Abstract
Background:
Mulberry (Morus, Moraceae) is widely distributed in the temperate, subtropical, or tropical regions of the world, while there are no conclusive reports on the chemical composition, nutritional value, and antioxidant properties of mulberry cultivars from China.
Objective:
To investigate chemical properties and to determine proximate nutritive compounds of the eight mulberry cultivars.
Materials and Methods:
Chemical properties (including moisture, ash, total dry matter, total soluble solids, pH, and total titratable acidity) of the eight mulberry cultivars were investigated. Proximate nutritive compounds (including crude protein, crude fat, mineral elements, total anthocyanins, total polyphenols, total flavonoids, and total sugars) were also determined.
Results:
The results indicated that the moisture contents were 70.0-87.4%, the crude protein contents 1.62-5.54%, and the crude fat contents from 1.23-2.23%. The major fatty acids in mulberry fruits were linoleic acid (C18:2) and palmitic acid (C16:0), 26.40-74.77% and 9.29-22.26%, respectively. Mulberry fruit is also a good source of minerals and the potassium content (521.37-1718.60 mg/100g DW) is especially higher than that of other elements. Compared with other species, the Morus atropurpurea Roxb. had relatively high total polyphenols content (189.67-246.00 mg GAE/100mg) and anthocyanins content (114.67-193.00 mg/100mg). There was a good linear correlation between antioxidant activity and total polyphenols content.
Conclusion:
Significant differences of the chemical composition, nutritional value, and antioxidant activities among the mulberry cultivars were observed, the Morus atropurpurea Roxb. showed considerable high nutritional value and antioxidant activity which could be developed for functional food that benefits human health.
Keywords: Anthocyanins, antioxidant activity, chemical composition, minerals, mulberry, polyphenols
INTRODUCTION
Mulberry (Morus, Moraceae) is widely distributed in the temperate, subtropical, or tropical regions of the world and can grow in a wide range of climatic, topographical, and soil conditions. There are 24 species of Morus and one subspecies with at least 100 known varieties.[1] Recently, the production and consumption of mulberry fruits are increasing rapidly because of their good taste, nutritional value, and biological activities. Mulberry fruits can be used as a worming agent, a remedy for dysentery, and a laxative, odontalgic, anthelmintic, expectorant, hypoglycemic, and emetic.[1] In traditional Chinese herbal medicine, mulberry fruits have been used in folk medicine to treat diabetes, hypertension, anemia, and arthritis.[2] Anthocyanins are the most important constituent of mulberry fruits, which are a group of naturally occurring phenolic compounds that are responsible for the color attribute and biological activities such as antioxidant, antimicrobial, and neuro-protective, anti-inflammatory properties.[3–6]
There are 29 mulberry varieties registered by the National Mulberry and Silkworm Apprising Committee and more than 10 by provincial mulberry variety identification have been released in China.[7] The mulberry fruits, even from the same species, may contain different amounts of chemical composition as well as different antioxidant properties. Different cultivars may be developed for their suitable application such as diet fruits consuming, juices, marmalades, liquors or food process, natural dyes, and cosmetics industry. Furthermore, it is unknown about the correlations between antioxidant activities and major active components in mulberry fruits. Among the known mulberry species in China, only a few species have been analyzed for their phytochemical profiles and developed for food or food additives. There are no conclusive report on the chemical composition, nutritional value, and antioxidant properties of mulberry cultivars from China.
In our study, a compositional comparison between different mulberry cultivars was undertaken aiming at exploiting the nutrient profiles of mulberry fruits and promoting the further development of the rich mulberry resources. The present work was to investigate the eight mulberry cultivars from China in terms of chemical properties (including moisture, ash, total dry matter, total soluble solids, pH, and total titratable acidity), to determine proximate nutritive compounds (including crude protein, crude fat, mineral elements, total anthocyanins, total polyphenols, total flavonoids, and total sugars), and to evaluate the correlations between the active components and their antioxidant activities.
MATERIALS AND METHODS
Materials
Eight mulberry cultivars, belonging to four species, were obtained from the National Mulberry Gene Bank of the Sericultural Research Institute, Chinese Academy of Agricultural Sciences (Zhenjiang, Jiangsu, China). Cultivars of Zhongsang 5801, Dashi, Guang 7200 and Jushensang belong to Morus atropurpurea Roxb., Damo4××guiV-4 and Hongyayizhilaishisheng99-6 belong to Morus multicaulis Perr., Yaan 3 is Morus cathayana Hemsl., and Lvshenzi is Morus alba Linn. All different cultivars of mulberry fruits were manually picked at ripe stage in May 2009 and immediately transported to our laboratory. On the same day, the mulberry fruits were selected according to uniformity of shape and color to eliminate damage material and then each cultivar were divided into three groups.
The first group was stored in polyethylene bags at -18 °C (up to 1 month) until proximate analysis. The second group was air-dried in laboratory and ground to fine powder, packed into new plastic bags and stored in a desiccator prior to crude protein, crude fat, fatty acid, and minerals analysis. The third group: each cultivar of fruits (10 g) were triturated, homogenized, and treated with 100 ml of 80% ethanol aqueous solution containing 0.1% hydrochloric acid. The extraction was carried out at 50 °C for 90 min and each sample was centrifuged at 4000 g for 10 min. The supernatant was used for the determination of total anthocyanins, total polyphenols, total flavonoids, total sugars, and antioxidant activity.
Proximate analysis
Proximate analysis was performed according to official methods procedures[8] including moisture, ash, total dry weight (TDW), total soluble solid contents (TSS), pH, total titratable acidity (TAC), crude fat, and crude protein.
The moisture of each cultivar was determined by weighing the fresh samples before and after drying at 103 ± 2°C for 24 h, cooling in a desiccator, and weighing until a constant weight. Ash was determined by weighing the incinerated residue obtained by Muffle Furnace at 550°C until they reached constant weight. TDW was determined by oven drying at 70°C until a constant weight and TSS was measured at 20°C on an Abbe refractometer (WYS-2S, Shanghai Precision Instrument Co., China). The pH was determined at 20°C with a digital pH meter calibrated with pH 4.00 and pH 6.86 buffer solutions. TAC was measured by potentiometric titration and calculated as % citric acid.
Crude fat was obtained by exhaustively extracting 5.0 g sample power in a Soxhlet apparatus using petroleum ether (boiling point range 60-90°C) as extractant. Nitrogen content was determined by using the Kjeldahl method and multiplied by a factor of 6.25 to determine the crude protein content.
Analysis for fatty acid content
The gas chromatography-mass spectrometry (GC-MS) was used to analyze the fatty acid compositions of each cultivar. The fatty acids after trans-esterification were separated on an Agilent HP-6890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) with a HP-5MS 5% phenylmethylsiloxane capillary column (30 m × 0.25 mm i.d., film thickness 0.25 μm; Restek, Bellefonte, PA). Injector and ion source temperatures were set at 250°C and 200°C, respectively. Oven temperature was held at 160°C for 1 min, increased to 210°C at 10°C·min-1 and held for 2 min, then increased to 215°C at 0.2°C·min-1 and held for 3 min, and last increased to 250°C at 5°C·min-1 and held for 1 min. Each sample (1 μl) was injected into the column at a split ratio of 15:1. Helium was carrier gas at a flow rate of 1.0 ml·min-1. The mass spectrometer was operated in electron-impact ionization (EI) mode by the energy of 70 eV. The scanning range was 30-450 amu and the rate was 0.2 s·scanning-1. The individual identification of components was based on the matching of their recorded mass spectra with those of NIST05.LIB and NIST05s. LIB (National Institute of Standards and Technology) libraries data provided by the software of GC-MS system.
Determination of mineral analysis
The mineral elements including potassium (K), sodium (Na), phosphorus (P), calcium (Ca), magnesium (Mg), zinc (Zn), iron (Fe), copper (Cu), manganese (Mn), chromium (Cr), selenium (Se), and arsenic (As) were determined after the wet-digestion according to the method described by Leterme et al,[9] with slight modification. Each sample (0.2 g) was digested with 8 ml mixture of HNO3 and HClO4 (4:1, v/v) at room temperature overnight and heated at 130°C for 1 h till a clear solution was obtained (about 2 ml). The solution was subsequently transferred to a 25 ml volumetric flask with ultrapure water after cooling down. A blank digest was carried out in the same way. For As and Se, the treatment was the same as above except the need of adding 5 ml HCl (6 mol·l-1) and incubating at 25°C for 20 min after cooling. The digested products were then used for the determination of K, Ca, Mg, Cu, Fe, Zn, and Mn by TAS-986 atomic absorption spectrometer (Purkinje General Instrument Co., Ltd, Beijing, China). The contents of Na, P, and Cr were determined by Vista-MPX Simultaneous ICP-OES (Varian, Inc. USA) and As and Se contents were measured by AFS-930 hydride generation atomic fluorescence spectrometry (Beijing Titan Instruments Co., Ltd., China).
Determination of total anthocyanins, polyphenols, flavonoids and sugars content
Total anthocyanins content was directly determined by pH differential method.[10] Absorbance was measured at 513 and 700 nm and the results were expressed as cyanidin-3-glucoside (Cy-3-glc, molar extinction coefficient of 26900 L·cm-1·mol-1 and molecular weight of 449.2 g·mol-1).
Total polyphenols content was determined by the Folin-Ciocalteu method[11] with some modifications. An aliquot of 1 ml sample solution was mixed with 1.5 ml of 7.5% sodium carbonate and 1 ml of 0.1 M Folin-Ciocalteu reagent. After incubation at room temperature for 30 min in the dark, the absorbance of the reaction mixture was measured at 765 nm against reagent blank. Gallic acid was chosen as a standard and got the standard curve. The results were presented in mg gallic acid equivalent (GAE)/100 g of sample on a dry weight basis (DW).
The total flavonoids content was determined by the aluminum chloride colorimetric method described by Makris et al,[12] with slight modification. An aliquot of 1 ml sample solution appropriately diluted with 95% alcohol was mixed with 4 ml distilled water in a tube, added 0.3 ml 5% NaNO2, and allowed to react for five min. Following this, 0.3 ml 10% AlCl3 was added and the mixture was allowed to stand for further five min. Finally, 2 ml 1 M NaOH and 2.4 ml distilled water were added to the reaction mixture. The absorbance was measured at 510 nm against a blank that had been prepared in a similar manner by replacing the extract with distilled water. Rutin was used as standard compound for the quantification of total flavonoids. All values were expressed as mg of rutin equivalent (RE)/100 g DW.
Total sugars content was determined according to the phenol-sulfuric method described by Masuko et al,[13] with slight modification. Briefly, an aliquot of 0.3 ml of sample solution mixed with 0.6 ml 5% phenol and 3 ml sulfuric acid. The absorbance at 490 nm was measured after the reaction mixture incubation for 30 min at 37°C. Anhydrous dextrose was used as standard compound for calibration. All values were presented in mg anhydrous dextrose equivalent (DE)/100 g DW.
Determination of antioxidant activity
DPPH assay
The DPPH scavenging activity of each sample was determined as described by Su et al,[14] with some modification. Extracts of mulberry fruits were tested at a polyphenols concentration of 0.1 mmol·l-1 (GAE). Briefly, 100 μmol/L of DPPH in ethanol was prepared and 2.0 ml of this solution was added to a test sample (2.0 ml). The reaction mixture was shaken well and incubated for 30 min at room temperature in the dark. The absorbance of the resulting solution was measured at 517 nm against a blank. Ascorbic acid was used as a positive antioxidant control. The radical-scavenging activity (RSA) was calculated as a percentage of DPPH discoloration using the equation I %= [1-(Ai-Aj)/A0] ×100, where A0 was the absorbance of the blank sample, Ai was the absorbance in the presence of the different test samples and Aj was the absorbance of the blank reagent.
Hydroxyl radical-scavenging assay
Hydroxyl radical-scavenging assay was carried out by using Fenton's reaction method described by Kuda and Ikemori[15] with some modifications. An aliquot of 1.0 ml sample solution, 2.0 ml of 0.75 mmol·l-1 phosphate buffer (pH 7.4), 1.0 ml 0.75 mmol·l-1 1, 10-phenanthroline ethanol solution were added in the tube and then 1.0 ml of 0.75 mmol·l-1 FeSO4 were added and mixed thoroughly. Hydroxyl radical was generated by adding of 1.0 ml 0.01% H2O2. The mixture was incubated at 37°C for 60 min and the absorbance was measured at 536 nm. The RSA was calculated as the percent reduction of OH I %= [(Ai-A0)/(A-A0)] ×100, where Ai was the absorbance of test samples, A0 was the absorbance of blank solution containing H2O2, A was the absorbance of control solution containing without H2O2 replaced with distilled water.
Statistical analysis
One way analysis of variance (ANOVA) with multiple ranges significant difference (LSD) test (P < 0.05; P < 0.01) were carried out by Statistical Package for the Social Sciences (SPSS) version 16.0 software (SPSS Inc., Chicago, USA). Data are reported as mean standard ± deviation (SD) for three replicates.
RESULTS AND DISCUSSION
Proximate analysis of mulberry fruits
The statistical differences in terms of moisture, ash, pH, TAC, TSS, TDW, crude protein, and crude fat contents of each mulberry cultivar were showed in Table 1. The moisture considered in this study varied from 70.0% (Yaan 3) to 87.4% (Guang 7200) and the fruits of Morus atropurpurea Roxb. tend to have comparable higher moisture contents for Zhongsang 5801, Dashi, Guang 7200, and Jushensang cultivars were all above 85%. The moisture of fresh mulberry grown in the East Anatolia Region of Turkey ranged from 71 to 75% with a very low variation among the three different cultivars (Morus alba, Morus rubra, and Morus nigra).[1]
Table 1.
Proximate composition of different mulberry cultivars*
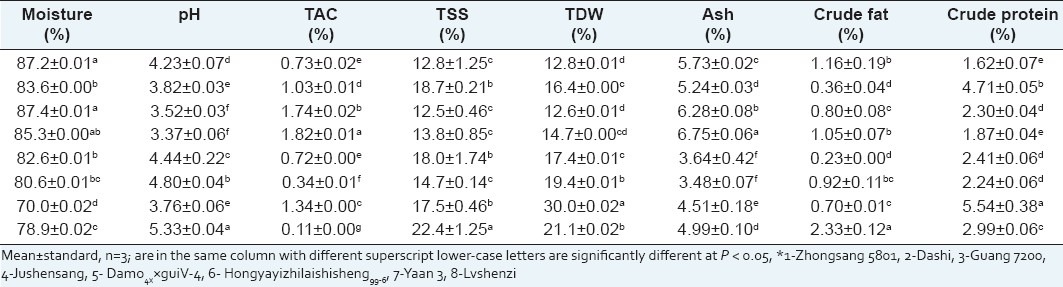
Ash values were significantly different amongst the cultivars and lowest contents were found for Morus multicaulis Perr. with the values of 3.64% (Damo4××guiV-4) and 3.48% (Hongyayizhilaishisheng99-6), respectively. Compared to other cultivars, Morus atropurpurea Roxb. had higher ash content for Zhongsang 5801, Dashi, Guang 7200, and Jushensang cultivars with 5.73%, 5.24%, 6.28%, and 6.75%, respectively.
The pH of the eight mulberry fruits were from 3.37 (Jushensang) to 5.33 (Lvshenzi) and the Morus multicaulis Perr. has relatively high pH values of 4.80 (Hongyayizhilaishisheng99-6) and 4.44 (Damo4××guiV-4). These values were consistent with previously reported results for mulberry cultivars grown in Turkey (pH 3.52-5.60).[1,16] As for TAC, the value was from 0.11% (Lvshenzi) to 1.82% (Jushensang). Both the amounts and compositions of the organic acids were found to be variable for the tested species, the main organic acids were citric and malic acids, a trace amounts of tartaric and ascorbic acid.[2] It might be presumed that the different contents of these organic acids in different mulberry cultivars might be related with the variation of their pH.
As for TDW, the cultivar of Yaan 3 showed the highest value 30.0%, followed by Lvshenzi, Hongyayizhilaishisheng99-6, and Damo4××guiV-4 with 21.1%, 19.4% and 17.4%, respectively. It could be observed that the cultivars of Morus atropurpurea Roxb. has comparable low TDW compared with other cultivars which might be related with their high moisture contents. The TSS values were significantly different amongst the cultivars varied from 12.5% (Guang 7200) to 22.4% (Lvshenzi). The cultivars of Morus atropurpurea Roxb. had relatively low TSS at average 13.0% except Dashi (18.7%).
The variation of TDW, TSS, pH, and TAC in different mulberry cultivars could be result of heterozygote nature of seed propagated genotypes, different species, the climate, growth and geographical conditions where grown.[17,18] According to the results, the Lvshenzi cultivar (Morus alba L.) fruit might be recommended for processing due to relatively high TSS and TDW contents. High fruit TSS and acid content is a combination for fruit juice process found only in a few other fruits such as pomegranate and kiwifruit. Therefore, the Dashi cultivar seems the most promising, combined for more bright and red fruits appreciably high TSS and acid content, which may be especially useful in developing cultivars with greater agronomic potential. The Morus atropurpurea Roxb. might be proposed for fresh fruit production, since it has attractive full fruits with flesh plump, bright color, bigger particle, and higher moisture.
The crude protein contents of the eight mulberry cultivars were given in Table 1. The cultivar Yaan 3 was found to be the highest protein values (5.54%) followed by Dashi (4.71%), Lvshenzi (2.99%), Damo4××guiV-4 (2.41%), Guang 7200 (2.30%), Hongyayizhilaishisheng99-6 (2.24%), Jushensang (1.87%), and Zhongsang 5801 (1.62%). The crude protein content of Yaan 3 was appreciably higher than other mulberry cultivars and thus was interesting for food products and dietary consumption.
The crude fat contents of the mulberry cultivars were low and ranged from 0.23% (Damo4××guiV-4) to 2.33% (Lvshenzi) as shown in Table 1. As for the Morus atropurpurea Roxb., the cultivars of Zhongsang 5801 and Jushensang have relatively high crude fat content 1.16 % and 1.05%, respectively. The results were close to a previous study that reported the total fat contents of the mulberry species were low, between 0.85% (Morus. rubra) and 1.10% (Morus. alba).[1]
The fatty acid composition
The fatty acid composition analysis was performed by GC-MS for the oil samples from mulberry fruits. There were 16 fatty acids found in test cultivars and significant variation showed in Table 2. Linoleic acid (C18:2) was the dominant fatty acid in all mulberry cultivars, ranged from 26.40% to 74.77%, followed by palmitic acid (C16:0) with the value 9.29%-22.26%. Linoleic acid is one of the two essential fatty acids that humans require because they cannot be produced by the human body. Deficiency symptoms may include dry hair, hair loss, and poor wound healing.[19]
Table 2.
Fatty acid composition of different mulberry cultivarsa
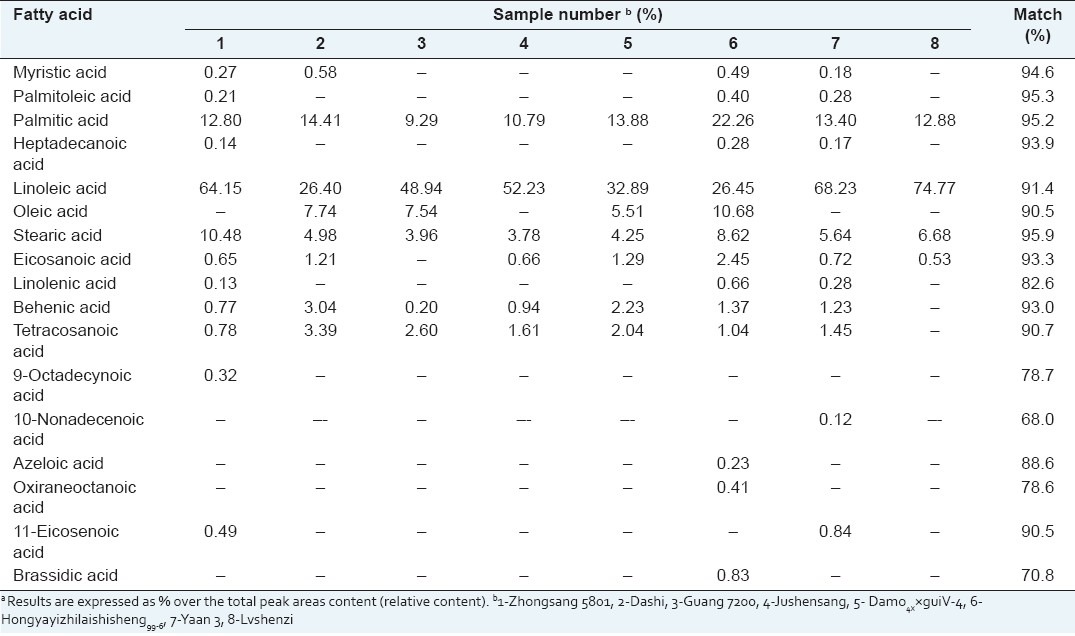
All cultivars contained stearic acid (C18:0) with the values of 3.78%-10.48%. Oleic acid (C18:1) was found with relatively high amount in Dashi, Guang 7200, Damo4××guiV-4, and Hongyayizhilaishisheng99-6, whereas not detected in other cultivars. Our results were comparable to the finding reported by Ercisli and Orhan,[1] who reported that the fatty acids found at the highest levels in three black mulberry fruits were linoleic acid (43.4%-61.9%), palmitic acid (12.1%-24.8%), and oleic acid (2.33%-16.01%), respectively.
Myristic acid (C14:0) was detected as minor amounts in Zhongsang 5801, Dashi, Hongyayizhilaishisheng99-6, and Yaan 3 fruits with 0.27%, 0.58%, 0.49%, and 0.18%, respectively. Palmitoleic acid (C16:1), margaric acid (C17:0), and linolenic acid were only found in Zhongsang 5801, Hongyayizhilaishisheng99-6, and Yaan 3 fruits. Behenic (C22:0) acid (0.20%-3.04%) and tetracosanoic (C24:0) acid (0.78%-3.39%) were detected in all mulberry cultivars except Lvshenzi and eicosanoic acid (0.53%-2.45%) was not found in Guang 7200. 11-Eicosenoic acid (C20:1) was only found in Zhongsang 5801 (0.49%) and Yaan 3 (0.84%). Hongyayizhilaishisheng99-6 was the only cultivar that contained brassidic acid (C22:1), azeloic acid (C11:0), and oxiraneoctanoic acid (C19:0) with the value of 0.23%, 0.41%, and 0.83%, respectively.
Linoleic acid, palmitic acid, and oleic acid generally constitute the major fatty acids of fruits. In any case, the fatty acid composition of the mulberry cultivars was of some similar (major compounds including palmitic, linoleic, stearic, behenic acid, tetracosanoic acid, and eicosanoic acid) in the mulberry cultivars studied and there were also appreciable differences among the fatty acids of minor amount. The differences among fatty acids might mostly depend on the species and physiological environments.
Mineral elements
The knowledge of the levels of trace elements in mulberry fruits is necessary because of their benefits for human health. Mulberry was a good source of minerals which could provide nutritionally useful amounts of most of them including K, Ca, Na, Mg, Cu, Fe, P, Mn, and Zn [Table 3]. There were significant differences in the levels of minerals among the test mulberry cultivars. The content of K was especially higher than other elements from 521.37 mg/100g DW (Hongyayizhilaishisheng99-6) to 1718.60 mg/100g DW (Guang 7200). The Na content was low and the K/Na ratio was relatively high which has been considered to be an advantage from the nutritional point of view, since the high content of K can be utilized beneficially in the diets of people who take diuretics to control hypertension and suffer from excessive excretion of K.[20]
Table 3.
Minerals contents of different mulberry cultivars*
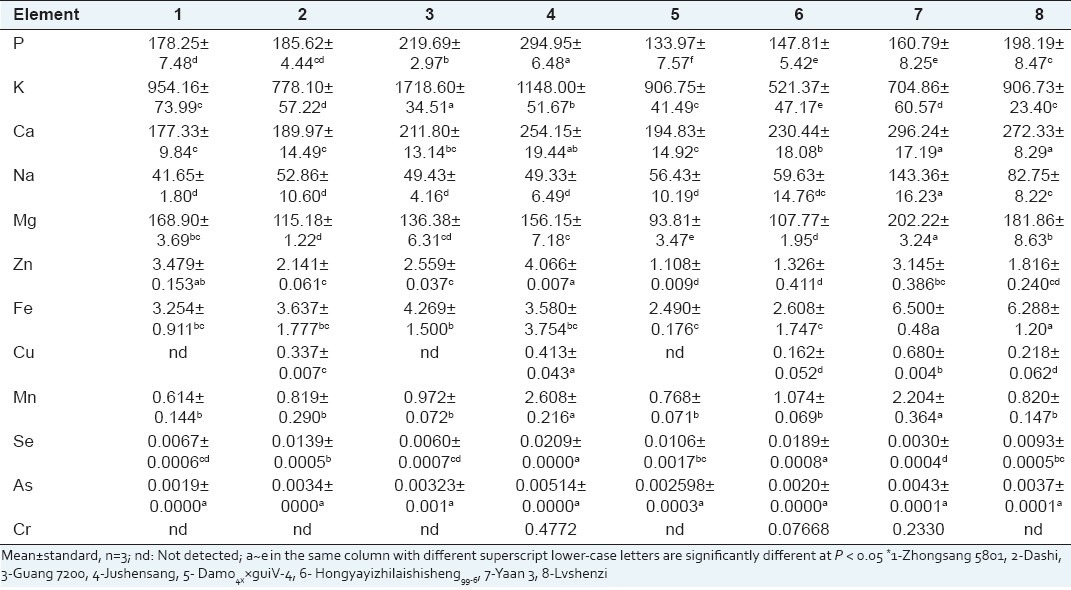
The content of P in mulberry fruits was also remarkable with the range of 133.97-294.95 mg/100g DW. The cultivar Jushensang showed the highest level (294.95 mg/100g DW) and the value was lower than that reported in strawberry.[21] Ca is important for bone growth and neurologic function, whereas Mg is required by many enzymes as cofactors and its dietary deficiency is linked with ischemic heart disease.[22] The relatively high amounts of Ca and Mg were also found in these fruits, and especially the Yaan3 cultivar, showed the highest contents, 296.24 mg/100g DW and 202.22 mg/100g DW, respectively.
Fe, Cu, Zn, and Mn are other minerals of nutritional importance in mulberry. Higher variations were observed for Fe among the mulberry cultivars ranged from 2.490 mg/100g DW (Damo4××guiV-4) to 6.500 mg/100g DW (Yaan 3). The Cu content varied from 0.162 mg/100g DW (Hongyayizhilaishisheng99-6) to 0.680 mg/100g DW (Yaan 3) with some exceptions for Zhongsang 5801, Guang 7200, and Damo4××guiV-4. Zn is especially important for the normal functioning of the immune system, whereas Mn could activate several enzymes which protect cells from free radicals attack and regulation of glucose homeostasis. The Zn and Mn contents among different cultivars were in the range of 1.108-4.066 mg/100g DW and 0.614-2.608 mg/100g DW, respectively. Their levels were very similar to other fruits such as strawberry, blackcurrant, redcurrant, and raspberry, etc.[23] Meanwhile, the Se, As, or Cr contents of all mulberry fruits were very low and the Se content was much higher compared to As. No Cr was detected in the five mulberry fruits such as Zhongsang 5801, Dashi, Guang 7200, Damo4××guiV-4, and Lvshenzi.
The mineral composition of mulberry fruits not only depended on the species or cultivars, but also on the growing conditions such as soil, climatic, geographical conditions, the addition of fertilizers, and cultural management techniques.[24] The results indicated that mulberry was a better source of minerals and could be recognized as a valuable horticultural product based on their rich and beneficial nutrient composition.
Total anthocyanins, polyphenols, flavonoids, and sugars content of mulberry cultivars
The total anthocyanins, polyphenols, flavonoids, and sugars content among different mulberry cultivars were statistically significant as given in Table 4. The total anthocyanins contents were from 19.00 mg/100g (Yaan 3) to 193.00 mg/100g (Jushensang), which were not detected in Lvshenzi. The Morus atropurpurea Roxb. was observed to be higher in anthocyanins content compared to other cultivars. Dashi contained comparable high amounts of anthocyanins (155.00 mg/100 g) which was in agreement with that reported by Song et al,[25] who also presented that the contents of anthocyanins in different species varied widely, and not only the cultivars, but also the plant growing environment could affect anthocyanins content.
Table 4.
Contents of total anthocyanins, polyphenols, flavonoids and sugars of mulberry cultivars*
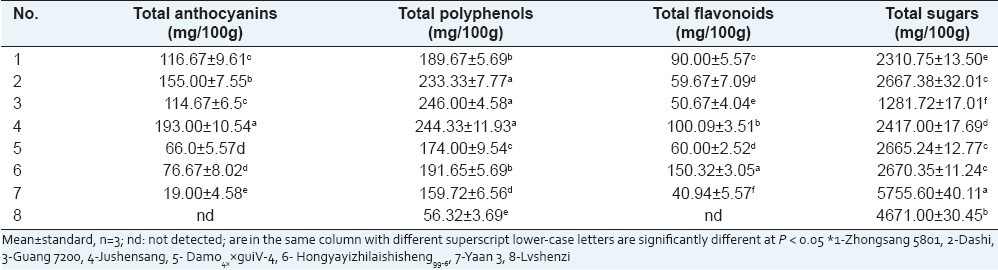
Mulberry was also a rich source of polyphenols and the total polyphenols clearly showed that fruits of Morus atropurpurea Roxb. (189.67-246.00 GAE mg/100mg DW) had high total polyphenols content and their wide differences among cultivars. Bae and Suh[26] found the similar amounts of total polyphenols in mulberries cultivated in Korea. The variation of phenolic compounds in the fruits depends on many factors such as the degree of maturity at harvest, genetic differences, and environmental conditions, etc. In red-colored fruits, phenolics increase during the last ripening stage due to the maximal accumulation of anthocyanins and flavonols.
Total flavonoids content in different cultivars widely varied from 40.94 RE mg/100g DW (Yaan 3) to 150.32 RE mg/100g DW (Hongyayizhilaishisheng99-6) and the exception was Lvshenzi cultivar. Previously published results of total flavonoids content of mulberry fruits in Turkey was higher than the results presented here probably because of the different species, growth areas, and extraction methods.[26]
Total sugars content is very important from a sensory viewpoint because it confers sweetness to the fruit and sweet mulberry are particularly appreciated for this sensory attribute. Among the considered cultivars, the Yaan 3 showed a total sugars content higher than the others. There was no obviously difference in Morus multicaulis Perr., and the total sugars contents of Damo4××guiV-4 and Hongyayizhilaishisheng99-6 were 2665.24 DE mg/100g DW and 2670.35 DE mg/100g DW, respectively. The Morus atropurpurea Roxb. was observed to be moderate level of total sugars (at average 2169.21 DE mg/100g) and the lowest content was detected in Guang 7200 with value of 1281.72 DE mg/100g [Table 4]. It could be observed that mulberry fruit contains moderate amounts of sugars compared to sweet cherry which averagely contains 150-230 g/kg.[27] It was slightly higher than that presented in previous study which showed the average sugar contents of M. nigra and M. rubra were 12.2% and 7.9%, respectively.[2]
Antioxidant activity
Phenolic compounds including anthocyanins, flavonoids, and phenolic acids are known to be responsible for antioxidant activities in fruits and the fruits with higher phenolic contents generally show stronger antioxidant activities.[28,29] The concept of antioxidant activities which describes the ability of different food antioxidants in scavenging preformed free radicals has been suggested as a tool for investigating the health effects of antioxidant-rich foods. Based on the DPPH and OH radicals scavenging assays, the mulberry extracts obtained from the eight cultivars had considerable high antioxidant activities [Figure 1]. The RSA of DPPH among the mulberry cultivars varied from 50% to 96%. The comparable high RSA was found in the cultivars Jushensang (96%) and Zhongsang 5801 (93%) in Morus atropurpurea Roxb., while Hongyayizhilaishisheng99-6 also had high DPPH RSA of 88%. The mulberry cultivars were found to have better ability to scavenge hydroxyl radicals and the four cultivars of Morus atropurpurea Roxb. exhibited considerable high RSA (92%-97%) with the same result of the DPPH assay. The result indicated that Jushensang exhibited the highest antioxidant activity, while the lowest was found in Lvshenzi which showed the lowest RSA values for both DPPH and OH radical scavenging assays (50.5% and 68.9%, respectively). It has been reported that the phytochemicals responsible for antioxidant activity most likely be accounted for phenolics (including non-anthocyanin phenolics), anthocyanins, and other flavonoid compounds.[30] Therefore, the antioxidant activity observed in the Lvshenzi cultivar which gave the absence of anthocyanins and flavonoids could be due to the small amount of total polyphenols content.
Figure 1.
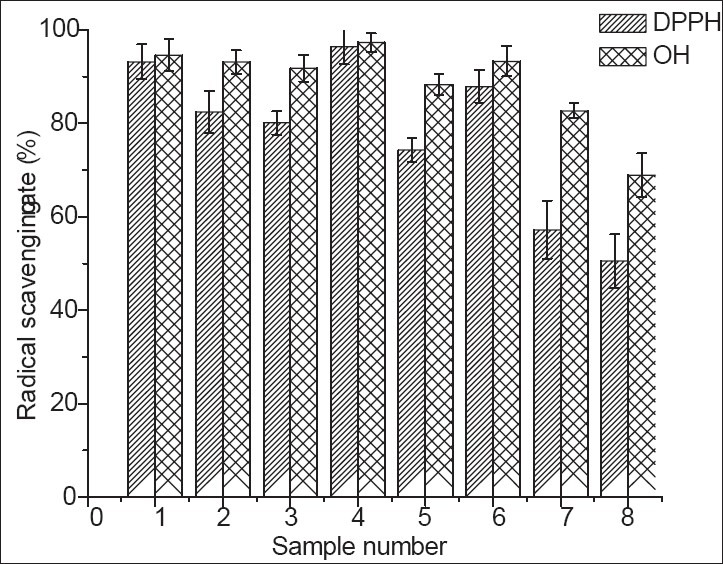
The scavenging radical ability of different mulberry cultivars on DPPH and OH. *1-Zhongsang 5801, 2-Dashi, 3-Guang 7200, 4-Jushensang, 5- Damo4××guiV-4, 6- Hongyayizhilaishisheng99-6, 7-Yaan 3, 8-Lvshenzi
It had been reported relatively higher levels of antioxidant activity in highly pigmented berries, pomegranate juices, red wine, green tea infusion,[31–33] and blueberries constituted one of the richest reported antioxidants.[34] Our results indicated that mulberry fruits could also be considered as a potential resource for antioxidants from dietary fruits consumption and the development of functional drinks.
Correlations between antioxidant activity and the contents of total anthocyanins (TA), total polyphenols (TP), total flavonoids (TF) and total sugars (TS)
Correlations between the antioxidant activities and the contents of TA, TP, TF, and TS were shown in Table 5. There was a good linear correlation between the antioxidant activity and TP in the extracts of mulberry determined by DPPH and OH radical scavenging assays. Previous literatures had shown that anthocyanins (especially monoglucosides of cyanidin and delphinidin) as well as non-anthocyanin phenolic (chlorogenic acid, kaempferol, quercetin, etc.) possess high antioxidant activity which could compare well with the activity of the well-known antioxidants α-tocopherol and trolox.[35] The antioxidant activity values were slightly better correlated to total polyphenols (TP vs. DPPH, R2= 0.8735: TP vs. OH, R2 = 0.9249) rather than to total anthocyanins (TP vs. DPPH, R2= 0.8463: TP vs. OH, R2= 0.7228). This conclusion was in agreement with previous findings obtained on blueberries and on red wines.[33,34] The correlations between the antioxidant activity and total flavonoids were TF vs. DPPH, R2=0.6432 and TF vs. OH, R2=0.5886, respectively. There was a negative linear correlation coefficient between the antioxidant activity and total sugars (TS vs. DPPH, R2=0.6373; TS vs. OH, R2=0.5318). There was a slightly better correlation between antioxidant activity and TP/TA as compared to that of TF/TS, which meant that polyphenols and anthocyanins significantly contributed to the antioxidant activities of mulberry fruits.
Table 5.
Correlations between antioxidant activity and the total anthocyanins, polyphenols, flavonoids and sugars

CONCLUSION
Significant differences of the chemical composition, nutritional value, and antioxidant activities among the mulberry cultivars were observed. The different cultivars could be exploited and made the best value according to their own nutritive value for different processes purpose. The mulberry fruits can be considered as a good dietary source of some nutrients and antioxidant compounds, especially some anthocyanins and polyphenols, which could provide nutritionally useful amounts of most of minerals as well. The total polyphenols content was observed in the mulberry cultivars between 56.32 mg/100g DW (Lvshenzi) and 246.00 mg/100g DW (Guang 7200), and the total anthocyanins content ranged from 19.00 mg/100g DW (Yaan 3) to 193.00 mg/100g DW (Jushensang), whereas not detected in Lvshenzi. The correlation analysis indicated that total polyphenols contribute significantly to the antioxidant activity. The results indicated that mulberry has the potential to be further developed into a nutritionally interesting raw material for food and beverage applications. Additionally, the Morus atropurpurea Roxb. showed considerable high nutritive value and antioxidant activity which could be chosen for functional food development that benefits human health.
ACKNOWLEDGMENT
The authors are thankful to Professor Yile Pan (Key Laboratory of Silkworm Biotechnology, Ministry of Agriculture, Sericultural Research Institute, Chinese Academy of Agricultural Sciences, Zhenjiang, Jiangsu, China) for providing us with mulberry samples and their identification.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ercisli S, Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007;103:1380–4. [Google Scholar]
- 2.Özgen M, Serçe S, Kaya C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci Hortic (Amsterdam) 2009;119:275–9. [Google Scholar]
- 3.Kang TH, Hur JY, Kim HB, Ryu JH, Kim SY. Neuroprotective effects of the cyanidin-3-O-[beta]-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci Lett. 2006;391:122–6. doi: 10.1016/j.neulet.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 4.Liu LK, Lee HJ, Shih YW, Chyau CC, Wang CJ. Mulberry anthocyanin extracts inhibit LDL oxidation and macrophage-derived foam cell formation induced by oxidative LDL. J Food Sci. 2008;73:113–21. doi: 10.1111/j.1750-3841.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 5.Butt MS, Nazir A, Sultan MT, Schroën K. Morus alba L. nature's functional tonic. Trends Food Sci Tech. 2008;19:505–12. [Google Scholar]
- 6.Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang CL, Hsieh YS. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006;235:248–59. doi: 10.1016/j.canlet.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Zhao WG, Miao XX, Zang B, Zhang L, Pan YL, Huang YP. Construction of fingerprinting and genetic diversity of mulberry cultivars in China by ISSR markers. Yi Chuan Xue Bao. 2006;33:851–60. doi: 10.1016/S0379-4172(06)60119-4. [DOI] [PubMed] [Google Scholar]
- 8.Association of Official Analytical Chemists Official methods of analysis. 15th ed. Washington, DC: 1995. AOAC. [Google Scholar]
- 9.Leterme P, Buldgen A, Estrada F, Londoño AM. Mineral content of tropical fruits and unconventional foods of the Andes and the rain forest of Colombia. Food Chem. 2006;95:644–52. [Google Scholar]
- 10.Hosseinian FS, Li W, Beta T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008;109:916–24. doi: 10.1016/j.foodchem.2007.12.083. [DOI] [PubMed] [Google Scholar]
- 11.Alonso ÁM, Guillén DA, Barroso CG, Puertas B, García A. Determination of antioxidant activity of wine byproducts and its correlation with polyphenolic content. J Agr Food Chem. 2002;50:5832–6. doi: 10.1021/jf025683b. [DOI] [PubMed] [Google Scholar]
- 12.Makris DP, Boskou G, Andrikopoulos NK. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J Food Compos Anal. 2007;20:125–32. [Google Scholar]
- 13.Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S-I, Lee YC. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Su MS, Shyu YT, Chien PJ. Antioxidant activities of citrus herbal product extracts. Food Chem. 2008;111:892–6. [Google Scholar]
- 15.Kuda T, Ikemori T. Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem. 2009;112:575–81. [Google Scholar]
- 16.Ercisli S, Orhan E. Some physico-chemical characteristics of black mulberry (Morus nigra L.) genotypes from Northeast Anatolia region of Turkey. Sci Hortic (Amsterdam) 2008;116:41–6. [Google Scholar]
- 17.Kafkas E, Ercisli S, Kemal KN, Baydar K, Yilmaz H. Chemical composition of blood orange varieties from Turkey:A comparative study. Pharmacog Mag. 2009;5:329–35. [Google Scholar]
- 18.Ercisli S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007;104:1379–84. [Google Scholar]
- 19.Evstatieva L, Todorova M, Antonova D, Staneva J. Chemical composition of the essential oils of Rhodiola rosea L. of three different origins. Pharmacog Mag. 2010;6:256–58. doi: 10.4103/0973-1296.71782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dini I, Tenore GC, Dini A. Chemical composition, nutritional value and antioxidant properties of Allium caepa L. Var. tropeana (red onion) seeds. Food Chem. 2008;107:613–21. doi: 10.1021/np800237w. [DOI] [PubMed] [Google Scholar]
- 21.Özcan MM, HacIseferogullarI H. The Strawberry (Arbutus unedo L.) fruits: Chemical composition, physical properties and mineral contents. J Food Eng. 2007;78:1022–8. [Google Scholar]
- 22.Chen XH, Xia LX, Zhou HB, Qiu GZ. Chemical composition and antioxidant activities of Russula griseocarnosa sp. nov. J Agr Food Chem. 2010;58:6966–71. doi: 10.1021/jf1011775. [DOI] [PubMed] [Google Scholar]
- 23.Ekholm P, Reinivuo H, Mattila P, Pakkala H, Koponen J, Happonen A, et al. Changes in the mineral and trace element contents of cereals, fruits and vegetables in Finland. J Food Compos Anal. 2007;20:487–95. [Google Scholar]
- 24.Ercisli S. Black table olives from northeastern region of Turkey: The composition and nutritive value. Pharmacog Mag. 2009;5:183–8. [Google Scholar]
- 25.Song W, Wang HJ, Bucheli P, Zhang PF, Wei DZ, Lu YH. Phytochemical profiles of different Mulberry (Morus sp.) Species from China. J Agr Food Chem. 2009;57:9133–40. doi: 10.1021/jf9022228. [DOI] [PubMed] [Google Scholar]
- 26.Bae SH, Suh HJ. Antioxidant activities of five different mulberry cultivars in Korea. LWT Food Sci Technol. 2007;40:955–62. [Google Scholar]
- 27.Usenik V, Fabcic J, Stampar F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.) Food Chem. 2008;107:185–92. [Google Scholar]
- 28.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 29.Sim K, Nurestri AS, Norhanom A. Phenolic content and antioxidant activity of Pereskia grandifolia Haw. (Cactaceae) extracts. Pharmacog Mag. 2010;6:248–54. doi: 10.4103/0973-1296.66945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christian KR, Jackson JC. Changes in total phenolic and monomeric anthocyanin composition and antioxidant activity of three varieties of sorrel (Hibiscus sabdariffa) during maturity. J Food Compos Anal. 2009;22:663–7. [Google Scholar]
- 31.Mousavinejad G, Emam-Djomeh Z, Rezaei K, Khodaparast MHH. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009;115:1274–8. [Google Scholar]
- 32.Fang Z, Zhang Y, Lü Y, Ma G, Chen J, Liu D, et al. Phenolic compounds and antioxidant capacities of bayberry juices. Food Chem. 2009;113:884–8. [Google Scholar]
- 33.Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, et al. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agr Food Chem. 2008;56:1415–22. doi: 10.1021/jf073035s. [DOI] [PubMed] [Google Scholar]
- 34.Giovanelli G, Buratti S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009;112:903–8. [Google Scholar]
- 35.Kähkönen MP, Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J Agr Food Chem. 2003;51:628–33. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]


