRNA, while the decreased adipocyte differentiation is determined by a reduction in Oil red O staining and the expressions of PPARγ2 and C/EBPα mRNA. Moreover, an increase in HO-1 expression by curcumin is consistent with the increased osteoblast differentiation from rMSCs. Thus, our findings suggest that curcumin promotes osteoblast differentiation of rMSCs by upregulation of HO-1.
Bone marrow MSCs are precursors of different mesenchymal cell lineages, including osteoblasts, adipocytes, and chondroblasts. Several factors have been reported to be involved in the differentiation of MSC such as VitD3,[24] bone morphogenetic proteins,[25] and osteogenic growth peptide (OGP). OPG can regulate the commitment of MSCs into osteogenic or adipogenic lineages,[26–28] suggesting factors that influence the balance between osteoblast and adipocyte differentiation could lead to changes in body composition. Understanding the role of these factors could have important implications for treatment of osteoporosis and other bone diseases.
Curcumin is a diferuloylmethane derived from Curcuma longa. The role of curcumin in bone metabolism has been the subject of studies. Some studies have reported the antiosteoclastogenic properties of curcumin. Others have reported that curcumin markedly inhibited the proliferation of rat calvarial osteoblastic cells and induced the death of human osteoblast.[26,29] However, the effect of curcumin on osteoblast differentiation from rMSCs remains unknown.
ALP can be detected in the early stage of osteogenic differentiation from MSCs,[30] during which MSCs have not acquire mature osteoblast phenotype. In this study, we found that curcumin increased ALP activity of rMSCs, which suggests that curcumin could increase the osteogenic differentiation of rMSCs. However, even at day 21 after cultured in osteogenic medium, we did not find the increased mineralization of rMSCs. This result is consistent with previous study that administration of curcumin cannot efficiently improve the bone mineralization in ovariectomized rats.[31] Considering the fact that mature osteoblasts perform the matrix production and mineralization,[32] our data indicate that curcumin promotes osteogenesis at the level of commitment rather than at the level of maturation. Although von Kossa staining showed that curcumin did not change the mineralization of rMSCs, real-time PCR showed that curcumin enhanced the expressions of Runx2 and osteocalcin mRNA. The transcription factor Runx2 is a critical regulator of osteoblast differentiation, and osteocalcin is a marker of osteoblastic differentiation. These results lend further evidence that curcumin can enhance the osteoblast differentiation of rMSCs.
In contrast to the osteogenic effect, curcumin significantly inhibited the adipogenic differentiation of rMSCs. Curcumin reduced Oil red O staining compared with nontreated group. Moreover, curcumin decreased the expression of the adipogenic differentiation gene. PPARγ2 is a critical transcription factor involved in adipogenic differentiation.[33] In our study, PPARγ2 was decreased markedly in rMSCs treated with curcumin. It has been reported that PPARγ2 indirectly inhibits osteoblast differentiation by suppressing Runx2 gene expression,[34] which is consistent with our findings that curcumin increased Runx2 gene expression. In the present study, curcumin corrected osteoblastogenesis and adipogenesis indicates that curcumin may promote osteoblast differentiation at the expense of the adipogenic differentiation.
A previous study has shown that HO-1 can positively regulate osteoblast differentiation, and negatively regulates adipocyte stem cell differentiation.[20] HO-1 expression is essential for osteoblast differentiation of MSCs. In our study, curcumin significantly increased the expressions of HO-1 mRNA and protein. Curcumin increased the expression of ALP activity and Runx2 mRNA, which is consistent with the role of HO-1 on the osteoblast differentiation of MSCs. The relationship between HO-1 and curcumin has received extensive attentions.[35,36] Our results suggest that curcumin may promote osteoblast differentiation of rMSCs through the upregulation of HO- 1. Curcumin can activate MAP kinase pathways in tumor cells resulting in the induction of the expression of HO-1.[37] It has also been reported that curcumin can enhance neurogenesis via MAP kinase-mediated mechanism,[38] suggesting curcumin can exert important effects on MAP kinase pathways. MAP kinase pathway is an important signal transduction pathway that might regulate differentiation of MSCs into osteoblasts. Many growth factors that activate MAP kinase can inhibit adipocyte differentiation. Leptin-induced MAP kinase activation may cause a reduction in the adipogenic differentiation by phosphorylating PPAR.[39] These findings suggest that curcumin may regulate the differentiation of rMSCs through MAP kinase pathway. However, to clarify the mechanism of curcumin regulating the differentiation of rMSCs needs further investigation.
CONCLUSION
We show here that curcumin, a naturally occurring compound, can directly increase rMSCs-mediated osteoblasts with reduction in adipocytes. Moreover, curcumin may promote osteoblast differentiation of rMSCs by upregulation of HO-1. The results of this study may offer strategy in the management of metabolic disorders related diseases such as osteoporosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Otto WR, Rao J. Tomorrow's skeleton staff: mesenchymal stem cells and the repair of bone and cartilage. Cell Prolif. 2004;37:97–110. doi: 10.1111/j.1365-2184.2004.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vats A, Tolley NS, Buttery LD, Polak JM. The stem cell in orthopaedic surgery. J Bone Joint Surg Br. 2004;86:159–64. doi: 10.1302/0301-620x.86b2.14756. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102:341–51. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 5.Hong JH, Yaffe MB. TAZ: a beta-catenin-like molecule that regulates mesenchymal stem cell differentiation. Cell Cycle. 2006;5:176–9. doi: 10.4161/cc.5.2.2362. [DOI] [PubMed] [Google Scholar]
- 6.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 8.Biswas S, Rahman I. Modulation of steroid activity in chronic inflammation: a novel anti-inflammatory role for curcumin. Mol Nutr Food Res. 2008;52:987–94. doi: 10.1002/mnfr.200700259. [DOI] [PubMed] [Google Scholar]
- 9.Bharti AC, Takada Y, Aggarwal BB. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol. 2004;172:5940–7. doi: 10.4049/jimmunol.172.10.5940. [DOI] [PubMed] [Google Scholar]
- 10.Ozaki K, Kawata Y, Amano S, Hanazawa S. Stimulatory effect of curcumin on osteoclast apoptosis. Biochem Pharmacol. 2000;59:1577–81. doi: 10.1016/s0006-2952(00)00277-x. [DOI] [PubMed] [Google Scholar]
- 11.Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am J Physiol Cell Physiol. 2010;298:C1510–6. doi: 10.1152/ajpcell.00369.2009. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173–99. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–12. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 14.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 15.Drechsler Y, Dolganiuc A, Norkina O, Romics L, Li W, Kodys K, et al. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J Immunol. 2006;177:2592–600. doi: 10.4049/jimmunol.177.4.2592. [DOI] [PubMed] [Google Scholar]
- 16.Ogborne RM, Rushworth SA, O’Connell MA. Alpha-lipoic acid-induced heme oxygenase-1 expression is mediated by nuclear factor erythroid 2-related factor 2 and p38 mitogen-activated protein kinase in human monocytic cells. Arterioscler Thromb Vasc Biol. 2005;25:2100–5. doi: 10.1161/01.ATV.0000183745.37161.6e. [DOI] [PubMed] [Google Scholar]
- 17.Rushworth SA, Chen XL, Mackman N, Ogborne RM, O’Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol. 2005;175:4408–15. doi: 10.4049/jimmunol.175.7.4408. [DOI] [PubMed] [Google Scholar]
- 18.Abraham NG. Molecular regulation--biological role of heme in hematopoiesis. Blood Rev. 1991;5:19–28. doi: 10.1016/0268-960x(91)90004-v. [DOI] [PubMed] [Google Scholar]
- 19.Abraham NG, Nelson JC, Ahmed T, Konwalinka G, Levere RD. Erythropoietin controls heme metabolic enzymes in normal human bone marrow culture. Exp Hematol. 1989;17:908–13. [PubMed] [Google Scholar]
- 20.Vanella L, Kim DH, Asprinio D, Peterson SJ, Barbagallo I, Vanella A, et al. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236–43. doi: 10.1016/j.bone.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu R, Ma T, Smith RL, Goodman SB. Polymethylmethacrylate particles inhibit osteoblastic differentiation of bone marrow osteoprogenitor cells. J Biomed Mater Res A. 2006;77:850–6. doi: 10.1002/jbm.a.30697. [DOI] [PubMed] [Google Scholar]
- 22.Bavendiek U, Zirlik A, LaClair S, MacFarlane L, Libby P, Schonbeck U. Atherogenesis in mice does not require CD40 ligand from bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2005;25:1244–9. doi: 10.1161/01.ATV.0000161420.55482.ef. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol Ther. 2005;12:247–53. doi: 10.1016/j.ymthe.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 24.D’Ippolito G, Schiller PC, Perez-stable C, Balkan W, Roos BA, Howard GA. Cooperative actions of hepatocyte growth factor and 1,25-dihydroxyvitamin D3 in osteoblastic differentiation of human vertebral bone marrow stromal cells. Bone. 2002;31:269–75. doi: 10.1016/s8756-3282(02)00820-7. [DOI] [PubMed] [Google Scholar]
- 25.Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS, Park SW, et al. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem. 2003;278:23270–7. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 26.Bab I, Gazit D, Chorev M, Muhlrad A, Shteyer A, Greenberg Z, et al. Histone H4-related osteogenic growth peptide (OGP): a novel circulating stimulator of osteoblastic activity. EMBO J. 1992;11:1867–73. doi: 10.1002/j.1460-2075.1992.tb05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg Z, Gavish H, Muhlrad A, Chorev M, Shteyer A, Attar-Namdar M, et al. Isolation of osteogenic growth peptide from osteoblastic MC3T3 E1 cell cultures and demonstration of osteogenic growth peptide binding proteins. J Cell Biochem. 1997;65:359–67. [PubMed] [Google Scholar]
- 28.Robinson D, Bab I, Nevo Z. Osteogenic growth peptide regulates proliferation and osteogenic maturation of human and rabbit bone marrow stromal cells. J Bone Miner Res. 1995;10:690–6. doi: 10.1002/jbmr.5650100504. [DOI] [PubMed] [Google Scholar]
- 29.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–8. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 30.Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995;17(2 Suppl):77S–83S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- 31.Folwarczna J, Zych M, Trzeciak HI. Effects of curcumin on the skeletal system in rats. Pharmacol Rep. 2010;62:900–9. doi: 10.1016/s1734-1140(10)70350-9. [DOI] [PubMed] [Google Scholar]
- 32.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103–8. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, et al. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–94. [PubMed] [Google Scholar]
- 34.Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS, Park SW, et al. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem. 2003;278:23270–7. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 35.Bao W, Li K, Rong S, Yao P, Hao L, Ying C, et al. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J Ethnopharmacol. 2010;128:549–53. doi: 10.1016/j.jep.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Lima CF, Pereira-Wilson C, Rattan SI. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: relevance for anti-aging intervention. Mol Nutr Food Res. 2011;55:430–42. doi: 10.1002/mnfr.201000221. [DOI] [PubMed] [Google Scholar]
- 37.McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19:165–72. [PubMed] [Google Scholar]
- 38.Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, et al. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283:14497–505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, et al. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem. 1997;272:12897–900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]


