Abstract
BACKGROUND
Cysteine-rich angiogenic inducer 61 (Cyr61) is an extracellular matrix protein involved in the transduction of growth factor and hormone signaling. Previously, we demonstrated that Cyr61 was highly expressed in prostate cancer (PCa) but that the expression levels were associated with a lower risk of PCa recurrence. In the present study, we demonstrate that serum Cyr61 is a potential biomarker that correlates with PCa aggressiveness. Furthermore, we also explore the potential mechanism underlying the changes in Cyr61 expression during PCa progression.
METHODS
Cyr61 concentrations in the medium from PCa cell lines and in serum samples obtained from PCa patients were measured by sandwich ELISA. Serum Cyr61 levels were correlated with disease characteristics and the association between Cyr61 expression changes by several types of stimulation or stress and cAMP/cAMP-dependent protein kinase (PKA) pathway were examined.
RESULTS
There was a positive correlation between Cyr61 levels in cell supernatants and mRNA expression in these cell lines. Serum Cyr61 levels were significantly higher in non-organ-confined PCa patients (116.3 ± 140.2 ng/ml) than in organ-confined PCa patients (79.7 ± 56.1 ng/ml) (P = 0.031). Cyr61 expression was up-regulated in response to both lysophosphatidic acid and androgen treatments which promoted PCa cell invasion. Serum starvation and phosphoinositide-3-kinase inhibition also resulted in Cyr61 up-regulation; however, they suppressed cell proliferation. Cyr61 up-regulation was correlated with an increase in cAMP and suppressed by PKA inhibition.
CONCLUSIONS
These findings suggest that Cyr61 expression in PCa is regulated by the cAMP/PKA pathway and that circulating Cyr61 levels are a potential serum-based biomarker for characterizing PCa.
Keywords: Cyr61, prostate cancer, serum marker, cAMP-dependent protein kinase
INTRODUCTION
Prostate cancer (PCa) is the most common malignancy and the second leading cause of cancer-related deaths in men in the United States [1]. The prostate specific antigen (PSA) revolution greatly improved the detection rate of early PCa. However, the PSA era has resulted in what is described as an over-diagnosis of the disease. At present, there are no markers in clinical use that can reliably differentiate indolent from aggressive PCa. Such a marker or panel of markers has the potential to both minimize over-treatment and radically alter existing PCa treatment practices [2].
Cystein-rich angiogenic inducer 61 (Cyr61), also called CCN1, is an extracellular matrix protein involved in growth factor transduction, hormone signaling, and mechanical stress response mediation [3]. Cyr61 participates in regulating many pathways, including cell adhesion, migration, proliferation, differentiation, and survival [4–6]. The specific function of Cyr61 remains largely unknown but its biological activity is believed to be contextual and cell-type dependent [7,8]. Cyr61 signals through interaction with integrins but downstream effects vary greatly depending on the combination of integrins bound [9].
Numerous genomic studies demonstrate altered Cyr61 expression in various cancers, including breast, ovarian, hepatocellular, lung, and colorectal cancer, and depending on the cancer type, Cyr61 may enhance or inhibit tumor growth [7–11]. A previous study showed that Cyr61 mRNA was down-regulated in PCa tissue when compared to normal tissue adjacent to the cancer lesion [12]. Previously, we found greater Cyr61 mRNA expression in PCa samples than in BPH or donor prostates [13]. Additional evidence substantiating the biological importance of Cyr61 in PCa indicates that knocking down Cyr61 expression suppresses PCa cell migration, invasion, and proliferation [14]. In an immunohistochemical study using tissue microarrays (TMA) we previously reported that Cyr61 protein expression is significantly up-regulated in PCa [15]. However, decreased expression of Cyr61 was associated with PCa recurrence after surgical treatment [16]. Although on the surface these results seem to reveal an inverse correlation, these differences may reflect the extracellular environmental status of the cancer. There are numerous extracellular signals that can increase the cellular concentrations of cyclic adenosine monophosphate (cAMP) by increasing the activity of adenylyl cyclase. We previously reported that Cyr61 expression levels in benign prostate cell lines were enhanced by lysophosphatidic acid (LPA) [17], which regulates cAMP concentrations [18]. Cyclic AMP is a second messenger involved in regulation of a variety of cellular functions. It acts mainly through its binding to cAMP-dependent protein kinase (PKA), which is suggested to participate in the progression of various tumors including PCa [19]. However, it can also inhibit proliferation and induce differentiation of cancer cells [20].
In this study, we developed an ELISA method for measuring serum Cyr61 levels and analyzed the correlation between serum Cyr61 levels and PCa characteristics. Further, we also explored the potential mechanisms underlying the changes in Cyr61 expression during PCa progression associating with the cAMP/PKA pathway.
MATREIALS AND METHODS
Cell Lines
PCa cells (LNCaP, DU145, and PC3) were routinely cultured in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (PAA Laboratories). For androgen-depleted conditions, the cells were cultured in phenol red-free RPMI 1640 (Invitrogen) supplemented with 10% charcoal-stripped fetal bovine serum (CSFBS) (Hyclone).
Antibodies and Reagents
Antibodies against Cyr61:N16 (goat polyclonal, sc-8560, Santa Cruz), Cyr61: 3H3 (mouse monoclonal, Abcam), phospho-Akt:Ser473:D9E (rabbit monoclonal, Cell Signaling), and β-actin:A5441 (mouse monoclonal, SIGMA) were purchased as indicated. Recombinant human Cyr61 protein (P-01, Abnova), LPA with unsaturated (18:1) acyl chains (Avanti Polar Lipids), R1881 (Perkin Elmer), the phosphoinositide-3-kinase (PI3K) inhibitor LY294002, and the PKA inhibitor H89 (Enzo Life Science) were also purchased commercially.
RNA Isolation and Real-time PCR
Total RNA was isolated using RNeasy Kit (QIAGEN). After quantification, 1 µg of RNA was reverse-transcribed into first strand cDNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc.). Quantitative real-time PCR was carried out with an i-Cycler iQ Real-Time Detection System using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc.). PCR primers were 5′-acttcatggtcccagtgctc-3′ (forward) and 5′-tggtcttgctgcatttcttg-3′ (reverse) for Cyr61 and 5′-gaatataatcccaagcggtttg-3′ (forward) and 5′-acttcacatcacagctcccc-3′ (reverse) for TATA-binding protein (TBP). Analysis and fold differences were determined using the comparative threshold cycle method. All experiments were performed in triplicate and data presented represents mean ± SD.
Western Blot Analysis
Western blotting was performed as described previously [21]. Briefly, cells were lysed with M-PER Mammalian Protein Extraction Reagent with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific). Protein (30 µg) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with the corresponding antibody at the appropriate dilution (polyclonal Cyr61, 1:500; monoclonal Cyr61, 1:1000; phopho-Akt, 1:1000; β-actin, 1:5000).
Sandwich ELISA
Briefly, 96-well immunoplates (Nunc) were coated with 100 µl of goat polyclonal (N16) anti-Cyr61 antibody (1:30) in coating solution (KPL) overnight at 4°C. After washing with 0.05% Tween-20 in TBS (TBST), unbound sites were blocked with Starting Block T20 (Thermo Scienntific) by incubation for 2 hr at room temperature. After washing with TBST, a 100 µl sample was added to each well, followed by incubating for 1.5 hr at 37°C. Cell supernatants were not diluted and serum samples were diluted 1:2 with Starting Block T20. After washing with TBST, 100 µl of mouse monoclonal (3H3) anti-Cyr61 antibody (1:100) in 0.5% BSA-TBST was added. After 2 hr incubation at room temperature, the wells were washed with TBST and then peroxidase-labeled human serum absorbed affinity purified antibody to rabbit IgG (KPL) was added. After 2 hr incubation, the wells were washed with TBST and then TMB substrate (KPL) was added to each well. After 30 min incubation at room temperature, the absorbance at 630 nm of each well was measured with a microplate reader (BMG Labtech). All experiments were performed in duplicate.
Clinical Samples
The study was approved by the Johns Hopkins Institutional Review Board and utilized retrospectively de-identified samples. Serum samples were obtained from men at the time of prostate biopsy. In a preliminary study, we used samples from controls (n = 10), organ-confined (OC) PCa (n = 10), non-organ-confined (NOC) PCa (n = 10), and metastatic (Met) PCa (n = 10). Controls represent samples from men with high prostate-specific antigen (PSA) levels but negative for PCa in prostate biopsies. NOC-PCa represent samples with positive surgical margin, capsular penetration, seminal vesicle involvement, or lymph node metastasis and OC-PCa samples are those wherein all these pathologic features are negative. Metastatic PCa is a group of patients with significant metastasis as evaluated by computed tomography (CT) or bone scan. In a validation study, we compared 58 OC-PCa and 57 NOC-PC. To predict PSA recurrence, 10 samples each with or without PSA recurrence were used. PSA recurrence means PSA increases >0.2 ng/ml after surgical treatment. The minimum and mean ± SD follow-up duration was 5.0 and 6.2 ± 2.2 years, respectively.
MTS Cell Proliferation Assay
In a 96-well plate, 3 × 103 cells were plated in 100 µl medium, incubated for the indicated hours, after which 20 µul of CellTiter 96 Aqueous One Solution (Promega) was added. After an additional 2 hr of incubation at 37°C, the absorbance at 490 nm of each well was measured.
Matrigel Invasion Assay
In vitro tumor cell invasion was measured with BD Biocoat Matrigel Invasion Chambers (BD). Invaded cell numbers were counted after a 72 hr incubation. Upper chamber: 1 × 105 cells in serum-free medium containing 0.1% BSA. Lower chamber: Serum-free medium containing 0.1% BSA with or without 1 µM LPA for PC3 cells and androgen-depleted medium with or without 1 nM R1881 for LNCaP cells. All experiments were performed three times in triplicate and data presented represent mean ± SD.
cAMP Assay
Cells were seeded at 5 × 104 cells/well in 96-well plates and incubated for 24 hr. Cells were washed once with PBS and cultured for 1 hr in medium with or without FBS. The intracellular cAMP concentrations were assayed using the cAMP-EIA kit (RPN225; Amersham-Pharmacia Biotech) in duplicate.
Statistical Analysis
Serum Cyr61 levels in each group were compared by an unpaired t-test. Correlations between serum Cyr61 and PSA levels were analyzed by a Pearson correlation test. Statistical tests were one-sided and P-values < 0.05 were considered to be statistically significant. The creation of receiver operating characteristics (ROC) curve and the calculation of area under the curve (AUC) were performed using a web-based calculator (http://www.rad.jhmi.edu/jeng/javarad/roc/JROCFITi). PSA recurrence was compared using log-rank test in Kaplan–Meier survival curves.
RESULTS
Cyr61 in PCa Cell Culture Medium and Human Serum Samples can be Measured by Sandwich ELISA
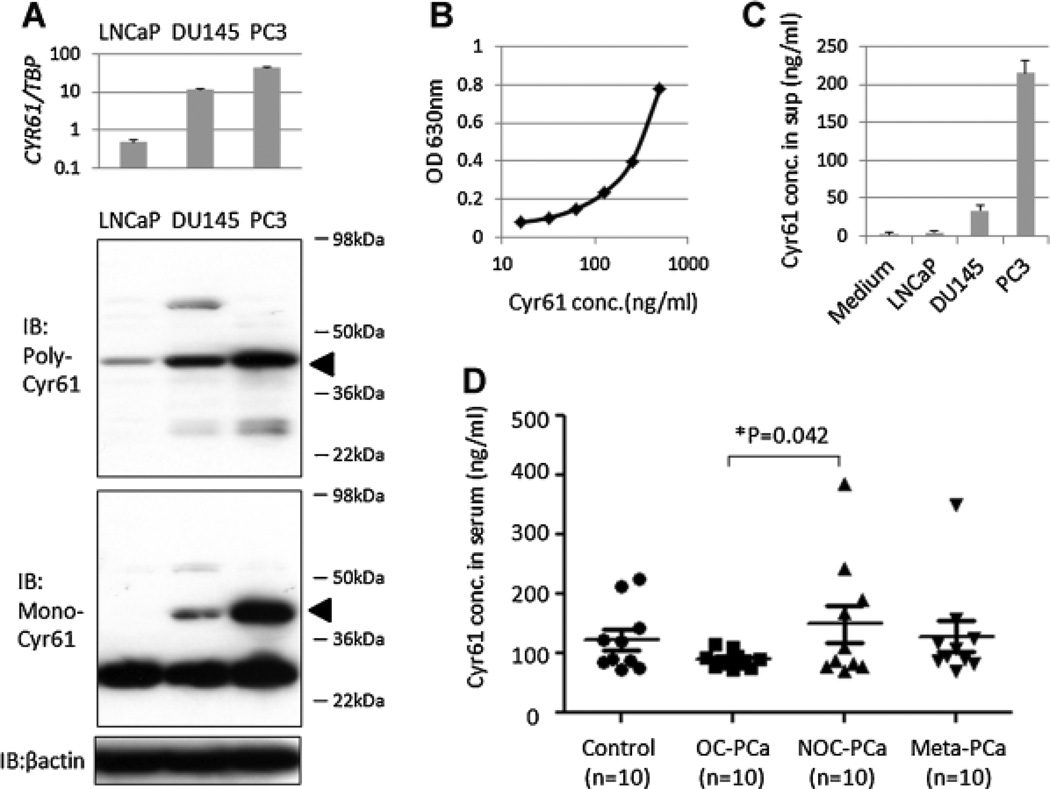
The possibility that Cyr61 was secreted by PCa cells into the culture medium and could be quantitatively determined was examined. As a first step in this direction, the expression of Cyr61 in these cell lines was evaluated. The mean Cyr61 mRNA expression ratios to TBP in the three PCa cell lines, namely LNCaP, DU145, and PC3, were examined and were 0.5, 11.5, and 42.7, respectively (Fig. 1A). Furthermore, there was a positive correlation between Cyr61 mRNA expression and the corresponding protein expression in these cells as detected by western blotting using two different anti-Cyr61 antibodies. The polyclonal antibody used as a capture antibody in a sandwich ELISA showed a specific Cyr61 band at 40 kDa. The monoclonal antibody used as a detection antibody showed a nonspecific band at 30 kDa. However, the expression levels of the Cyr61 band at 40 kDa were more significantly different among the cell lines (Fig. 1A).
Fig. 1.
Cyr61 levels in PCa cell supernatants and human serum samples can be measured by sandwich ELISA. A: Cyr61 mRNA expression levels in real-time PCR (upper) and Cyr61 protein expression levels in western blot using the polyclonal anti-Cyr61 (middle) and monoclonal anti-Cyr61 antibodies (lower) in LNCaP, DU145, and PC3 cells. The estimated molecular weight of Cyr61 is 40 kDa (arrows). B: Standard curve of Cyr61 protein concentrations and optical density (OD) in 630 nmusing Cyr61 recombinant protein. C: Cyr61 concentrations in the supernatant of no cells (medium), LNCaP, DU145, and PC3 cells. All cells are 80% confluent in 10-cm dishes. D: Cyr61 concentrations in serum samples obtained from control (n = 10), OC-PCa (n = 10), NOC-PCa (n = 10), and metastatic (Meta)-PCa(n = 10) patients, demonstrating significant difference between OC-PCa and NOC-PCa (*P = 0.042).
A sandwich ELISA using these two antibodies was established to determine the amount of Cyr61 protein secreted by these cell lines. Based on the standard curve (Fig. 1B), the Cyr61 concentrations in media alone was 1.1 ng/ml and those in the supernatants containing 80% confluent LNCaP, DU145, and PC3 cells were 4.0, 33.5, and 215.1 ng/ml, respectively (Fig. 1C), indicating that Cyr61 protein secreted by the PCa cells could be measured by the sandwich ELISA. Cyr61 levels were then measured in human serum samples utilizing the same method. The mean ± SD Cyr61 levels in the serum obtained from controls, OC-PCa, NOC-PCa, and metastatic-PCa patients were 123.4 ± 17.6 (reference), 89.8 ± 4.5 (P = 0.040 vs. controls), 149.4 ± 39.2 (P = 0.244 vs. controls), and 128.7 ± 26.0 (P = 0.434 vs. controls) ng/ml, respectively (Fig. 1D). In these patient groups the mean ± SD PSA levels were 6.1 ± 2.2 (reference), 5.3 ± 1.0 (P = 0.360 vs. controls), 5.2 ± 0.5 (P = 0.347 vs. controls) and 204.5 ± 99.0 (P = 0.030 vs. controls) ng/ml, respectively. Cyr61 levels were significantly different among OC-PCa and NOC-PCa patients (P = 0.042), although PSA levels were not significantly different (P = 0.472).
Serum Cyr61 Levels Differentiate NOC-PCa From OC-PCa More AccuratelyThan PSA
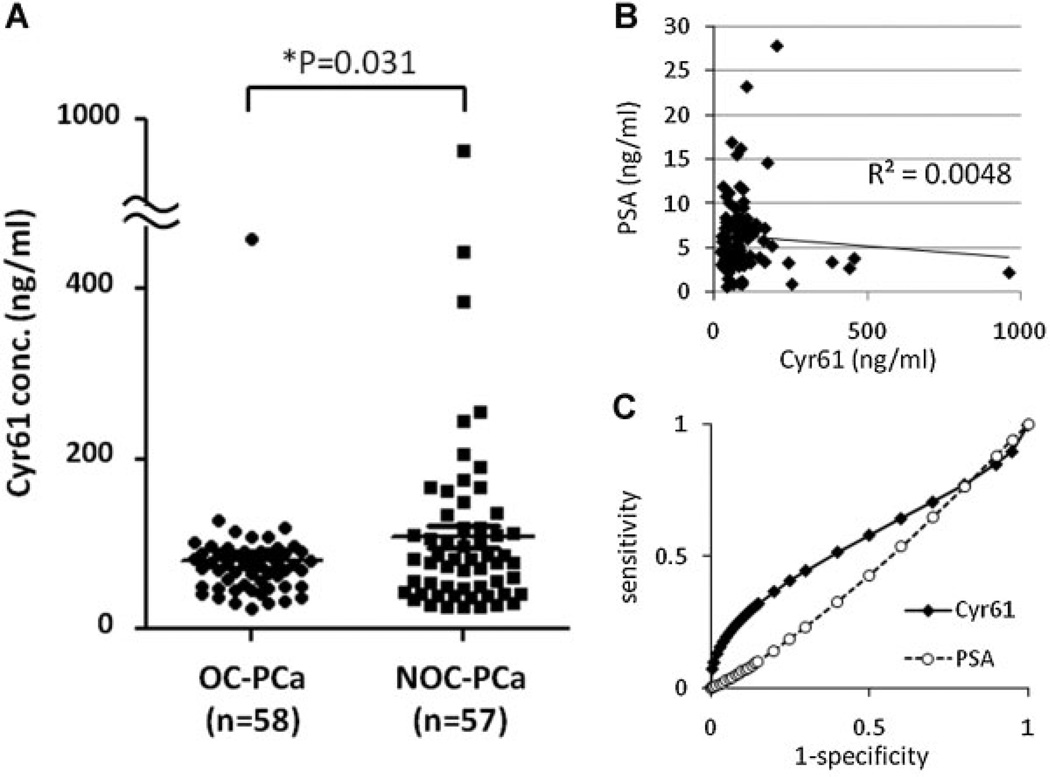
To further assess the ability of serum Cyr61 levels to predict PCa aggressiveness, additional serum samples were obtained from OC-PCa (n = 58) and NOC-PCa (n = 57) patients. The characteristics of the patients in these groups are shown in Table I. As expected, Gleason sum scores at the time of surgery were significantly higher in NOC-PCa than in OC-PCa (P < 0.001). Interestingly, serum Cyr61 levels were also significantly higher in NOC-PCa (116.3 ± 140.2 ng/ml) than in OC-PCa (79.7 ± 56.0) (P = 0.031) although there was no significant difference in serum PSA levels (P = 0.376) (Fig. 2A) and as a result, there was no significant correlation between serum PSA and Cyr61 levels (R2 = 0.0048) (Fig. 2B). Utilizing ROC curves for differentiating NOC-PCa from OC-PCa, the AUC of PSA and Cyr61 were 0.451 and 0.568, respectively (Fig. 2C). Using the cut-off value of 100 ng/ml, the sensitivity and specificity of Cyr61 for differentiating NOC-PCa from OC-PCa were 75.9 and 59.3%, respectively. The sensitivity and specificity of PSA using the cut-off value of 6 ng/ml were 38.5 and 51.9%, respectively. Together, these results indicate that in the patient sets evaluated, serum Cyr61 levels can more accurately differentiate NOC-PCa from OC-PCa than serum PSA.
TABLE I.
Characteristics of PCa Patients
| OC-PCa | NOC-PCa | P* | |
|---|---|---|---|
| Number | 58 | 57 | |
| Age (years) | 58.1 ± 7.6 | 59.6 ± 6.5 | 0.132 |
| PSA (ng/ml) | 6.4 ± 3.8 | 6.1 ± 4.7 | 0.376 |
| Gleason sam | 6.2 ± 0.5 | 6.7 ± 0.7 | <0.001 |
| Clinical stage | 0.976 | ||
| T1c | 45 | 40 | |
| T2 | 10 | 11 | |
| T3 | 1 | 0 | |
| NA | 2 | 6 | |
| Non-rec | Rec | P* | |
| Number | 10 | 10 | |
| Age (years) | 56.0 ± 7.6 | 59.6 ± 4.8 | 0.110 |
| PSA (ng/ml) | 6.7 ± 3.5 | 11.0 ± 9.0 | 0.090 |
| Gleason sam | 6.3 ± 0.5 | 7.1 ± 0.6 | 0.002 |
| Clinical stage | 0.626 | ||
| T1c | 8 | 6 | |
| T2 | 2 | 4 | |
| T3 | 0 | 0 | |
unpaired t-test and chi-square test.
Fig. 2.
Serum Cyr61 levels can differentiate NOC-PCa from OC-PCa more accurately than serum PSA. A: Cyr61 concentrations in serum samples obtained from organ-confined (OC)-PCa (n = 58) and non-organ-confined (NOC)-PCa (n = 57) patients, demonstrating significant difference (*P = 0.031). B: Scatter diagram of serum Cyr61 and PSA levels in PCa patients, demonstrating no significant correlation (R2 = 0.0048). C: Receiver operating characteristics (ROC) curve for differentiating NOC-PCa from OC-PCa.
PCa Patients With Lower Serum Cyr61 Levels May Have a Higher Risk of Disease Recurrence
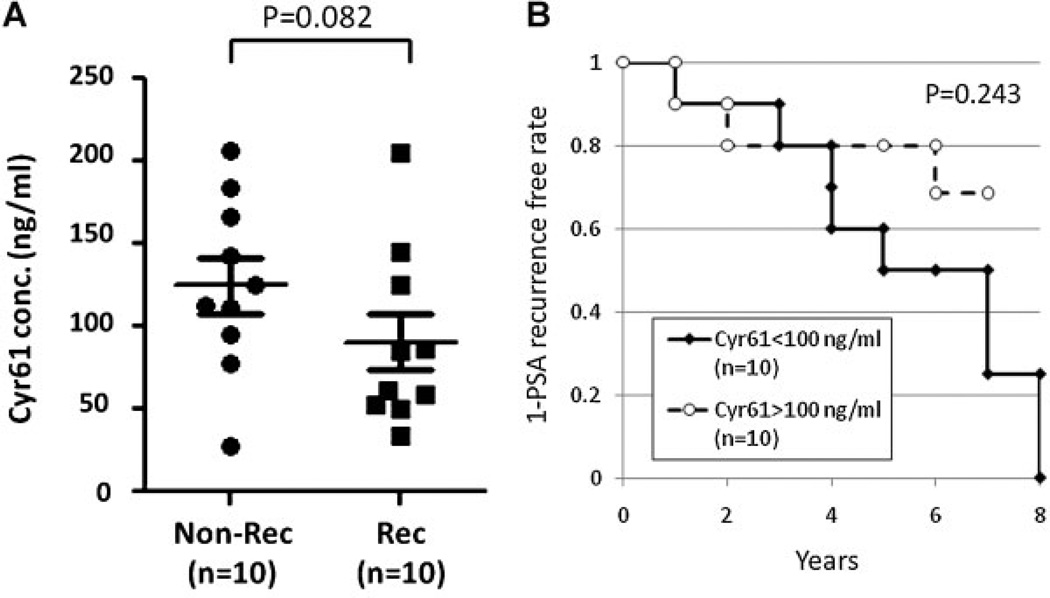
The Gleason score of the surgical specimens remains the most prognostic pathological feature [22]. When serum Cyr61 levels were compared among each Gleason sum group, they tended to be lower in high Gleason sum groups although the differences were not significant (Supplement Fig. 1S). To further address the question of whether serum Cyr61 levels could predict disease progression after surgery, we collected serum samples from PCa patients that later recurred (Rec; n = 10) and those that did not recur within a similar time frame (Non-Rec; n = 10). The characteristics of the patients in these two groups are shown in Table I. The mean ± SD time to PSA recurrence after surgery was 3.6 ± 2.1 year. The serum Cyr61 levels in the Rec patients (90.5 ± 17.0 ng/ml) tended to be lower than in the non-rec patients (125.0 ± 16.7 ng/ml) although these differences were not statistically significant (P = 0.082) (Fig. 3A). A Kaplan–Meier curve for PSA recurrence free survival between high serum Cyr61 (>100 ng/ml) and low serum Cyr61 (<100 ng/ml) also did not show a significant difference (P = 0.243). However, the mean ± SD Cyr61 levels in patients with PSA recurrence at more than 3 years after surgery (n = 6) were 69.0 ± 53.4 ng/ml, which was significantly lower than those in patients without PSA recurrence (P = 0.020). These results indicate that PCa patients with lower serum Cyr61 levels might have PCa with high Gleason score and have a higher risk of PSA recurrence.
Fig. 3.
PCa patients with lower serum Cyr61 levels tend to have a higher risk of PSA recurrence. A: Cyr61 concentrations in serum samples obtained from PCa patients without PSA recurrence (Non-Rec, n = 10) and those with PSA recurrence (Rec, n = 10), demonstrating no significant difference (P = 0.082). B: Kaplan–Meier curve for PSA recurrence-free survival in PCa patients with lower (<100 ng/ml) and higher (>100 ng/ml) Cyr61 levels (P = 0.243).
LPA and Androgen Stimulation Enhance Cell Invasion and Induce Cyr61 Up-regulation via cAMP/PKA Pathway Activation
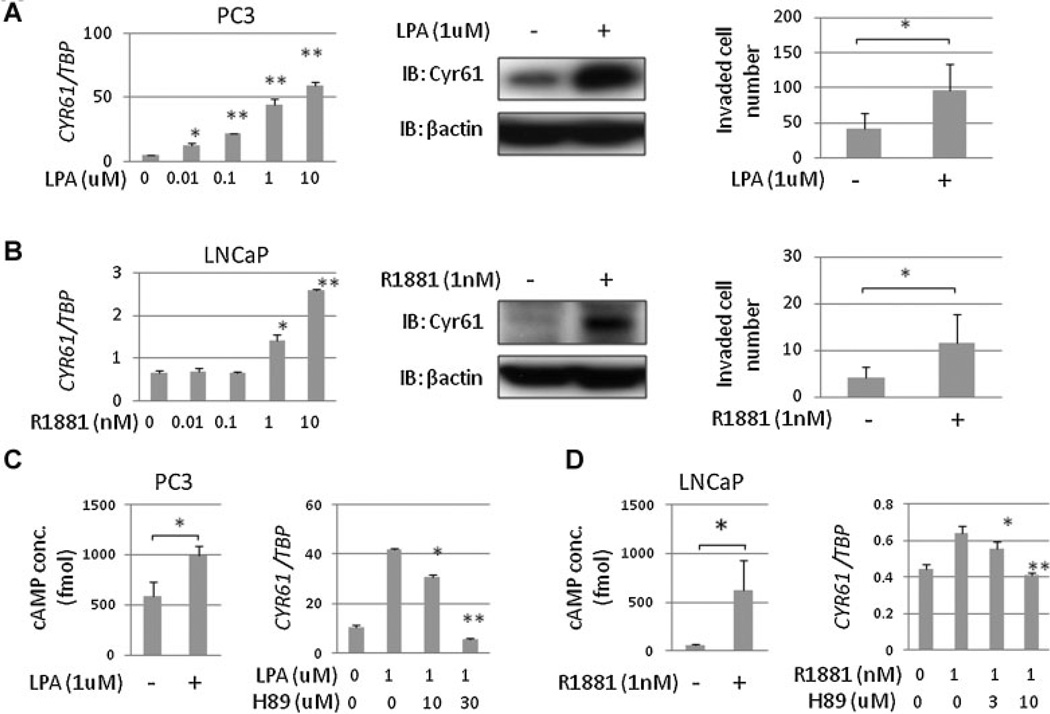
Based upon our immunohistochemical analysis of PCa tissue samples reported previously [15,16] together with serum Cyr61 levels of PCa patients in the present study, we hypothesized that Cyr61 expression increases during early stage PCa development but decreases during late stage PCa progression. To test this hypothesis, we performed in vitro experiments using PCa cell lines. Previous studies demonstrated that Cyr61 expression is enhanced by several kinds of growth factors, cytokines, and hormones [23]. We explored oncogenic (stimulating) and anti-oncogenic (stress) conditions to determine if they regulate Cyr61 expression. LPA is a serum phospholipid that has been associated with progression in several types of cancer including PCa [24,25]. In PC3 cells, LPA enhanced Cyr61 expression levels in a dose-dependent manner. In Matrigel invasion assays, cell invasion was promoted by 1 µM of LPA under serum-free medium (Fig. 4A). On the other hand, androgens have been well demonstrated to play a major role both in normal prostatic regulation and in PCa progression [26]. In LNCaP cells Cyr61 expression levels were enhanced by the synthetic androgen R1881 at concentrations of 1 nM or higher, which also promoted cell invasion (Fig. 4B). LPA and androgen stimulation increased intracellular cAMP levels and LPA- and androgen-induced Cyr61 up-regulation was suppressed by PKA inhibition with H-89 (Fig. 4C). These results indicate that “stimulation” with LPA or androgen enhanced Cyr61 expression levels via cAMP/PKA pathway activation in PCa cells.
Fig. 4.
Cyr61 up-regulation and cell invasion were induced by LPA or androgen stimulation via cAMP/PKA pathway. A: Cyr61 mRNA levels in PC3 cells under 0.01–10 µM lysophosphatidic acid (LPA) stimulation for 2 hr in serum-free medium containing 0.1% BSA (left). Cyr61 protein expression in PC3 cells ±1 µM LPA (middle). Invaded cell numbers in Matrigel invasion assays of PC3 cells ±1 µM LPA stimulation for 72 hr (right). B: Cyr61 mRNA levels in LNCaP cells under 0.01–10 nM R1881 stimulation for 24 hr in androgen-depleted medium (left). Cyr61 Protein expression in LNCaP cells ±1 nM R1881 (middle). Invaded cell numbers in Matrigel invasion assays of LNCaP cells ±1 nM R1881 stimulation for 72 hr (right). C: Intracellular cAMP concentrations in PC3 cells ±1 µM LPA stimulation for 1 hr (left) and Cyr61 mRNA expression levels with H-89 administration under 1 µM LPA stimulation for 2 hr. D: Intracellular cAMP concentrations in LNCaP cells ±1 nM R1881 stimulation for 1 hr (left) and Cyr61 mRNA expression levels with H-89 administration under 1 nM R1881 stimulation for 24 hr. *P < 0.05, **P < 0.005.
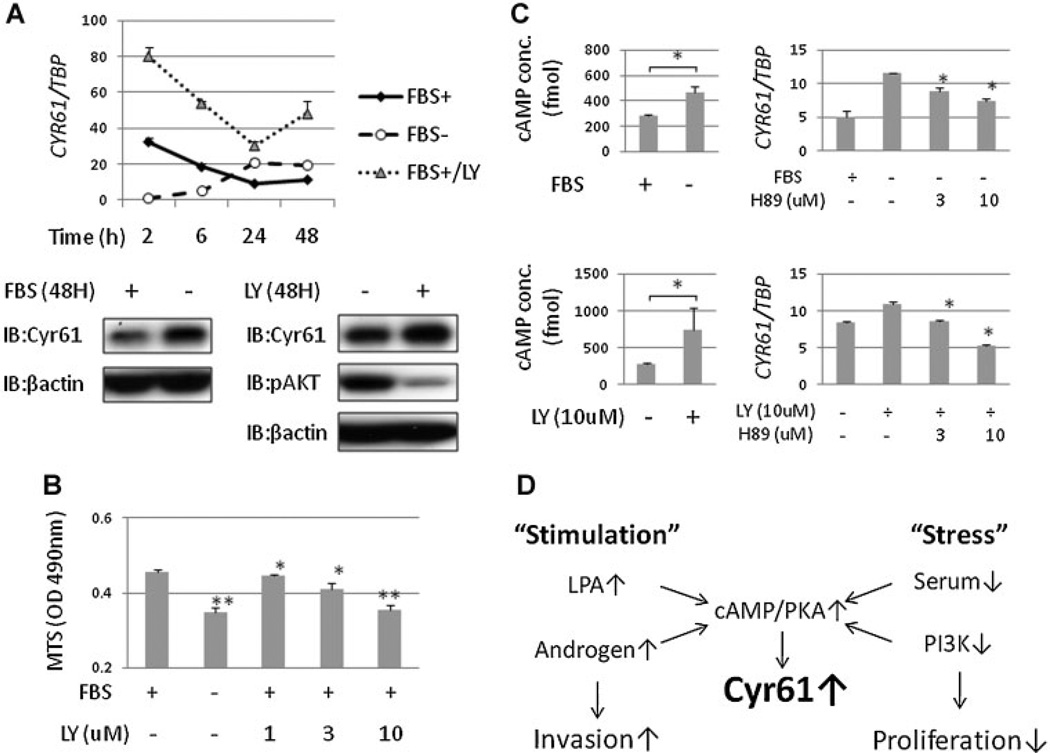
Serum Starvation and PI3K Inhibition Suppresses PCa Cell Proliferation and Induces Cyr61 Up-Regulation
We previously reported that Cyr61 expression is enhanced by short-term FBS stimulation in a dose-dependent manner [27]. In this study, we examined the sequential changes in Cyr61 expression in PC3 cells. Two hours after adding fresh medium, Cyr61 expression levels were higher in the medium with 10% FBS than without FBS. However, the levels decreased after incubation for longer than 6 hr. Cyr61 expression levels upon exposure to medium without FBS increased after incubation for longer than 24 hr (Fig. 5A). On the other hand, the PI3K/AKT pathway has been implicated in prostate carcinogenesis and PCa progression [28]. PI3K inhibition with LY294002 more significantly enhanced Cyr61 expression levels than serum starvation (Fig. 5A). Cell proliferation was significantly suppressed by serum starvation or PI3K inhibition (Fig. 5B). Serum starvation and PI3K inhibition increased intracellular cAMP concentrations and Cyr61 up-regulation was suppressed by PKA inhibition with H-89 (Fig. 5C). These results indicated that “stress” with serum starvation or PI3K inhibition enhanced Cyr61 expression levels via the cAMP/PKA pathway activation. Taken together, oncogenic “stimulation” such as LPA or androgen and anti-oncogenic “stress” such as serum starvation or PI3K inhibition activates the cAMP/PKA pathway and induces Cyr61 up-regulation. These mechanisms may partially explain the biphasic change of Cyr61 during PCa progression (Fig. 5D).
Fig. 5.
Cyr61 expression is up-regulated by long-term serum starvation and PI3K inhibition via cAMP/PKA pathway.A: Cyr61 mRNA levels in PC3 cells incubated with or without 10% FBS and with LY294002 (LY) for 2, 6, 24, and 48 hours after medium change. B: Optical density (OD) measured by MTS cell proliferation assay of PC3 with or without FBS and 1–10 µM LY for 48 hours. C: Intracellular cAMP concentrations in PC3 cells ±10% FBS (upper left) and ±10 µM LY (lower left) for 1 hr and Cyr61 mRNA expression levels with H-89 administration under serum starvation (upper right) and LY administration (lower right) for 24 hr. D: “Stimulation” with LPA or androgen enhances cell invasion and “stress” with serum starvation or PI3K inhibition suppresses cell proliferation. They activate cAMP/PKA pathway and induce Cyr61 up-regulation.
DISCUSSION
Recent studies from our laboratory suggest that Cyr61 might serve as a tissue biomarker for characterizing the aggressiveness of PCa [15,16]. Cyr61 is an extracellular matrix protein that is secreted and therefore, represents a good candidate as a serum biomarker. Previously, we had established a sandwich ELISA for measuring Cyr61 concentrations in cell supernatants using polyclonal anti-Cyr61 antibodies [27]. Using the same method, serum Cyr61 levels were measured but failed to show any significant difference between the various patient cohorts, presumably due to high background signal. In contrast, in the present study, using monoclonal Cyr61 antibodies, we observed that the serum Cyr61 levels were significantly higher in NOC-PCa than in OC-PCa patients. As serum Cyr61 levels are not correlated with serum PSA levels, they may increase the accuracy for differentiating NOC-PCa from OC-PCa combined with serum PSA levels and other clinical parameters used in PCa staging nomograms [29].
However, as shown in Figure 1D, serum Cyr61 levels tended to be higher in control patients compared with OC-PCa patients. This may be due to the fact that in these control patients, PSA levels are high although their prostate biopsies were found to be negative, suggesting that inflammation in their prostate tissues might have induced PSA production [30]. Interestingly, Cyr61 expression in benign prostate cells was enhanced by treating with prostaglandin E2 (PGE2) or transforming growth factor β (TGFβ) [31]. Therefore, it is quite possible that inflammation in benign prostate tissues induced Cyr61 production in control patients. These results indicated that serum Cyr61 levels could differentiate NOC-PCa from OC-PCa but could not differentiate PCa patients from control individuals.
Previous reports showed that Cyr61 knock down suppressed cell proliferation in DU145 and PC3 cells [14]. We also found that Cyr61 knock down by siRNA suppressed proliferation and LPA-induced invasion of PC3 cells (Supplement Fig. S2). In other benign prostate (BPH1 and BRF55T) and PCa (DU145) cell lines, LPA enhanced Cyr61 expression and cell invasion but not in LNCaP cells (Supplement Fig. S3). A recent report demonstrated that mice transgenic for LPA receptors and autotoxin, the primary enzyme producing LPA, in mammary epithelium induced a high frequency of late-onset, invasive, and metastatic mammary cancer, indicating that LPA could contribute to the initiation and progression of breast cancer [32]. Interestingly, LPA also appears to be associated with PCa initiation and progression [25]. In this study, LPA stimulation enhanced both cell invasion and Cyr61 expression in benign prostate and PCa cell lines. However, LPA stimulation did not enhance cell proliferation (data not shown). LPA receptor (LPAR) is one of the G-protein-coupled receptors (GPCRs) and some GPCRs were reported to be associated with cancer initiation and progression [33,34]. GPCR regulates intracellular cAMP concentrations and PKA activation [18]. We show that LPA-induced Cyr61 up-regulation was associated with cAMP/PKA pathway activation. It has also been reported that most GPCRs expressed in PCa can stimulate extracellular signal regulated kinases (ERK) [35]. LPA enhanced ERK phosphorylation and LPA-induced Cyr61 up-regulation was slightly suppressed by the ERK inhibitor (PD98059) (data not shown), indicating that the ERK pathway is also associated with LPA-induced Cyr61 up-regulation. It was reported that prostate and PCa tissues express LPAR 1, 2, and 3 [36]. In LPARs, LPAR1 expression is mostly correlated with Cyr61 expression and only knocking down LPAR1 significantly suppressed LPA induced Cyr61 up-regulation (Supplement Fig. S4), indicating that LPAR1 is associated with LPA-induced Cyr61 up-regulation. LPA causes only a marginal increase in Cyr61 protein levels in DU145 cells, even when they express LPAR1. Intracellular cAMP concentrations in PC3 cells were significantly increased by LPA stimulation. However, those were not changed in DU145 cells (data not shown). Moreover, knocking down Cyr61 did not suppress LPA-induced cell invasion in DU145 cells. Interestingly, LPA-induced ERK phosphorylation was more significant in DU145 than in PC3 cells (data not shown). It was suggested that downstream signals of LPAR1 were different between PC3 and DU145 cells. To elucidate the exact mechanisms for the difference, further examination is needed.
Androgen ablation therapy has been the mainstay in the treatment of advanced metastatic PCa, indicating that androgens are associated with PCa progression [37]. To our knowledge, there has been no previous report showing the association between androgens and Cyr61. We found that 1 nM or more of androgen stimulation enhanced Cyr61 expression levels in LNCaP cells. However, androgen depletion or androgen stimulation of lower than 1 nM changed PSA expression but did not alter Cyr61 expression levels (Fig. 4B). Interestingly, 1 nM or more of androgen stimulation enhanced cell invasion but did not enhance cell proliferation, as it did with the LPA stimulation, indicating that stimulation-enhancing Cyr61 expression associates with cell invasion and not with cell proliferation. Androgen-induced Cyr61 up-regulation was slightly but significantly suppressed by androgen receptor (AR) knockdown (Supplement Fig. S5). However, no AR response element could be found in the promoter region of Cyr61. Therefore, it appears that AR regulates Cyr61 not directly but via other signal transductions such as the cAMP/PKA pathway. That might be a reason why there is no correlation between serum PSA and Cyr61 levels in PCa patients.
In our study population, Cyr61 could more accurately differentiate NOC-PCa from OC-PCa than PSA. Pretreatment knowledge of OC-PCa or NOC-PCa is important for treatment selection and planning. However, it was reported that approximately 10% of OC-PCa patients and 50% of NOC-PCa patients experienced disease progression within 10 years after surgery [38,39]. It is challenging to find serum markers that can identify patients with disease progression preoperatively. Most recently, we observed that decreased expression of Cyr61 is associated with PCa recurrence after surgical treatment [16]. In this study, serum Cyr61 levels tended to be lower in Rec-PCa patients than in Non-rec-PCa patients although these differences were not significant. To elucidate whether serum Cyr61 levels can predict PCa recurrence, further examination in a larger number of patients is needed.
The tumor micro-environment has been associated with PCa progression [40]. Cyr61 is regulated by various growth factors, chemokines, and hormones [41]. In our study, Cyr61 expression levels are enhanced by long-term serum starvation and PI3K inhibition which suppressed cell proliferation. These changes were transduced by the same cAMP/PKA pathway as LPA- or androgen-induced Cyr61 up-regulation. It was reported that short-term PKA activation enhanced AR activation and induced PCa progression. However, long term PKA activation induced PCa cell differentiation and suppressed their proliferation [20,42]. These reports suggested that PKA activation might be increased in early stage well-differentiated PCa with AR expression but decreased in late stage poorly differentiated PCa. Gene expression data from the Gene Expression Omnibus (GEO) database show that Cyr61 expression levels are higher in PCa and benign prostate tissues adjacent to tumor compared with normal prostate tissues, but lower in metastatic tissues (Supplement Fig. S6). As shown in Figure 1D, Cyr61 levels in metastatic-PCa patients tended to be lower than in NOC-PCa patients. Therefore, it is possible that Cyr61 expression levels in PCa cells increase during local progression but decrease after acquiring metastatic potential and subsequent metastasis, correlating with PKA activation.
Based on our results, we hypothesize that one of the mechanisms for biphasic changes in Cyr61 during PCa progression is as follows: LPA or androgen stimulation increases PCa cell invasiveness and also up-regulates Cyr61 expression via cAMP/PKA pathway activation. Cyr61 is an angiogenic inducer and enhances tumor vascularization, which makes PCa cells free from ischemic stress and decreases Cyr61 expression. PI3K/AKT activation caused by loss of PTEN or other signal transductions enhances PCa cell aggressiveness, but suppresses Cyr61 expression via cAMP/PKA pathway inactivation.
CONCLUSIONS
We developed an ELISA method for measuring serum Cyr61 levels in PCa patients. Serum Cyr61 levels could differentiate NOC-PCa from OC-PCa more accurately than PSA. However, those with lower serum Cyr61 levels might have a higher risk of disease recurrence. The changes in Cyr61 expression during PCa progression were associated with the cAMP/PKA pathway activation. Cyr61 expression in PCa appears to be modulated by oncogenic “stimulation” as well as certain anti-oncogenic “stress” factors in the tumor environment, and thus may represent a novel serum marker for PCa aggressiveness.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Mario Eisenberger and Elizabeth Humphreys (The James Buchanan Brady Urological Institute, The Johns Hopkins University School of Medicine) for sample collection and members of the Getzenberg lab for many helpful discussions.
Supported by the NCI Prostate Cancer SPORE grant (P50 CA058236), the Patana Fund of the Brady Urological Institute, and a grant from the Patrick C. Walsh Fund of the Brady Urological Institute.
Grant sponsor: NCI Prostate Cancer SPORE; Grant numbers: P50; CA058236; Grant sponsor: Patana Fund of the Brady Urological Institute; Grant sponsor: Patrick C. Walsh Fund of the Brady Urological Institute.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Parekh DJ, Ankerst DP, Troyer D, Srivastava S, Thompson IM. Biomarkers for prostate cancer detection. J Urol. 2007;178(6):2252–2259. doi: 10.1016/j.juro.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 3.Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grzeszkiewicz TM, Lindner V, Chen N, Lam SC, Lau LF. The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemo-taxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology. 2002;143(4):1441–1450. doi: 10.1210/endo.143.4.8731. [DOI] [PubMed] [Google Scholar]
- 5.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1998;95(11):6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menendez JA, Mehmi I, Griggs DW, Lupu R. The angiogenic factor CYR61 in breast cancer: Molecular pathology and therapeutic perspectives. Endocr Relat Cancer. 2003;10(2):141–152. doi: 10.1677/erc.0.0100141. [DOI] [PubMed] [Google Scholar]
- 7.Lin MT, Zuon CY, Chang CC, Chen ST, Chen CP, Lin BR, Wang MY, Jeng YM, Chang KJ, Lee PH, Chen WJ, Kuo ML. Cyr61 induces gastric cancer cell motility/invasion via activation of the integrin/nuclear factor-kappaB/cyclooxygenase-2 signaling pathway. Clin Cancer Res. 2005;11(16):5809–5820. doi: 10.1158/1078-0432.CCR-04-2639. [DOI] [PubMed] [Google Scholar]
- 8.Chien W, Kumagai T, Miller CW, Desmond JC, Frank JM, Said JW, Koeffler HP. Cyr61 suppresses growth of human endometrial cancer cells. J Biol Chem. 2004;279(51):53087–53096. doi: 10.1074/jbc.M410254200. [DOI] [PubMed] [Google Scholar]
- 9.Bleau AM, Planque N, Perbal B. CCN proteins and cancer: Two to tango. Front Biosci. 2005;10:998–1009. doi: 10.2741/1594. [DOI] [PubMed] [Google Scholar]
- 10.Feng P, Wang B, Ren EC. Cyr61/CCN1 is a tumor suppressor in human hepatocellular carcinoma and involved in DNA damage response. Int J Biochem Cell Biol. 2008;40(1):98–109. doi: 10.1016/j.biocel.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Holloway SE, Beck AW, Girard L, Jaber MR, Barnett CC, Jr, Brekken RA, Fleming JB. Increased expression of Cyr61 (CCN1) identified in peritoneal metastases from human pancreatic cancer. J Am Coll Surg. 2005;200(3):371–377. doi: 10.1016/j.jamcollsurg.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Pilarsky CP, Schmidt U, Eissrich C, Stade J, Froschermaier SE, Haase M, Faller G, Kirchner TW, Wirth MP. Expression of the extracellular matrix signaling molecule Cyr61 is downregulated in prostate cancer. Prostate. 1998;36(2):85–91. doi: 10.1002/(sici)1097-0045(19980701)36:2<85::aid-pros3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Prakash K, Pirozzi G, Elashoff M, Munger W, Waga I, Dhir R, Kakehi Y, Getzenberg RH. Symptomatic and asymptomatic benign prostatic hyperplasia: Molecular differentiation by using microarrays. Proc Natl Acad Sci USA. 2002;99(11):7598–7603. doi: 10.1073/pnas.112191399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun ZJ, Wang Y, Cai Z, Chen PP, Tong XJ, Xie D. Involvement of Cyr61 in growth, migration, and metastasis of prostate cancer cells. Br J Cancer. 2008;99(10):1656–1667. doi: 10.1038/sj.bjc.6604712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Antonio KB, Toubaji A, Albadine R, Mondul AM, Platz EA, Netto GJ, Getzenberg RH. Extracellular matrix associated protein CYR61 is linked to prostate cancer development. J Urol. 2010;183(4):1604–1610. doi: 10.1016/j.juro.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Antonio KB, Schultz L, Albadine R, Mondul AM, Platz EA, Netto GJ, Getzenberg RH. Decreased expression of cyr61 is associated with prostate cancer recurrence after surgical treatment. Clin Cancer Res. 2010;16(23):5908–5913. doi: 10.1158/1078-0432.CCR-10-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamoto S, Yokoyama M, Zhang X, Prakash K, Nagao K, Hatanaka T, Getzenberg RH, Kakehi Y. Increased expression of CYR61, an extracellular matrix signaling protein, in human benign prostatic hyperplasia and its regulation by lysophosphatidic acid. Endocrinology. 2004;145(6):2929–2940. doi: 10.1210/en.2003-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: Subtypes and biological actions. Annu Rev Pharmacol Toxicol. 50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 19.Merkle D, Hoffmann R. Roles of cAMP and cAMP-dependent protein kinase in the progression of prostate cancer: Cross-talk with the androgen receptor. Cell Signal. 2011;23(3):507–515. doi: 10.1016/j.cellsig.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Cox ME, Deeble PD, Lakhani S, Parsons SJ. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: Implications for prostate cancer progression. Cancer Res. 1999;59(15):3821–3830. [PubMed] [Google Scholar]
- 21.Inoue T, Leman ES, Yeater DB, Getzenberg RH. The potential role of purine-rich element binding protein (PUR) alpha as a novel treatment target for hormone-refractory prostate cancer. Prostate. 2008;68(10):1048–1056. doi: 10.1002/pros.20764. [DOI] [PubMed] [Google Scholar]
- 22.Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003;170(5):1872–1876. doi: 10.1097/01.ju.0000091876.13656.2e. [DOI] [PubMed] [Google Scholar]
- 23.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41(4):771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3(8):582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni P, Getzenberg RH. High-fat diet, obesity and prostate disease: The ATX-LPA axis? Nat Clin Pract Urol. 2009;6(3):128–131. doi: 10.1038/ncpuro1311. [DOI] [PubMed] [Google Scholar]
- 26.Chuan YC, Pang ST, Cedazo-Minguez A, Norstedt G, Pousette A, Flores-Morales A. Androgen induction of prostate cancer cell invasion is mediated by ezrin. J Biol Chem. 2006;281(40):29938–29948. doi: 10.1074/jbc.M602237200. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto S, Yokoyama M, Prakash K, Tsuruha J, Masamoto S, Getzenberg RH, Kakehi Y. Development of quantitative detection assays for CYR61 as a new marker for benign prostatic hyperplasia. J Biomol Screen. 2003;8(6):701–711. doi: 10.1177/1087057103259159. [DOI] [PubMed] [Google Scholar]
- 28.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15(15):4799–4805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 29.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58(6):843–848. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 30.Kandirali E, Boran C, Serin E, Semercioz A, Metin A. Association of extent and aggressiveness of inflammation with serum PSA levels and PSA density in asymptomatic patients. Urology. 2007;70(4):743–747. doi: 10.1016/j.urology.2007.06.1102. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto S, Yokoyama M, Aoki M, Suzuki K, Kakehi Y, Saito Y. Induction and function of CYR61 (CCN1) in prostatic stromal and epithelial cells: CYR61 is required for prostatic cell proliferation. Prostate. 2004;61(4):305–317. doi: 10.1002/pros.20098. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC, Cui X, Murrow G, Coombes K, Muller W, Hung MC, Perou CM, Lee AV, Fang X, Mills GB. Expression of auto-taxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15(6):539–550. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7(2):79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 34.Terada N, Shimizu Y, Kamba T, Inoue T, Maeno A, Kobayashi T, Nakamura E, Kamoto T, Kanaji T, Maruyama T, Mikami Y, Toda Y, Matsuoka T, Okuno Y, Tsujimoto G, Narumiya S, Ogawa O. Identification of EP4 as a potential target for the treatment of castration-resistant prostate cancer using a novel xenograft model. Cancer Res. 2010;70(4):1606–1615. doi: 10.1158/0008-5472.CAN-09-2984. [DOI] [PubMed] [Google Scholar]
- 35.Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: Emerging paradigms. Trends Pharmacol Sci. 2001;22(7):368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Y, Kakehi Y, Nouh MA, Tsunemori H, Sugimoto M, Wu XX. Gene expression profiles of lysophosphatidic acid-related molecules in the prostate: Relevance to prostate cancer and benign hyperplasia. Prostate. 2009;69(3):283–292. doi: 10.1002/pros.20879. [DOI] [PubMed] [Google Scholar]
- 37.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 38.Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993;71(11):3582–3593. doi: 10.1002/1097-0142(19930601)71:11<3582::aid-cncr2820711120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 39.Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167(2 Pt 1):528–534. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 40.Karan D, Thrasher JB, Lubaroff D. Prostate cancer: Genes, environment, immunity and the use of immunotherapy. Prostate Cancer Prostatic Dis. 2008;11(3):230–236. doi: 10.1038/pcan.2008.3. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Du XY. Functional properties and intracellular signaling of CCN1/Cyr61. J Cell Biochem. 2007;100(6):1337–1345. doi: 10.1002/jcb.21194. [DOI] [PubMed] [Google Scholar]
- 42.Kasbohm EA, Guo R, Yowell CW, Bagchi G, Kelly P, Arora P, Casey PJ, Daaka Y. Androgen receptor activation by G(s) signaling in prostate cancer cells. J Biol Chem. 2005;280(12):11583–11589. doi: 10.1074/jbc.M414423200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.