Abstract
Administration of peptides i.n. induces peripheral tolerance in Tg4 myelin basic protein-specific TCR-Tg mice. This is characterized by the generation of anergic, IL-10-secreting CD4+ T cells with regulatory function (IL-10 Treg). Myelin basic protein Ac1–9 peptide analogs, displaying a hierarchy of affinities for H-2 Au (Ac1–9[4K]<<[4A]<[4Y]), were used to investigate the mechanisms of tolerance induction, focusing on IL-10 Treg generation. Repeated i.n. administration of the highest affinity peptide, Ac1–9[4Y], provided complete protection against EAE, while i.n. Ac1–9[4A] and Ac1–9[4K] treatment resulted in only partial protection. Ac1–9[4Y] was also the most potent stimulus for IL-10 Treg generation. Although i.n. treatment with Ac1–9[4A] gave rise to IL-10-secreting CD4+ T cells, the population as a whole was also capable of secreting IFN-γ after an in vitro recall response to Ac1–9[4A] or [4Y]. In addition to IL-10 production, other facets of tolerance, namely, anergy and suppression (both in vitro and in vivo), were affinity dependent, with i.n. Ac1–9[4Y]-, [4A]- or [4K]-treated CD4+ T cells being the most, intermediate and least anergic/suppressive, respectively. These findings demonstrate that the generation of IL-10 Treg in vivo is driven by high signal strength.
Keywords: Autoimmunity, Cytokines, Rodent, T cells, Tolerance
Introduction
Antigen administered in a tolerogenic form has long been known to result in down-regulation of immune responses. Our previous studies demonstrated tolerance induction in WT B10.PL mice by i.n. administration of the N-terminal peptide of myelin basic protein (MBP), Ac1–9[4K], the immunodominant encephalitogenic epitope in H-2u mice, as measured by decreased EAE severity upon subsequent challenge [1]. MBP Ac1–9[4K] forms highly unstable complexes with the MHC class II molecule H-2 Au [2]. Using MBP Ac1–9 peptide analogs with an alanine or tyrosine substitution at position four, displaying a hierarchy in affinity for H-2 Au (MBP Ac1–9[4K]<<[4A]<[4Y]), we previously found that protection from EAE correlated with peptide affinity for H-2 Au [1]. The Tg4 TCR Tg mouse was generated so as to circumvent the limitations imposed by low T-cell precursor frequency in the WT mice [3]. The use of the Tg4 mouse model demonstrated that T-cell deletion was only transient and incomplete after a single dose of a high-affinity analog of the MBP epitope, Ac1–9[4Y]. Repeated administration resulted in down-regulation of the capacity of Tg4 CD4+ T cells to proliferate and a shift in cytokine secretion from IL-2, IL-4 and IFN-γ to IL-10 (but not TGF-β) production [4, 5]. In addition to protection against EAE, the peptide-induced tolerant cells were shown to suppress proliferation of responder Tg4 CD4+ T cells, both in vitro and in vivo [6]. The role of IL-10 in suppression was subsequently confirmed by administration of blocking anti-IL-10R and anti-IL-10 antibodies [4, 6]. Of note, peptide-induced IL-10-secreting CD4+ T regulatory cells (IL-10 Treg) were found to be distinct from naturally occurring Treg in that they did not express Foxp3 [7]. Furthermore, genetic depletion of FoxP31 Treg from the CD4+ T-cell repertoire in the RAG-deficient Tg4 mouse gave rise to spontaneous EAE, the onset of which could be prevented by repetitive treatment with i.n. peptide, correlating with the generation of IL-10 Treg [8]. In our most recent study, we have shown that repeated i.n. peptide treatment gave rise to IL-10 Treg that originated from Th1 cells [9].
Thus, in view of the apparent correlation between protection from EAE and the affinity of MBP Ac1–9 analogs for H-2 Au, as well as the role of IL-10 in tolerance, it was of interest to investigate the ability of the analogs to induce IL-10 production. In this study, we use MBP Ac1–9 analogs as a tool to study the role of signal strength in peripherally induced IL-10 Treg generation. Our results demonstrate that antigenic strength is a key factor in the generation of IL-10 Treg in vivo, as characterized by changes in proliferative capacity, cytokine secretion, acquisition of regulatory function and protection from EAE.
Results
Treatment with peptide i.n. protects against EAE in an affinity-dependent manner
Administration of MBP Ac1–9[4K] i. n. limits induction of EAE in H2u mice, with higher affinity analogs Ac1–9[4A] and Ac1–9[4Y] providing greater protection [1]. A TCR Tg mouse on the H2u background (Tg4) was generated in order to circumvent the limitations imposed by low T-cell precursor frequency in the WT mouse [3]. As shown in Fig. 1, repeated administration of the highest affinity peptide, Ac1–9[4Y], provided complete protection against the disease, while i.n. Ac1–9[4A] and Ac1–9[4K] treatment were less effective. This included a graded effect on incidence, day of onset and peak of clinical disease score that correlated with individual peptide affinity for H-2 Au (Table 1). However, the Tg4 CD4+ T-cell repertoire is heterogeneous with respect to TCR expression whereby a proportion of the cells express endogenous α chains as a result of gene recombination [10]. It follows that preferential selection of CD4+ T cells with the alternatively rearranged TCR-α genes could provide a possible explanation for tolerance induction in the Tg4 mouse model. These experiments were therefore repeated using Tg4 mice on the Rag1−/− deficient background and provided similar results (Table 1). These findings show that, similar to the WT model, the affinity of the i.n. administered peptide for MHC also influences the effectiveness of tolerance induction in Tg4 mice as well as Tg4 Rag1−/− mice.
Figure 1.
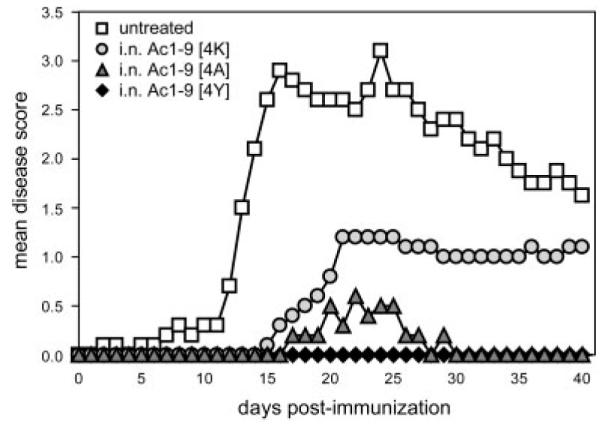
Decrease in the susceptibility to EAE induction upon i.n. treatment with MBP peptides of increasing affinity for H-2 Au. Tg4 mice were treated with ten i.n. doses of MBP Ac1–9[4K], [4A] or [4Y] peptides. EAE was induced 3 days after the last peptide treatment in peptide-treated and untreated mice by s.c. immunization with SCH emulsified in PBS/CFA containing M. tuberculosis on day 0 followed by two i.p. injections of PT administered on days 0 and 2. Individual mice were monitored daily for the development of EAE and scored for 40 days post immunization. Values represent mean EAE clinical scores from five mice per treatment group; this experiment was repeated in Tg4 Rag1−/− mice with similar results.
Table 1.
The effect of i.n. MBP Ac1–9[4K], [4A] or [4Y] administration on the development of EAE
| Groupa) | Incidenceb) | Mortalityc) | Mean day of onsetd) | Mean maximum scoree) |
|---|---|---|---|---|
| Tg4 | ||||
| untreated | 5/5 (100%) | 1/5 (20%) | 8.8±2.2 | 3.8±0.4 |
| Ac1–9 [4K] | 2/5 (60%) | 1/5 (20%) | 17.0±2.0 | 3.0±2.0 |
| Ac1–9 [4A] | 3/5 (80%) | 0/5 (0%) | 18.0±0.8 | 1.0±0.0 |
| Ac1–9 [4Y] | 0/5 (0%) | 0/5 (0%) | 0.0±0.0 | 0.0±0.0 |
| Tg4 Rag1−/− | ||||
| untreated | 3/3 (100%) | 1/3 (33%) | 2.7±1.2 | 3.8±1.6 |
| Ac1–9 [4K] | 1/3 (33%) | 1/3 (33%) | 11.0 | 5.0 |
| Ac1–9 [4A] | 1/3 (33%) | 1/3 (33%) | 26.0 | 1.0 |
| Ac1–9 [4Y] | 0/3 (0%) | 0/3 (0%) | 0.0±0.0 | 0.0±0.0 |
Tg4 and Tg4Rag1−/− mice were treated with 10 i.n. doses of MBP Ac1–9[4K], [4A] or [4Y] peptides. EAE was induced 3 days after the last peptide treatment in peptide-treated and untreated mice by s.c. immunization with SCH (Tg4) or MBP Ac1-25 [4K] (Tg4Rag1−/−) emulsified in PBS/CFA containing M. tuberculosis on day 0 followed by two i.p. injections of PT administered on days 0 and 2. Individual mice were monitored daily for the development of EAE and scored for 40 days post immunization.
Number (percentage) of mice that have developed disease.
Number (percentage) of mice that have died.
Day of disease onset (mean±SEM).
Maximum clinical score (mean±SEM).
Antigenic activity of i.n. peptides correlates with their affinity
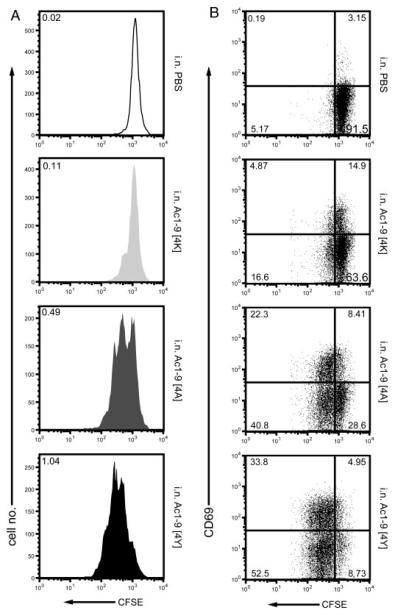
In order to interpret the EAE protection data, we first examined the effect of i.n. peptide treatment on the extent of Tg4 cell activation in vivo using a CFSE-labeled cell transfer model. As shown in Fig. 2, administration of a single i.n. dose of MBP Ac1–9[4K], [4A] or [4Y] to mice previously injected with naïve Tg4 CFSE labeled splenocytes resulted in their activation, albeit to varying degrees. CFSE+CD4+ T cells from the peptide-treated recipient mice displayed at least one round of division and up-regulated the expression of CD69 on their surface relative to PBS controls (Fig. 2A and B, respectively). Upon challenge with Ac1–9[4K], [4A] or [4Y], CFSE+CD4+ T cells proliferated with a division index, i.e. the average number of times that each responding cell had divided, of 0.11, 0.49 and 1.04, respectively, compared with that of 0.02 upon PBS challenge (Fig. 2A). The percentage of activated, CD69 expressing CFSE+CD4+ T cells (both divided and undivided) increased accordingly, with a total of around 19.8, 30.7 and 38.8% observed in Ac1–9[4K]-, [4A]- and [4Y]-treated compared with 3.3% in PBS-treated recipient mice. Thus, the ability of individual MBP Ac1–9 analogs to activate naïve Tg4 CD4+ T cells in vivo correlates with their affinity.
Figure 2.
Increasing in vivo activation of CD4+ T cells by i.n. treatment with Ac1–9 analogs of higher affinity. Splenocytes from naïve Tg4 mice were labeled with CFSE and adoptively transferred into untreated Tg4 recipients by i.p. injection on day 0. On day 1 post cell transfer, the recipient mice were challenged with a single i.n. dose of PBS as a control or MBP Ac1–9[4K], [4A] or [4Y]. Spleens from recipient mice were collected on day 3 and stained with anti-CD4, anti-CD69 mAb and PI prior to flow cytometry. Analysis was performed gating on live CD4+CFSE+ lymphocytes. (A) CFSE profile of CD4+ cells. Numbers represent mean division index values. (B) CSFE versus CD69 profile of CD4+ cells. Data are shown for one recipient mouse and are representative of two independent assays with similar results.
Increasing affinity of i.n. peptides is associated with anergy and switch towards IL-10 secretion
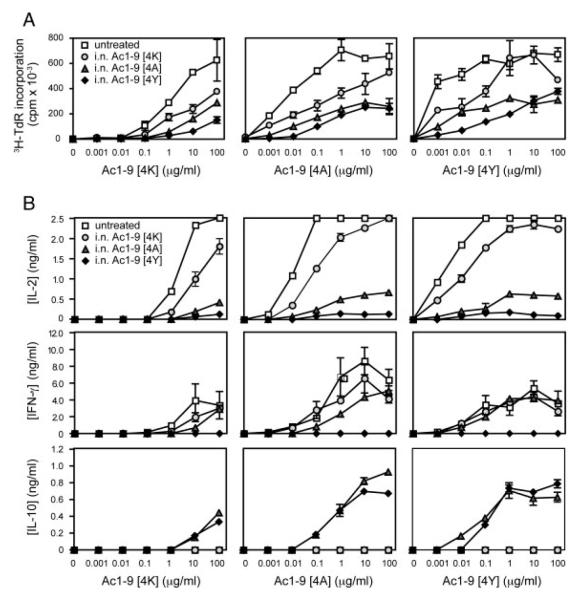
We next investigated whether the differential effects of i.n. Ac1–9[4K], [4A] and [4Y] treatment on disease resulted from differences in the ability of these peptides to induce IL-10 production. In order to determine their tolerogenic activity, as characterized by anergy induction and change in the cytokine secretion profile, Tg4 mice were treated with a minimum of ten i.n. doses of Ac1–9[4K], [4A] or [4Y] and the extent of tolerance induction was examined in vitro. The proliferative response of CD4+ T cells from untreated and peptide-treated Tg4 mice to Ac1–9[4K], [4A] and [4Y] in vitro is shown in Fig. 3A. Naïve CD4+ T cells responded optimally to the cognate Ac1–9[4K] peptide at a concentration of 100 μg/mL, while Ac1–9[4A] and [4Y] acted as superagonists, requiring 100- and 10 000-fold lower concentrations than MBP Ac1–9[4K] to optimally stimulate naïve Tg4 CD4+ T cells, respectively. Administration of either of the three peptides i. n. resulted in a reduced proliferative response of the treated compared with the untreated Tg4 CD4+ T cells. CD4+ T cells from mice treated i.n. with Ac1–9[4K], [4A] or [4Y] required 10-, 100- and 1000-fold higher concentration of Ac1–9[4K], respectively, to proliferate (Fig. 3A). The maximum proliferation of CD4+ T cells from treated mice remained below half the value observed from untreated Tg4 mice over a wide range of peptide concentration and affinity. Furthermore, Fig. 3A shows that neither could the hierarchy be altered nor could the relative degree of unresponsiveness be overcome by stimulating with higher affinity analogues.
Figure 3.
The influence of affinity on CD4+ T-cell responsiveness and cytokine secretion profile. Tg4 mice were treated with ten i.n. doses of MBP Ac1–9[4K], [4A] or [4Y] peptides. Splenic CD4+ cells were positively selected from either untreated Tg4 or peptide-treated Tg4 mice 3 days after the last peptide treatment. In total, 5 × 104 CD4+ T cells per well were cultured with 1 × 105 irradiated B10.PL splenocytes as APC in the presence of a tenfold titration of MBP Ac1–9[4K], [4A] or [4Y] ranging from 0.001 to 100 μg/mL. (A) Proliferative responses were measured at 72 h by 3[H]-thymidine incorporation. (B) Cytokine responses of CD4+ T cells from the above cultures were measured by ELISA at 24 h (IL–2) and 72 h (IFN-γ and IL-10) after in vitro restimulation. Data show mean ± SEM (n = 3). Data are representative of three independent experiments.
Changes in the cytokine secretion profiles of CD4+ T cells from untreated compared with peptide-treated Tg4 mice in response to in vitro peptide stimulation are shown in Fig. 3B. Supernatants from the above cultures were collected and analyzed for levels of IL-2, IFN-γ and IL-10 by sandwich ELISA. CD4+ T cells from untreated mice responded to in vitro stimulation with Ac1–9[4K], [4A] and [4Y] by increasing IL-2 secretion (top row, Fig. 3B), correlating directly with the proliferative response shown in Fig. 3A. This was also the case for IFN-γ secretion (middle row, Fig. 3B). No IL-10 was detected in cultures of untreated CD4+ T cells upon Ac1–9[4K], [4A] or [4Y] stimulation in vitro (bottom row, Fig. 3B). The cytokine secretion profile of CD4+ T cells from mice treated with i.n. Ac1–9[4K] was similar to that of untreated mice, albeit with lower IL-2 production. CD4+ T cells from mice treated with i.n. Ac1–9[4A] and [4Y] responded by much reduced IL-2 production in response to Ac1–9[4K], [4A] or [4Y] stimulation compared with those from untreated and Ac1–9[4K]-treated mice. IFN-γ was produced by CD4+ T cells from mice treated with i.n. Ac1–9[4K] and [4A] but not [4Y]. CD4+ T cells from both the i.n. Ac1–9[4A]- and [4Y]-treated mice produced large amounts of IL-10 in response to stimulation with Ac1–9[4K], [4A] or [4Y]. These results suggest that an active Th1 response is the dominant or default effector pathway in the Tg4 mouse model in response to MBP Ac1–9 peptides. Furthermore, they demonstrate that the decrease in responsiveness and Th1 cytokine secretion by CD4+ T cells, following a course of tolerization with increasing affinity peptides, coincides with induction of IL-10 production. Interestingly, while the affinity of Ac1–9[4A] reaches the required threshold for IL-10 secretion, it is not sufficient for IFN-γ down-regulation. Therefore, we observe a signal strength-dependent hierarchy of changes in cytokine production following i.n. administration of the panel of peptide analogues. In vivo treatment with [4K] reduces IL-2 and IFN-γ production without inducing IL-10, among cells responding to antigen in vitro; [4A] substantially inhibits IL-2, reduces IFN-γ while inducing IL-10; treatment with [4Y], on the other hand, inhibits both IL-2 and IFN-γ while enhancing IL-10 secretion. Increasing antigenic signal strength sequentially inhibits IL-2 followed by IFN-γ while simultaneously enhancing propensity towards secretion of IL-10 in response to antigen.
Induction of IL-10-secreting T cells is affinity-dependent and occurs via the Th1 pathway
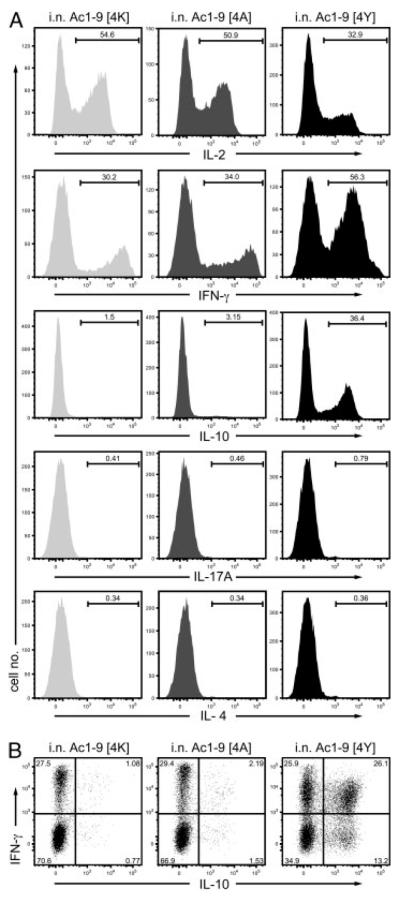
The proportion of CD4+ T cells producing IL-2, IL-4, IL-17A, IFN-γ and/or IL-10 was determined by intracellular cytokine staining (ICCS) at 2 h after the last i.n. peptide administration, the time of peak cytokine secretion in vivo [6]. As shown in the left panel of Fig. 4A, comparable proportions of Tg4 CD4+ T cells from mice treated with i.n. MBP Ac1–9[4K] or [4A] (~50%) produced IL-2, whereas CD4+ T cells from mice treated with i.n. MBP Ac1–9[4Y] showed reduced numbers of IL-2-producing cells (~33%) upon subsequent stimulation with PMA and ionomycin. This result is consistent with previous findings that the combination of PMA and ionomycin is a sufficiently potent stimulus to induce synthesis of cytokines that had been inhibited through anergy induction [11]; this explains why results from ICCS analysis differ from the cytokine secretion observed in vitro and shown in Fig. 3. Correspondingly, IFN-γ-producing cells were observed in all three peptide treatment groups, with CD4+ T cells from i.n. Ac1–9[4Y]-treated mice comprising the highest proportion (~30% of CD4+ T cells from i.n. Ac1–9[4K]- or [4A]- and 56% of [4Y]-treated mice) (Fig. 4A). CD4+ T cells from i.n. MBP Ac1–9[4Y]-treated mice also comprised the largest number of IL-10-producing cells (36%) (Fig. 4A). Interestingly, the majority of IL-10-producing CD4+ T cells co-produced IFN-γ Fig. 4B). Although i.n. Ac1–9[4A] treatment did not increase the IL-10-secreting T-cell frequency much above that of [4K]-treated mice, it “predisposed” T cells to IL-10 secretion so that they were able to secrete IL-10 following an antigenic challenge in vitro (Fig. 3B). These results demonstrate that i.n. treatment with peptides of increasing affinity drives CD4+ T cells to secrete IFN-γ and that high affinity peptides induce most IL-10 production from previous IFN-γ producers.
Figure 4.
Affinity dependent IL-10 Treg generation during tolerization. Tg4 mice were treated with 14 i.n. doses of MBP Ac1–9 Ac1–9[4K], [4A] or [4Y] peptides. Spleens were isolated from peptide-treated Tg4 mice 2 h after the last peptide treatment. ICCS for IL-2, IFN-γ, IL-4 and IL-10 was performed after an additional 4-h stimulation with PMA and ionomycin. (A) Cells are gated on V8+ cells from each peptide treatment group and analyzed for IL-2, IFN-γ and IL-10 expression with cytokine gates based on isotype controls. (B) Dot plots show co-secretion of IFN-γ and IL-10 by Vβ8+ T cells from each peptide treatment group. Data are representative of at least three independent experiments.
Treatment with increasing affinity peptides i. n. correlates with suppression in vitro
In order to determine the correlation between the affinity of tolerizing MBP peptides and antigen-driven Treg induction, the suppressive function of CD4+ T cells from peptide-treated mice was assessed in vitro. For the in vitro suppression assay, CD4+ T cells from untreated Tg4 mice were stimulated either alone or in the presence of a titrated number of CD4+ T cells from i.n. Ac1–9[4K]-, [4A]- or [4Y]-treated Tg4 mice that had been restimulated in vitro in order to maximize IL-10 secretion [12]. As shown in Fig. 5A, T cells from untreated mice proliferated optimally in response to Ac1–9[4K] stimulation, whereas CD4+ T cells from i.n. Ac1–9[4K]-, [4A]- or [4Y]-treated Tg4 mice responded poorly. When co-cultured with CD4+ T cells from untreated mice at a 1:1 ratio, CD4+ T cells from Tg4 mice treated with i.n. Ac1–9[4A] or [4Y] appeared suppressive, inhibiting naïve CD4+ T-cell proliferation by 55 and 64% at a ratio of 1:1, titrating out to 1:2 and 1:4, respectively (Fig. 5A).
Figure 5.
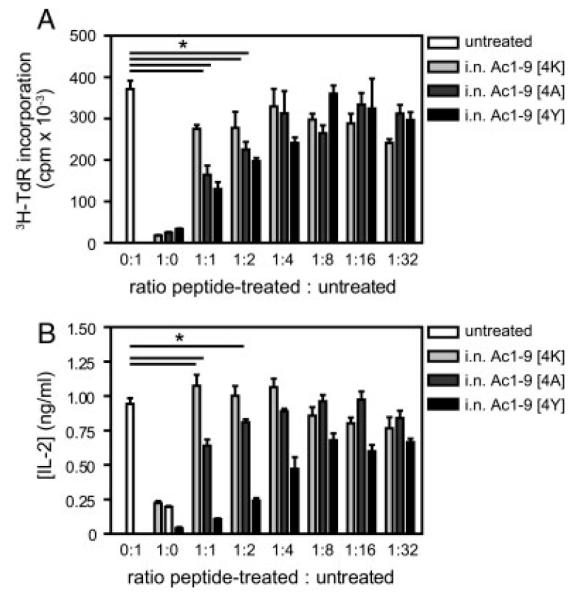
In vitro suppression of naïve CD4+ T-cell proliferation by CD4+ T cells from peptide-treated mice. Tg4 mice were treated with 14 i.n. doses of MBP Ac1–9[4K], [4A] or [4Y] peptides. Splenocytes were isolated 3 days after the last treatment and expanded in complete medium containing 10 μg/mL of MBP Ac1–9[4K] and 20 U/mL of rhIL-2 for 5 days. CD4+ T cells were positively selected from untreated mouse spleens as well as IL-2-expanded splenocytes from i.n. Ac1–9[4K]-, [4A]- and [4Y]-treated mice. (A) In total, 5 × 104 of each untreated and peptide-treated CD4+ T cells were either cultured alone or co-cultured at decreasing ratios from 1:1 to 1:32 of peptide-treated to untreated CD4+ T cells in the presence of 1 × 105 irradiated B10.PL splenocytes as APC and 100 μg/mL of MBP Ac1–9[4K]. Proliferative responses were measured at 72 h by 3[H]-thymidine incorporation. (B) IL-2 secretion by CD4+ T cells from the above cultures was measured by ELISA at 24 h after in vitro stimulation. Data show mean ± SEM (n = 3). Data are representative of three independent experiments. *p<0.05 versus untreated control.
Supernatants from the in vitro suppression assays were collected and analyzed for IL-2 levels by sandwich ELISA. As shown in Fig. 5B, CD4+ T cells from all three peptide-treated groups produced very small amounts of IL-2 when compared with untreated CD4+ T cells. The amount of IL-2 detected in the co-cultures reflected the amount of suppression observed in Fig. 5A. Taken together, these results demonstrate a hierarchy in the ability of the tolerizing peptides to induce Treg as significant suppression of T-cell proliferation and IL-2 secretion was only detected in co-cultures containing CD4+ T cells from i.n. Ac1–9 [4A]- and [4Y]-treated Tg4 mice.
Treatment with increasing affinity peptides i. n. correlates with suppression in vivo
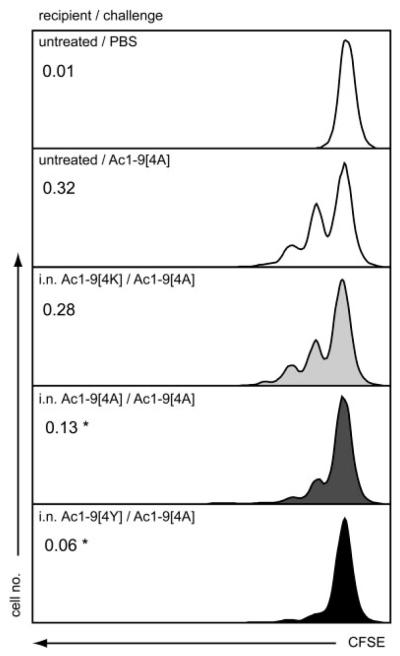
An in vivo model of T-cell-mediated suppression has been described previously [6] whereby CFSE-labeled Tg4 cells were transferred into either untreated or peptide-treated recipient mice and their proliferation to subsequent peptide challenge assessed by CFSE dilution. This assay was used here to address the capacity of the different affinity peptides to mediate suppression in vivo. Figure 6 shows the proliferation of naïve Tg4 CD4+ T cells adoptively transferred to untreated or peptide-treated recipient mice. The baseline CFSE level was determined by administering a single dose of i.n. PBS to untreated recipient mice. Upon challenge with Ac1–9[4A], CFSE+CD4+ T cells divided in the untreated recipient mice with a division index of 0.32. The division index of CFSE+CD4+ T cells transferred to i.n. Ac1–9[4K]-treated recipients was lower (0.28) but not significantly different from the above. However, when transferred to i.n. Ac1–9[4A]- or [4Y]-treated recipient mice, the division index of the same cells was only 0.13 and 0.06, respectively. Thus, the proliferation of transferred T cells was significantly suppressed upon transfer to i.n. Ac1–9[4A]- and [4Y]-treated recipient mice. These results are consistent with those depicted in Fig. 5 and demonstrate that the observed hierarchy in the ability of the tolerizing peptides to induce Treg and thus mediate suppression extends to in vivo suppression of T-cell proliferation.
Figure 6.
Peptide affinity associated inhibition of proliferation of Tg4 CD4+ T cells in vivo. Tg4 mice were either left untreated or treated with ten i.n. doses of MBP Ac1–9[4K], [4A] or [4Y] peptides. Splenocytes from naïve Tg4 mice were labeled with CFSE and 5 × 107 cells were transferred into untreated or i.n. MBP[4K]-, [4A]- or [4Y]-treated Tg4 recipients by i.p. injection 3 days after the last peptide treatment. One day after cell transfer, recipient mice were challenged with a single dose of MBP Ac1–9[4A]; a separate group of untreated recipient mice was challenged with a single dose of PBS. Spleens from recipient mice were collected on day 3 and stained with anti-CD4 and PI prior to flow cytometry. CFSE profile of CD4+ cells: Data show results from one recipient mouse and are representative of four independent experiments. Numbers represent mean division index values. *p<0.05 versus untreated control.
Discussion
Analysis of the phenotypic and functional changes of CD4+ T cells that take place upon i.n. MBP Ac1–9[4K], [4A] or [4Y] treatment revealed an association between peptide affinity and the ability to activate CD4+ T cells in vivo. This translates into an affinity-dependant loss of responsiveness to antigenic stimulation by CD4+ T cells following repeated peptide treatment, which is most likely due to the decreased ability of these cells to secrete IL-2. Indeed, the non-responsive state of CD4+ T cells from i.n. MBP Ac1–9[4Y]-treated mice could be reversed by the addition of exogenous IL-2 [5]. Exogenous IL-2 also reverses the anergy of CD4+ T cells from i.n. MBP Ac1–9[4K]- and [4A]-treated mice (Supporting Information Fig. 1). Lack of secreted IFN-γ in CD4+ T-cell cultures from i.n. Ac1–9[4Y]-treated mice is in turn likely to be the result of their anergy. This is supported by the observation that CD4+ T cells remain able to produce IFN-γ upon PMA and ionomycin stimulation. Interestingly, although anergy abrogates the production of IL-2 and IFN-γ in these cells, it allows the production of IL-10. By studying the effect of repeated i.n. administration of either MBP Ac1–9[4K], [4A] or [4Y], we reveal a correlation between the affinity of peptide binding to H-2 Au and acquisition of a regulatory phenotype by CD4+ T cells, as demonstrated by IL-10 secretion and naïve T-cell suppression, both in vitro and in vivo. Of note, the mechanism of in vitro suppression by CD4+ T cells from i.n. MBP Ac19[4Y]-treated mice has been shown to be cell contact-dependent, as determined by loss of suppression when using a transwell cell culture system, and cytokine independent, since neither anti-IL-10R or anti-TGF-β (or both) reversed suppression [6]. Moreover, Vieira et al. showed reduced IL-2 expression in co-cultures, indicating that CD4+ T cells from i.n. Ac19[4Y]-treated mice actively suppress naïve T cells in vitro [7]. Interestingly, there is no direct correlation between anergy and in vitro suppression [13]; cells from Ac1–9[4K]-treated mice were anergic but failed to suppress in vitro. Conversely, the observed EAE protection [4] and inhibition of T-cell proliferation in vivo afforded by i.n. MBP Ac1–9[4Y] treatment [6] has previously been attributed to IL-10. Our results show that i.n. treatment with high affinity peptides, which drive the production of IL-10 amongst CD4+ T cells, correlates with their ability to mediate suppression, both in vitro and in vivo, and to protect against EAE development. However, administration of i.n. MBP Ac1–9[4K], which does not lead to IL-10 secretion, can also limit disease, albeit to a lesser degree. Thus, other facets of tolerance apart from IL-10, such as anergy and/or reduction in the ability to secrete IL-2 and IFN-γ, are likely to play a role. Taken together, our data point to a model in which repeated treatment with peptide antigen induces anergy in T cells, which is sufficient for debilitating their own effector function. Induction of IL-10 secretion following repeated encounter with high affinity antigen is, however, required for the generation of cells capable of suppressing other naïve T cells in their vicinity.
Huang et al. showed that peripheral tolerance induction requires activation, proliferation and an effector phase [14]. Here, we show that i.n. treatment with all three MBP Ac1–9 position analogs induces CD4+ T-cell activation and proliferation in an adoptive transfer model in vivo. Furthermore, we have recently demonstrated that i.n. MBP Ac1–9[4Y] treatment induced IL-10 Treg are of Th1 origin [9], as alluded to here by the ability of CD4+ T cells from i.n. MBP Ac1–9[4Y]-treated mice to co-secrete IFN-γ and IL-10 at the single cell level. This is in direct contrast to the IL-10-secreting T cells generated by treatment with the random amino acid copolymer poly (F,Y,A,K,)n, which also secrete IL-4 and are, therefore, likely of the Th2 lineage [15, 16]. Thus, i.n. administration of MBP Ac1–9 does not result in a Th1 to Th2 immune deviation, which, in some cases, can lead to disease exacerbation [17]. Instead, the potentially pathogenic Th1 response is driven in a controlled manner by i.n. peptide treatment towards IL-10 secretion. This process mimics chronic infections with intracellular pathogens, where IL-10 plays a role in protecting against excessive inflammation-associated pathology [18]. In fact, it is now clear that all known Th cell subsets, including Th1 [19], Th2 [20], Th17 [21–23] and Th9 cells [24] are able to secrete IL-10 regardless of their commitment to a given lineage, thus granting them with suppressive activity. Of note, Saraiva et al. have shown recently that both high levels of TCR ligation and/or repeated TCR triggering leads to enhanced IL-10 production by Th1 cells in vitro [25].
Although high affinity peptide analogs have also been implicated in other murine models of autoimmune diseases such as collagen induced arthritis [26], insulin-dependent diabetes [27], experimental myasthenia gravis [28] or lupus [29], their exact mode of action remains unclear. Our data demonstrate that high signal strength is required for effective induction of IL-10 secretion by CD4+ T cells. Inducing IL-10 is important for regulating Th1 responses, thus ensuring tolerance in the face of epitope spreading, which is especially relevant to the development of therapeutic vaccines for autoimmune diseases.
Materials and methods
Mice
Mice were bred and maintained under specific pathogen-free conditions. B10.PL mice were obtained from The Jackson Laboratory. Tg4 TCR Tg mice were described previously [3] and backcrossed onto the B10.PL (H2u) background. All experiments were carried out in accordance with a UK Home Office Project License and animal welfare codes directed by the University of Bristol ethical review committee.
Peptides
The acetylated N-terminal peptide Ac1–9[4K] (AcASQKRPSQR) (or Ac1-25[4K] (AcASQKRPSQRSKYLATASTMDHARHG)) of murine MBP and its higher MHC affinity position four analogs Ac1–9[4A] (AcASQARPSQR) and Ac1–9[4Y] (AcASQYR-PSQR) were synthesized on an AMS 422 multiple peptide synthesizer (Abimed Analyes-Technik) using standard fluorenyl methoxycarbonyl chemistry. The purity of the peptides was >95%.
Administration of MBP Ac1–9 peptides i. n
MBP Ac1–9 analog peptides were administered i.n. at 100 μg of peptide in 25 μL of PBS under light either halothane or isoflurane anesthesia at 3–4 day intervals over a period of 5 wk. Mice used for experiments were treated with 10 to 14 doses of MBP Ac1–9 peptides.
EAE induction and assessment
EAE was induced in mice on day 0 by s.c. injection of 1 mg spinal cord homogenate (SCH) or 50 μg of MBP Ac1-25[4K] in 0.1 mL of emulsion consisting of equal volumes of PBS and CFA (BD Biosciences) containing heat-killed Mycobacterium tuberculosis (BD Biosciences) at 4 mg/mL. Pertussis toxin (PT) (200 ng) (Sigma-Aldrich) was administered by i.p. injection in 0.5 mL of PBS on days 0 and 2. Mice were monitored for disease for 40 days post-immunization. Clinical signs of EAE were assessed daily with a 0 to 5 scoring system as follows: 0, no disease; 1, flaccid tail; 2, impaired righting reflex and/or partial hind leg paralysis; 3, total hind limb paralysis; 4, fore and hind limb paralysis; 5, moribund or dead.
Transfer of CFSE-labeled splenocytes
Splenocytes from naïve Tg4 mice were labeled with 5- (and-6)-carboxyfluorescein diacetate succinimidyl ester as described previously [30] and suspended in PBS at 1 × 108cells/mL. On day 0, 0.5 mL of the cell suspension was transferred to untreated or 10× MBP Ac1–9[4K]-, [4A]- or [4Y]-treated recipient Tg4 mice i.p. Mice received a challenge of one i.n. dose of PBS or MBP Ac1–9 peptides on day 1 after transfer. On day 3, the spleens from the recipient mice were harvested, cells stained with anti-CD4 APC, anti-CD69 PE and PI (BD Biosciences), and the CD4+ T-cell proliferation/activation, as measured by CFSE dilution/CD69 expression, determined by flow cytometry. The division index, the average number of times that each responding cell has divided, was calculated using FlowJo (Tree Star) FACS analysis software.
Cell separation for in vitro culture
Purified CD4+ T cells were isolated from spleens by magnetic separation using mouse CD4 (L3T4) MicroBeads (Miltenyi Biotec) according to the manufacturer’s instructions.
T-cell proliferation assays
CD4+ T cells were cultured at 5 × 104 per well in complete RPMI medium (RPMI-1640 medium (Cambrex Bio Science) supplemented with 20 mM Hepes Buffer, 50 mM 2-Mercaptoethanol (Sigma-Aldrich), 100 U/mL penicillin and 100 μg/mL streptomycin sulfate, 4 mM l-Glutamine (Cambrex Bio Science) and 5% heat-inactivated fetal bovine serum (Sigma-Aldrich)) in round-bottomed 96-well plates at 37°C and 5% CO2 humidified atmosphere in the presence of 1 × 105 irradiated B10.PL splenocytes as APC. MBP Ac1–9[4K], [4A] or [4Y] ranging from 0.01 to 100 μg/mL were added to the cultures where indicated. Prior to in vitro suppression assays, splenocytes from i.n. peptide-treated mice were routinely expanded in vitro in the presence of recombinant human (rhIL-2) (R&D Systems) as follows. Spleens were isolated 3 days after the last i.n. peptide treatment and disaggregated to form single cell suspension and re-suspended at 1.5 × 106 cells/mL of complete medium. MBP Ac1–9[4K] and rhIL-2 at a final concentration of 10 μg/mL and 20 U/mL, respectively, were added to the cell suspension, and cultures were incubated in 6-well plates at 37°C and 5% CO2 humidified atmosphere. After at least 5 days, the cultured splenocytes were washed and CD4+ T cells were isolated by positive selection. 5 × 104 in vitro expanded CD4+ T cells from peptide-treated Tg4 mice were co-cultured with an equal number of untreated CD4+ T cells, or at ratios from 1:1 to 1:32 of peptide-treated to untreated CD4+ T cells, at 100 μg/mL of MBP Ac1–9[4K] in the presence of 1 × 105 APC/mL. After 72 h, wells were pulsed with 0.5 μCi [3H] thymidine overnight and the incorporated radio-activity was measured on a liquid scintillation β-counter (1450 Microbeta; Wallac).
Flow cytometry
Staining for intracellular cytokine expression was performed using BD CytoFix/CytoPerm Plus Kit (BD Biosciences). Splenocytes from peptide-treated mice were collected 2 h after the last treatment and restimulated with PMA and ionomycin (Sigma-Aldrich) at a final concentration of 5 ng/mL and 500 ng/mL of culture, respectively, for 4 h in the presence of GolgiStop (BD Biosciences). After the incubation, cells were stained with anti-Vβ8 FITC (BD Biosciences), fixed, permeabilized and stained intracellularly with anti-IL-2 APC, anti-IL-4 PE, anti-IL-10 APC, anti-IL-17 PE and anti-IFN-γ PE antibodies, or Ig isotype controls (BD Biosciences). Fluorescence intensity was measured on a FACSCalibur or BD™ LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Cytokine protein levels
Conventional sandwich ELISA was carried out according to instructions from the manufacturer using paired antibodies to assay the quantity of IL-2, IL-10 and IFN-γ (BD Biosciences) in cell culture supernatants. Optical densities were measured at 450/595 nm on a SpectraMax 190 microplate reader and the amount of cytokine present quantified with standard curves using SoftMax Pro software (both from Molecular Devices).
Statistical analysis
Statistical analyses were performed where stated using GraphPad Prism (GraphPad Software) software. The statistical significance of differences between data groups was determined by an unpaired t-test. A p value of ≤0.05 was considered to be significant.
Acknowledgements
We thank Drs. C.A. Sabatos-Peyton and J. Verhagen for critical reading of this manuscript. We also thank Miss L.E.L. Falk and ASU staff for assistance with the breeding of mice. This work was supported by the Wellcome Trust and the MS Society of Great Britain and Northern Ireland.
Abbreviations
- ICCS
intracellular cytokine staining
- IL-10 Treg
IL-10-secreting
- CD4+
T regulatroy cell
- MBP
myelin basic protein
- PT
Pertussis toxin
- rhIL-2
recombinant human IL-2
- SCH
spinal cord homogenate
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
Supporting Information available online
References
- 1.Metzler B, Wraith DC. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int. Immunol. 1993;5:1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 2.Mason K, Denney DW, Jr., McConnell HM. Myelin basic peptide complexes with class II MHC molecules I-Au and I-Ak form and dissociate rapidly at neutral pH. J. Immunol. 1995;154:5216–5227. [PubMed] [Google Scholar]
- 3.Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 4.Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC. Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int. Immunol. 1999;11:1625–1634. doi: 10.1093/intimm/11.10.1625. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PO, Sundstedt A, Yazici Z, Minaee S, Woolf R, Nicolson K, Whitley N, et al. IL-2 overcomes the unresponsiveness but fails to reverse the regulatory function of antigen-induced T regulatory cells. J. Immunol. 2005;174:310–319. doi: 10.4049/jimmunol.174.1.310. [DOI] [PubMed] [Google Scholar]
- 6.Sundstedt A, O’Neill EJ, Nicolson KS, Wraith DC. Role for IL-10 in suppression mediated by Peptide-induced regulatory T cells in vivo. J. Immunol. 2003;170:1240–1248. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- 7.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+regulatory T cells. J. Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 8.Nicolson KS, O’Neill EJ, Sundstedt A, Streeter HB, Minaee S, Wraith DC. Antigen-induced IL-10+regulatory T cells are independent of CD25+regulatory cells for their growth, differentiation, and function. J. Immunol. 2006;176:5329–5337. doi: 10.4049/jimmunol.176.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabrysova L, Nicolson KS, Streeter HB, Verhagen J, Sabatos-Peyton CA, Morgan DJ, Wraith DC. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J. Exp. Med. 2009;206:1755–1767. doi: 10.1084/jem.20082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 12.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, et al. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz RH. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 14.Huang CT, Huso DL, Lu Z, Wang T, Zhou G, Kennedy EP, Drake CG, et al. CD4+T cells pass through an effector phase during the process of in vivo tolerance induction. J. Immunol. 2003;170:3945–3953. doi: 10.4049/jimmunol.170.8.3945. [DOI] [PubMed] [Google Scholar]
- 15.Stern JN, Keskin DB, Zhang H, Lv H, Kato Z, Strominger JL. Amino acid copolymer-specific IL-10-secreting regulatory T cells that ameliorate autoimmune diseases in mice. Proc. Natl. Acad. Sci. USA. 2008;105:5172–5176. doi: 10.1073/pnas.0712131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Stern JN, Strominger JL. T cell receptors in an IL-10-secreting amino acid copolymer-specific regulatory T cell line that mediates bystander immunosuppression. Proc. Natl. Acad. Sci. USA. 2009;106:3336–3341. doi: 10.1073/pnas.0813197106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafaille JJ, Van de Keere F, Hsu AL, Baron JL, Haas W, Raine CS, Tonegawa S. Myelin basic protein-specific T helper 2 cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinchieri G. Regulatory role of T cells producing both interferon gamma and interleukin 10 in persistent infection. J. Exp. Med. 2001;194:53–57. doi: 10.1084/jem.194.10.f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 23.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 24.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 25.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staines NA, Harper N, Ward FJ, Malmstrom V, Holmdahl R, Bansal S. Mucosal tolerance and suppression of collagen-induced arthritis (CIA) induced by nasal inhalation of synthetic peptide 184–198 of bovine type II collagen (CII) expressing a dominant T cell epitope. Clin. Exp. Immunol. 1996;103:368–375. doi: 10.1111/j.1365-2249.1996.tb08289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, Kaufman DL. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J. Exp. Med. 1996;183:1561–1567. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karachunski PI, Ostlie NS, Okita DK, Conti-Fine BM. Prevention of experimental myasthenia gravis by nasal administration of synthetic acetylcholine receptor T epitope sequences. J. Clin. Invest. 1997;100:3027–3035. doi: 10.1172/JCI119857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu HY, Ward FJ, Staines NA. Histone peptide-induced nasal tolerance: suppression of murine lupus. J. Immunol. 2002;169:1126–1134. doi: 10.4049/jimmunol.169.2.1126. [DOI] [PubMed] [Google Scholar]
- 30.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]