Abstract
Pulmonary arterial hypertension is a serious disease with significant morbidity and mortality. While it can occur idiopathically, it is more commonly associated with other cardiac or lung diseases. While most of the available therapies were tested in adult populations, and most therapies in children remain off-label, new reports and randomized trials are emerging that inform the treatment of pediatric populations. This review discusses currently available therapies for pediatric pulmonary hypertension, their biologic rationales, and evidence for their clinical effectiveness.
Keywords: Pulmonary hypertension, Pulmonary vasculature, Vasodilator, Nitric Oxide, Phosphodiesterase, Prostacyclin, Endothelin
NORMAL PULMONARY VASCULAR TRANSITION
During fetal life, the placenta serves as the organ of gas exchange, and the fetal circulation uses a series of adaptive mechanisms to maximize delivery of oxygen to metabolically active organs such as the brain, gut and kidney. The most highly oxygenated blood enters the fetus via the umbilical vein. To efficiently direct oxygenated blood to the systemic circulation and minimize oxygen loss, almost 90% of fetal blood flow is diverted past the lungs through anatomic shunts through the foramen ovale and the ductus arteriosus. While the lung rapidly grows its network of small pulmonary arteries during the second half of gestation [1], blood flow in the pulmonary circulation remains highly restricted by hypoxic pulmonary vasoconstriction of small pulmonary arteries, which is reversed at birth by the sudden increase in lung oxygenation when the newborn takes his or her first breath. After birth, the first few breaths induce a rapid decrease in pulmonary vascular resistance and increase in pulmonary vascular flow in response to lung expansion, increased oxygen tension, and increased pH. Pulmonary artery pressure and vascular resistance decrease more slowly, with pulmonary arterial pressure reaching its nadir ~2–3 weeks after birth. However, the responsiveness to hypoxia is retained into adulthood, and pulmonary hypertension can be easily triggered in the newborn period by hypoxic lung disease, apnea, or other causes.
DEFINITION AND CLASSIFICATION OF PEDIATRIC PULMONARY HYPERTENSION
Pulmonary hypertension is necessary to support gas exchange in the fetus, but if pulmonary arterial pressure is elevated after birth or during infancy or childhood, then pulmonary hypertension becomes a serious medical problem with substantial mortality and morbidity. Pulmonary hypertension is classically defined as a mean pulmonary artery pressure of ≥25 mmHg at rest, with a pulmonary capillary wedge pressure of ≤ 15 mmHg. Numerous disease processes can produce pulmonary hypertension in both adults and children, but these two populations are quite different when considering classification, genetic causes, and in some cases, treatment. This is largely because the exposure of the developing lung to pathological and/or environmental insults affects lung adaptation, development, and growth, leading to far greater complexity of phenotypes [2]. The classifications of pulmonary hypertension introduced at the WHO Symposium in 1998 [3] and subsequently modified at the Venice and Dana Point Symposia [4, 5] were primarily designed for use in adult diseases, and have been difficult to apply to pediatric populations. A new pediatric classification scheme was developed by an expert panel in Panama City in 2011 to better address the developmental underpinnings of pulmonary vascular disease in children [2, 6]. For instance, while idiopathic pulmonary arterial hypertension occurs in children, pulmonary hypertension very commonly occurs in association with congenital heart disease, or other lung diseases such as lung hypoplasia or bronchopulmonary dysplasia (BPD). The latter group appears to be growing, and represents a significant proportion of patients followed by pediatric pulmonary hypertension programs. Pulmonary hypertension affects roughly one-third of infants with moderate to severe BPD [7, 8], and results in greater morbidity and mortality, poor growth and neurodevelopmental outcome, long term mechanical ventilation support, and death due to right heart dysfunction and multi-organ failure [9, 10]. Pulmonary vascular disease also contributes to the morbidity and mortality of other pediatric diseases such as sickle cell disease, interstitial lung diseases, and cystic fibrosis [11]. Relatively little is known about the epidemiology of pediatric pulmonary hypertension, and comprehensive registries to support phenotyping and clinical research are needed [12].
Persistent pulmonary hypertension of the newborn (PPHN) is a unique and specific type of pulmonary hypertension that occurs in the neonatal period as a failure to achieve the normal drop in pulmonary vascular resistance in the early neonatal period. PPHN is a syndrome that complicates a wide range of neonatal cardiopulmonary diseases, and affects up to 10% of all preterm and term neonates that require NICU support for respiratory failure. Moderate or severe PPHN affects up to 2–6 per 1,000 live births, and complicates the course of 10% of all infants admitted to neonatal intensive care [13]. These circulatory abnormalities are also responsible for an 8–10% risk of death and a 25% risk of long-term neurodevelopmental morbidity. The long-term impact of PPHN on pediatric and adult pulmonary vascular function is another important area for future research.
PHARMACOTHERAPY OF PULMONARY HYPERTENSION
The aims of therapy for pulmonary arterial hypertension are selective pulmonary vasodilation, restoration of normal endothelial function, and reversal of remodeling of the pulmonary vasculature. All of these serve to reduce right ventricular afterload and prevent right ventricular failure. The choice of agents will often depend on the severity and acuity of illness – for instance, acute pulmonary vasodilation is needed for PPHN and acute pulmonary hypertensive crises after cardiopulmonary bypass, but long-term therapy may focus more on vascular remodeling. The main therapeutic avenues involve the nitric oxide (NO), prostacyclin, and endothelin pathways, which are summarized in excellent recent comprehensive reviews [11, 14, 15]. It is also important to note that the scientific understanding and therapeutic management of pulmonary hypertension are changing rapidly.
Nitric oxide
Nitric oxide (NO) is synthesized from the terminal nitrogen of L-arginine by the enzyme nitric oxide synthase (NOS). Three isoforms of NOS are present in the lung, although endothelial NOS is regarded as the most important regulator of NO production in the lung vasculature. NO is a gas molecule that diffuses freely from the endothelium to the vascular smooth muscle cell. The biologic effects of NO in vascular smooth muscle are mediated primarily through activation of soluble guanylate cyclase, which converts GTP to cGMP (Figure 1). cGMP serves as a second messenger that relaxes vascular smooth muscle through activation of cGMP-gated ion channels and activation of cGMP-dependent protein kinase. However, recent studies indicate that alternative NO signaling pathways may also exist through reaction of NO with protein thiols to form S-nitrosothiols (SNO), which may induce vasodilation or protein modification [16].
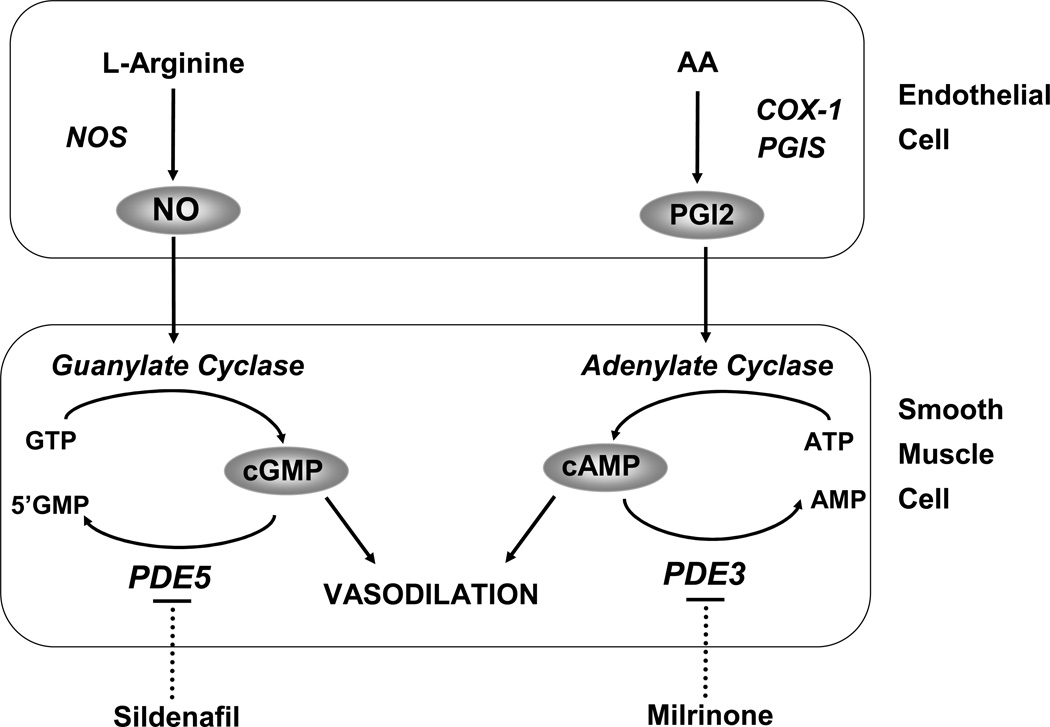
Figure 1. Nitric oxide (NO) and prostacyclin (PGI2) signaling pathways in the regulation of pulmonary vascular tone.
NO is synthesized by nitric oxide synthase (NOS) from the terminal nitrogen of L-arginine. NO stimulates soluble guanylate cyclase (sGC) to increase intracellular cGMP. PGI2 is an arachidonic acid (AA) metabolite formed by cyclooxygenase (COX-1) and prostacyclin synthase (PGIS) in the vascular endothelium. PGI2 stimulates adenylate cyclase in vascular smooth muscle cells, which increases intracellular cAMP. Both cGMP and cAMP indirectly decrease free cytosolic calcium, resulting in smooth muscle relaxation. Specific phosphodiesterases hydrolyze cGMP and cAMP, thus regulating the intensity and duration of their vascular effects. Inhibition of these phosphodiesterases with agents such as sildenafil and milrinone may enhance pulmonary vasodilation. Reproduced from [84], with permission.
Lung eNOS mRNA and protein are present in the early fetus, but both increase toward the end of gestation, readying the lung to adapt to the postnatal need for pulmonary vasodilation. This increase in lung endothelial NOS content explains the emergence of responsiveness to endothelium-dependent vasodilators, such as oxygen and acetylcholine, in late gestation [17]. Many factors associated with pulmonary hypertension have the capacity to perturb eNOS function, even if protein levels are sufficient. Presumably, this is because the normal catalytic function of eNOS depends on numerous post-translational modifications, including association with the chaperone protein Hsp90 and availability of essential substrates and cofactors including L-arginine, tetrahydrobiopterin (BH4), NADPH and calcium/calmodulin. Depletion of Hsp90 or biopterin will reduce production or bioavailability of NO, and will also “uncouple” eNOS, resulting in incomplete reduction of molecular oxygen with subsequent formation of superoxide, essentially turning the enzyme into a source of oxidant stress [18, 19].
Nitric oxide is currently FDA approved only for treatment of PPHN in the neonatal period. Since eNOS is decreased or dysfunctional in PPHN, iNO is thought to provide specific replacement therapy that is inhaled directly to airspaces approximating the pulmonary vascular bed. While it is most commonly administered with mechanical ventilation, iNO can also be provided via CPAP or nasal cannula devices, although the concentration may need to be increased to account for the entrainment of room air [20].
The safety and efficacy of iNO for PPHN have been particularly well studied through large placebo-controlled trials. iNO significantly decreases the need for ECMO support in newborns with PPHN, although mortality or length of hospitalization was not affected in any study. Data from the large registry maintained by the Extracorporeal Life Support Organization also indicate a 40% reduction in the number of neonates cannulated for ECMO, coincident with the FDA approval of iNO. By encompassing a range of disease severity, these studies also highlight that starting iNO for respiratory failure that is in earlier stages of evolution (for an oxygenation index of 15 to 25) does not decrease the incidence of ECMO and/or death or improve other patient outcomes [21, 22]. On the other hand, delaying iNO initiation until respiratory failure is advanced (oxygenation index of >40) may increase length of time on oxygen [23]. In longer term follow-up, iNO did not significantly alter the incidence of chronic lung disease or neurodevelopmental impairment relative to placebo.
Based on the available data, iNO should be initiated at a dose of 20 ppm after confirming PPHN and ruling out congenital heart disease and left ventricular dysfunction. A failure to respond to 20 ppm is only rarely followed by improvement at higher doses [24]. The greatest improvement in oxygenation occurs in infants with extra-pulmonary right-to-left shunting but without significant pulmonary parenchymal disease. Even if the total PVR does not decrease, iNO can improve oxygenation through a “microselective” effect that reduces intrapulmonary shunting. When iNO is stopped abruptly, “rebound” pulmonary hypertension can develop [25]. This potentially life-threatening phenomenon can occur even if no improvement in oxygenation was observed, and has raised questions about whether vascular cells respond to NO by upregulating vasoconstrictor pathways [26, 27]. However, from a practical standpoint, the clinical problem can usually be overcome by weaning iNO to 1 ppm prior to discontinuation.
iNO is used to treat many other forms of pediatric and neonatal pulmonary hypertension, although its role in those settings is less well defined. For instance, randomized studies have not demonstrated a consistent improvement in oxygenation in infants with congenital diaphragmatic hernia [28], although there appear to be benefits of stabilizing infants and preventing cardiac arrest before ECMO [29]. Up to one third of premature infants with bronchopulmonary dysplasia (BPD) will develop some degree of pulmonary hypertension or cor pulmonale [7, 8], and alterations in NO signaling appear to play a role in the vascular and lung injury [30]. A recent case series indicates that iNO may reduce established BPD-associated pulmonary hypertension to a greater degree than oxygen alone [31]. On the other hand, large clinical trials have shown inconsistent benefit when iNO is used to prevent BPD [32–35].
Outside of the NICU setting, the most common clinical use of iNO is for the infant or child with congenital heart disease with associated pulmonary hypertension. Postoperatively, iNO is frequently used to prevent or treat pulmonary hypertensive crises, improve oxygenation, and increase cardiac output. Pulmonary hypertension commonly develops in infants with congenital heart lesions associated with pulmonary overcirculation, such as truncus arteriosus or atrioventricular canal. If the heart lesion is not corrected, medial and intimal thickening occur in the pulmonary vasculature over time, which eventually leads to luminal obliteration. Acute life-threatening exacerbation of pulmonary hypertension following cardiopulmonary bypass is a serious clinical problem even in very young infants. In addition, children undergoing cavopulmonary connections for single ventricle lesions require low pulmonary vascular resistance for surgical success. A number of small single-center studies indicate that iNO can attenuate pulmonary hypertension in at-risk postoperative patients, reduce the number of pulmonary hypertension crises, and shorten time on mechanical ventilation [36]. In one larger randomized trial of pediatric patients, iNO (20 ppm) significantly decreased PVR and pulmonary hypertensive crises, and shortened the time to extubation readiness [37]. Responsivity to iNO has also been proposed as predictive of successful operability in patients with pulmonary hypertension associated with congenital heart disease [38].
In clinical studies of pediatric patients with severe acute respiratory distress syndrome (ARDS), inhaled NO produced selective pulmonary vasodilation and improved systemic oxygenation [39]. However, subsequent randomized controlled trials did not demonstrate a beneficial effect on mortality or duration of mechanical ventilation in pediatric or adult patients [40, 41]. While a transient improvement in oxygenation was observed in most of these studies, it has been speculated that since most patients dying from acute respiratory distress syndrome suffer from multiple organ systems failure, lung-selective therapy such as inhaled NO may not improve the overall survival rate [42]. These results likely indicate that the problem of pediatric respiratory failure involves much more than vascular dysfunction, and that there is still much to be learned about the role of NO in primary lung injury.
As our understanding of nitric oxide signaling evolves, strategies will emerge that enhance function of the native NOS enzyme. For instance, sufficient synthesis of L-arginine is necessary for optimal NOS function [43], and exogenous L-arginine supplementation enhances NOS activity in vitro. While arginine supplementation has been less successful when attempted in vivo, L-arginine can be endogenously synthesized from L-citrulline by a recycling pathway consisting of two enzymes, argininosuccinate synthase (AS) and argininosuccinate lyase (AL). Recent studies indicate that providing exogenous L-citrulline may reverse NOS dysfunction in animal models of neonatal pulmonary hypertension [44]. In clinical studies, oral L-citrulline increased both plasma citrulline and arginine levels in high-risk children undergoing cardiopulmonary bypass [45]. Intravenous L-citrulline has been shown to be safe and well tolerated in children undergoing cardiopulmonary bypass [46], and clinical trials are underway.
Sildenafil
Cyclic GMP is the second messenger that regulates contractility of smooth muscle through activation of cGMP-dependent kinases, phosphodiesterases and ion channels. In vascular smooth muscle cells, NO-mediated activation of soluble guanylate cyclase is a major source of cGMP production. Because cGMP is such a central mediator of vascular contractility, it is not surprising that its concentrations are regulated within a relatively narrow range to allow fine-tuning of vascular responses to oxygen, nitric oxide, and other stimuli.
Phosphodiesterases are a large family of enzymes that hydrolyze and inactivate cGMP and cAMP, thus regulating their concentrations and effects, as well as facilitating “cross-talk” between the two cyclic nucleotides. The type 5 phosphodiesterase (PDE5) is especially highly expressed in the lung, and not only uses cGMP as a substrate but also contains a specific cGMP binding domain that serves to activate its catabolic activity. As the primary enzyme responsible for regulating cGMP, PDE5 may well represent the most important regulator of NO-mediated vascular relaxation in the normal pulmonary vascular transition after birth [47].
Fetal and neonatal lung development, along with commonly used therapies, appears to regulate PDE expression and activity. In developing lambs and rats, PDE5 is expressed according to specific developmental trajectories that result in a peak of expression during late fetal life, followed by an acute fall around the time of birth [48, 49]. This drop in PDE5 activity would be expected to amplify the effects of nitric oxide produced by birth-related stimuli such as oxygen and shear stress . In contrast, when pulmonary vessels of fetal lambs undergo remodeling by chronic intrauterine pulmonary hypertension, PDE5 activity increases relative to controls [50, 51]. Even more striking is that after birth, PDE5 activity does not fall in this lamb model of PPHN, but rather increases dramatically to levels well over those observed in spontaneously breathing or ventilated control lambs [48, 51]. This abnormal increase in activity would be expected to diminish responses to both endogenous and exogenous NO, and could explain the incomplete clinical efficacy of iNO in some patients. It is also interesting to note that recent reports indicate that PDE5 is highly expressed in the remodeled human right ventricle, raising the possibility that sildenafil therapy may improve right ventricular function [52].
While sildenafil is presently only approved by the FDA for treatment of pulmonary hypertension in adult patients, it is frequently used in children. The first clinical report of sildenafil use in children was to facilitate weaning from iNO following corrective surgery for congenital heart disease [53]. In this initial case series, enteral sildenafil increased circulating cGMP and allowed two of three infants to wean from iNO without rebound pulmonary hypertension. Subsequent case series expanded these initial observations to show that enteral sildenafil may facilitate iNO discontinuation in infants with critical illness [54], and may also reduce duration of mechanical ventilation and ICU length of stay [55].
A recent large trial randomized children (approximately 2/3 of which had pulmonary hypertension associated with cardiac disease) to low, medium, or high dose sildenafil vs. placebo for 16 weeks [56]. The investigators concluded that sildenafil monotherapy for 16 weeks is well tolerated for pediatric pulmonary hypertension. The primary endpoint of improvement in peak oxygen consumption during exercise testing was only marginally different between sildenafil and placebo (p=0.056), but greater effects were noted in the medium and high-dose groups. Adverse events included pyrexia, upper respiratory infections, increased erections, and nausea. Patients who completed the 16-week trial were eligible to receive sildenafil for extended treatment (STARTS-2). While there was not a placebo arm, the authors noted that survival rates compared favorably with historical rates. They also suggest that the overall profile favors the medium dose (roughly 0.5 – 1 mg/kg given three times daily), and there should be consideration for adjusting sildenafil to a medium dose for long-term use. The clinical use of enteral sildenafil has also been reported in infants with PPHN, including one small, randomized controlled trial with oral sildenafil that showed a dramatic improvement in oxygenation and survival [57].
Enteral administration of sildenafil could raise concerns about gastrointestinal absorption, particularly in critically ill patients that may have compromised intestinal perfusion. An intravenous preparation of sildenafil was approved for clinical use in 2010. Two studies have been reported using intravenous sildenafil in critically ill children. One enrolled pediatric patients with postoperative pulmonary hypertension after cardiac surgery to one of three intravenous doses of sildenafil or placebo for a minimum of 24 hours [58]. While the study was underpowered, median time to extubation and length of time in intensive care was reduced in the sildenafil arm of the study. An open-label pilot trial in infants with PPHN demonstrated that intravenous sildenafil, delivered as a continuous infusion, improved oxygenation [59]. Furthermore, seven infants received sildenafil without prior use of iNO, and all experienced a significant improvement in oxygenation within four hours after sildenafil administration. Only one of these seven infants required iNO, and the other six infants improved and survived to hospital discharge without requiring either iNO or ECMO [59]. A randomized controlled trial is currently underway to evaluate the efficacy of intravenous sildenafil in infants with moderate PPHN (NCT01409031).
Sildenafil is also an attractive therapeutic option for infants with chronic pulmonary hypertension due to congenital diaphragmatic hernia or bronchopulmonary dysplasia because it can be given orally, and over longer periods of time with apparent low toxicity. In a rat model of hyperoxia-induced BPD, chronic use of sildenafil decreased medial wall thickness and RVH and improved lung alveolarization [60]. A clinical case series examined the effect of oral sildenafil in 25 infants and children (<2 years of age) with pulmonary hypertension due to chronic lung disease (mostly BPD). Most patients showed some improvement after a median treatment interval of 40 days, and the majority of infants were able to wean off iNO [61]. Five patients died after initiation of sildenafil treatment, but none died from refractory pulmonary hypertension or right heart failure. A similar approach might benefit some infants with chronic pulmonary hypertension associated with lung hypoplasia [62]. These important pilot studies suggest that sildenafil is well tolerated in infants with pulmonary hypertension due to chronic lung disease, and paves the way to further studies in this especially challenging population.
An interesting recent study showed that in a rat model of congenital diaphragmatic hernia, antenatal administration of sildenafil to the dam reduced PDE5 activity and increased cGMP, and produced striking reductions in the vascular findings of persistent pulmonary hypertension [63]. This is the first indication that pulmonary hypertension can be treated before birth, and will likely open up a productive line of investigation in antenatal diagnosis and treatment.
Tadalafil is a longer-acting selective phosphodiesterase type 5 inhibitor recently approved by the FDA for treatment of adult pulmonary hypertension. A recent retrospective report examined pediatric patients with pulmonary hypertension that were converted from sildenafil to tadalafil to achieve once-daily dosing at 1 mg/kg/day [64]. Interestingly, in addition to the ease of administration, about half of the patients exhibited significant improvements in mean pulmonary arterial pressure and pulmonary vascular resistance index measured at heart catheterization. Side effect profiles were similar for the two agents. These results indicate that tadalafil may be safe for pediatric patients with PAH and has the advantages of only once-daily dosing.
Prostanoids
A complementary vasodilatory pathway in the fetal lung is mediated by prostacyclin (PGI2) and cAMP (Figure 1). Prostacyclin is a metabolite of arachidonic acid that is endogenously produced by the vascular endothelium. The vascular effects of PGI2 are mediated through its binding to a membrane IP receptor which activates adenylate cyclase and increases cAMP, which triggers smooth muscle cell relaxation through reducing intracellular calcium concentrations. Prostacyclin production appears to increase in late gestation and early postnatal life [65, 66], indicating its importance in promoting the neonatal pulmonary vascular transition. Pulmonary hypertension in both neonates and older children is characterized by an decrease in the biosynthesis of prostacyclin accompanied by increased synthesis of the vasoconstrictor thromboxane A2 [67]. Furthermore, the PGI2 receptor (IP) is decreased in adult and pediatric patients with pulmonary hypertension, and animal studies point to its contribution to altered vasodilation in PPHN [68]. Prostacyclin is a potent vasodilator in both the systemic and pulmonary circulations and also has anti-platelet effects [69]. Prostacyclin was one of the earliest pulmonary vasodilators used for clinical treatment of pulmonary hypertension, and was approved by the FDA in 1995 for the treatment of severe chronic pulmonary arterial hypertension.
Currently, several prostanoid drug preparations are available for clinical use. Epoprostenol, delivered as a continuous intravenous infusion, remains a mainstay of therapy, and has been shown to improve pulmonary hemodynamics, quality of life, exercise capacity, and survival in adults and children with idiopathic and secondary pulmonary hypertension [70–75]. These significant long-term effects may occur even if short-term vasodilation is not observed, suggesting that a role for beneficial effects on platelet aggregation, inhibition of smooth muscle cell growth, and/or protection of right ventricular function [76]. The optimal dose of intravenous epoprostenol is not well defined, although children usually need higher doses than adults. In some children, rapid dosage escalation of infusions of PGI2 may be necessary to promote acute pulmonary vasodilation, and tolerance can develop that requires periodic dose escalation. Adverse effects include inhibition of platelet aggregation, systemic vasodilation and alterations in hepatic enzymes, and common side effects include diarrhea and jaw pain. Epoprostenol requires dedicated venous access, typically through a central venous line. The drug is chemically unstable at neutral pH and room temperature and its short half-life 0 requires continuous intravenous infusion with cold packs to maintain drug stability. Sudden interruption of the medication can lead to severe rebound pulmonary hypertension [77].
While used commonly for chronic management, intravenous epoprostenol is less frequently used for acute pulmonary hypertension due to risks of ventilation-perfusion mismatch and systemic hypotension. For this reason, inhaled, oral and subcutaneous routes of delivery have been pursued. When given as an inhaled aerosol, the vasodilator effects of PGI2 tend to be limited to the pulmonary circulation, making this strategy appealing when acute pulmonary vasodilation is needed [78]. Reports in children have been generally positive, although to date, there are few studies reporting the use of inhaled PGI2 in neonates with PPHN [79–81]. Our experience suggests that inhaled PGI2 is well tolerated and may assist in recovery without ECMO in infants with severe pulmonary hypertension and inadequate response to iNO [82]. Although the optimal dosing of inhaled PGI2 in critically ill mechanically ventilated infants is not known, short-term studies in newborn lambs with pulmonary hypertension suggest that doses up to 500 ng/kg/min produce progressive improvements in PA pressure, PVR and pulmonary blood flow [83]. In our clinical experience, doses of 50–100 ng/kg/min produce rapid improvement in oxygenation in infants that are refractory to iNO [84]. Concerns regarding the use of inhaled PGI2 include airway irritation from the alkaline solution needed to maintain drug stability, rebound pulmonary hypertension if the drug is abruptly discontinued, loss of medication into the circuit due to condensation, and alteration of characteristics of mechanical ventilation from the added gas flow needed for the nebulization. Prolonged use of continuous inhaled PGI2 could also lead to damage of mechanical ventilator valves. Further investigations will likely focus on preparations specifically designed for inhalation, such as iloprost or treprostinil, as discussed below. An oral agent, Beraprost, is a prostacyclin analogue that is less potent than epoprostenol, but chemically stable with a longer half-life of approximately one hour. Beraprost is currently only approved for the treatment of pulmonary hypertension in Japan and South Korea, although new oral prostanoids are currently under investigation in adult populations.
Iloprost is a chemically stable prostacyclin analogue with a half-life to 20–30 minutes that has been studied as an inhaled compound [85]. The advantages of an inhaled agent include improved selectivity for the pulmonary vascular bed, which is superior for patients with acute pulmonary hypertensive crisis with hemodynamic compromise and low cardiac output. This approach may improve ventilation/perfusion mismatch and oxygenation, and successful use has been reported in pediatric populations, including those with persistent pulmonary hypertension of the newborn and post cardiopulmonary bypass [85–92], and those refractory to inhaled nitric oxide or sildenafil [93, 94]. Acute bronchoconstriction is a potential adverse event and compliance can be poor due to the need for frequent aerosol administrations, up to 8–12 times daily [11].
Treprostinil is a prostacyclin analogue that is stable at room temperature and neutral pH, and with a longer half-life of about 3 hours. Treprostinil is approved by the FDA for the treatment of chronic pulmonary hypertension in adults, and can be delivered via continuous subcutaneous or intravenous infusion as well as by inhalation. Subcutaneous treprostinil produces short-term improvements in exercise tolerance and hemodynamics in some adults with PAH, but severe local site discomfort and induration can limit its tolerance. Subcutaneous treprostinil has been used to transition some children who were chronically stable on intravenous epoprostenol [95], and may improve clinical course in children as an add-on therapy [96]. Placebo-controlled trials have demonstrated improved exercise tolerance, clinical symptoms, and hemodynamics in patients with pulmonary hypertension, including children [97].
PDE3 Inhibition - Milrinone
Similar to the NO–cGMP pathway, prostacyclin–cAMP signaling is regulated by cAMP-hydrolyzing PDE isoforms such as PDE3 and PDE4. We recently reported that PDE3A expression and activity in the resistance pulmonary arteries increase dramatically by 24 h after birth [98]. These results were unexpected, as we would have predicted that PDE3 activity would decrease after birth to facilitate cAMP accumulation, similar to the patterns reported for PDE5 [47, 84]. We also observed that addition of inhaled nitric oxide dramatically increased PDE3 levels, which suggests that inhibition of PDE3 activity might enhance the vasodilatory effects of iNO/cGMP signaling in addition to its expected effects on the cAMP pathways [68].
Milrinone is an inhibitor of PDE3 activity that is frequently used in pediatric patients to improve myocardial contractility after cardiac surgery [99, 100]. Milrinone may also bypass abnormalities in endogenous PGI2 production and/or enhance availability of cAMP, which could be useful for acute treatment of pulmonary hypertension. Recent studies have shown a potential effect on the pulmonary vascular bed as well as synergistic effects with inhaled prostanoids [68, 100, 101]. In animal studies, milrinone decreases pulmonary artery pressure and resistance and acts additively with iNO [83, 102]. Clinical reports indicate that milrinone may decrease rebound pulmonary hypertension after iNO is stopped [27], and may enhance pulmonary vasodilation of infants with PPHN refractory to iNO [103]. A study to better define the pharmacokinetic profile of milrinone in infants with PPHN is ongoing (NCT01088997), and should lead to clinical trials designed to test its efficacy.
Endothelin Receptor Antagonists - Bosentan
Endothelin-1 (ET-1) is a 21 amino acid protein formed by serial enzymatic cleavage of a larger prepropeptide to the vasoactive form, and is one of the most potent vasoconstrictors described in the pulmonary vasculature (Figure 2). ET-1 is principally produced in endothelial cells in response to hypoxia, and is known to promote endothelial cell dysfunction, smooth muscle cell proliferation and remodeling, as well as inflammation and fibrosis [104]. ET-1 binds to two receptor subtypes, ET receptors A and B, and the binding of ET-1 to the ETA receptor on smooth muscle cells produces vasoconstriction. Increased ET-1 production and altered ET receptor activity have been consistently reported in neonatal and adult animal models of pulmonary hypertension, and lung ET-1 expression and plasma ET levels were elevated in severe PAH in adults [105]. In several animal and human studies, plasma endothelin-1 concentrations are consistently increased during and following cardiopulmonary bypass, suggesting a role for endothelin-1 in the pathophysiology of cardiopulmonary bypass-induced pulmonary hypertension [106–109]. Endothelin-1 is believed to play a role in the pathogenesis of neonatal pulmonary hypertension, and endothelin blockade augments pulmonary vasodilation [104]. A recent prospective examination of 40 newborns with congenital diaphragmatic hernia and poor outcome also indicated that plasma ET-1 levels were highly correlated with the severity of pulmonary hypertension [110].
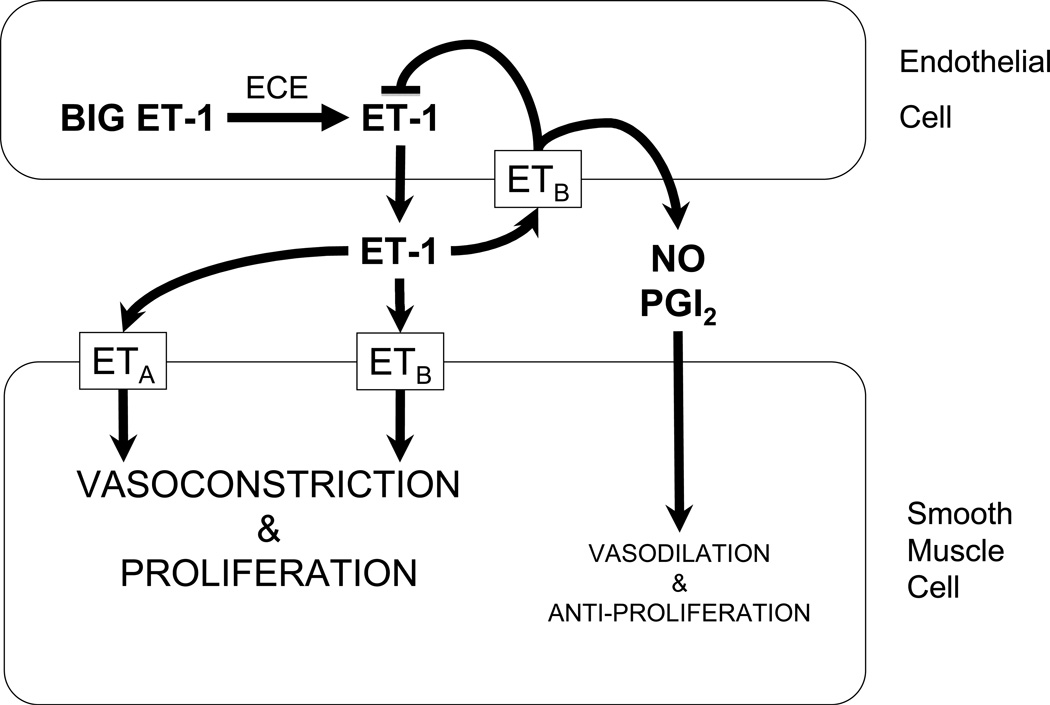
Figure 2. Endothelin-1 (ET-1) signaling pathway in the regulation of pulmonary vascular tone.
Big ET-1 is cleaved to ET-1 by endothelin converting enzyme (ECE) in endothelial cells. ET-1 binds its specific receptors ETA and ETB with differential effects. Binding of ET-1 to ETA or ETB on smooth muscle cells leads to vasoconstrictive and proliferative effects. ETB are transiently expressed on endothelial cells after birth; binding of ET-1 to ETB on endothelial cells leads to down regulation of ECE activity and increased production of nitric oxide (NO) and prostacyclin (PGI2) which lead to vasodilation and anti-proliferation. Reproduced from [84], with permission.
Bosentan is an oral endothelin receptor antagonist (ERA) that improves exercise capacity, pulmonary vascular resistance, and quality of life in adults [111, 112] and children with pulmonary hypertension [113, 114]. Bosentan lowers pulmonary artery pressure and pulmonary vascular resistance in children with diverse causes of pulmonary hypertension [113, 115]. Recent case reports also suggest that bosentan may improve oxygenation in neonates with PPHN [116, 117], and a clinical trial is currently underway in that population (NCT01389856). Bosentan is generally well-tolerated in children, and has also been successfully used as adjunctive treatment for children receiving long-term epoprostenol therapy [118]. Bosentan may improve functional status and survival estimates for up to two years [113], although these long-term improvements may not be observed in patients with cardiac disease and systemic to pulmonary shunts [119]. Risks include hepatotoxicity (dose-dependent), teratogenicity and possibly male infertility [120]. Liver function should be monitored monthly, although elevated aminotransferases and drug discontinuation rates are less common in young children compared to patients ≥12 years of age [120]. A specific pediatric formulation of bosentan was recently approved for use in children by the European authorities.
Ambrisentan is a selective ETA receptor antagonist with high oral bioavailability and a long half-life [121]. This agent blocks the vasoconstrictor effect of ETA receptors while maintaining the vasodilator and clearance effects of ETB receptors. Ambrisentan has been shown to improve exercise capacity and delay clinical worsening in PAH patients [122, 123], and is thought to produce less liver toxicity than bosentan, but further study is needed in children. Of note, another selective ETA receptor antagonist, Sitaxentan, was recently withdrawn from the market due to concerns about severe liver toxicity.
Summary
Pediatric pulmonary hypertension remains a devastating illness that requires much more study if we are to adapt therapies for the unique features of the pediatric lung and its vasculature. Still, much progress has been made over the last decade, and prognosis appears to be improving with the new therapies now available. Future study will likely focus on a better understanding of the signaling pathways and how they interact. For example, the combination of strategies that increase cGMP and cAMP together may be more effective than either treatment alone. In addition, earlier identification combined with the development of novel, and more specific therapies, will hopefully improve the lifespan and quality of life for these children.
Pediatric pulmonary hypertension remains a devastating illness that requires much more study if we are to adapt therapies for the unique features of the pediatric lung and its vasculature.
Still, much progress has been made over the last decade, and prognosis appears to be improving with the new therapies now available.
Future study will likely focus on a better understanding of the signaling pathways and how they interact. For example, the combination of strategies that increase cGMP and cAMP together may be more effective than either treatment alone.
Earlier identification combined with the development of novel, and more specific therapies, will hopefully improve the lifespan and quality of life for these children.
Acknowledgments
The preparation of this manuscript was supported in part by R01HL54705 (NHLBI) and U01HL102235 (NHLBI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levin DL, Rudolph AM, Heymann MA, et al. Morphological development of the pulmonary vascular bed in fetal lambs. Circulation. 1976;53:144–151. doi: 10.1161/01.cir.53.1.144. [DOI] [PubMed] [Google Scholar]
- 2.Cerro MJ, Abman S, Diaz G, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: Report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ. 2011;1:286–298. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, McLaughlin VV. The 4th World Symposium on Pulmonary Hypertension. Introduction. J Am Coll Cardiol. 2009;54:S1–S2. doi: 10.1016/j.jacc.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Lammers AE, Adatia I, Cerro MJ, et al. Functional classification of pulmonary hypertension in children: Report from the PVRI pediatric taskforce, Panama 2011. Pulm Circ. 2011;1:280–285. doi: 10.4103/2045-8932.83445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slaughter JL, Pakrashi T, Jones DE, et al. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. 2011;31:635–640. doi: 10.1038/jp.2010.213. [DOI] [PubMed] [Google Scholar]
- 8.An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40:131–136. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farquhar M, Fitzgerald DA. Pulmonary hypertension in chronic neonatal lung disease. Paediatr Respir Rev. 2010;11:149–153. doi: 10.1016/j.prrv.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 11.Abman SH, Ivy DD. Recent progress in understanding pediatric pulmonary hypertension. Curr Opin Pediatr. 2011;23:298–304. doi: 10.1097/MOP.0b013e3283464a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins IM, Moore TM, Blaisdell CJ, et al. Improving outcomes for pulmonary vascular disease. Am J Respir Crit Care Med. 2012;185:1015–1020. doi: 10.1164/rccm.201201-0049WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatr. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 14.Tissot C, Ivy DD, Beghetti M. Medical therapy for pediatric pulmonary arterial hypertension. J Pediatr. 2010;157:528–532. doi: 10.1016/j.jpeds.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oishi P, Datar SA, Fineman JR. Advances in the management of pediatric pulmonary hypertension. Respir Care. 2011;56:1314–1339. doi: 10.4187/respcare.01297. discussion 39-40. [DOI] [PubMed] [Google Scholar]
- 16.Gaston BM, Carver J, Doctor A, et al. S-nitrosylation signaling in cell biology. Mol Interv. 2003;3:253–263. doi: 10.1124/mi.3.5.253. [DOI] [PubMed] [Google Scholar]
- 17.Tiktinsky MH, Morin III FC. Increasing oxygen tension dilates fetal pulmonary circulation via endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1993;265:H376-H80. doi: 10.1152/ajpheart.1993.265.1.H376. [DOI] [PubMed] [Google Scholar]
- 18.Mata-Greenwood E, Jenkins C, Farrow KN, et al. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol. 2006;290:L232–L241. doi: 10.1152/ajplung.00393.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrow KN, Lakshminrusimha S, Reda WJ, et al. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L979–L987. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsella JP, Parker TA, Ivy DD, et al. Noninvasive delivery of inhaled nitric oxide therapy for late pulmonary hypertension in newborn infants with congenital diaphragmatic hernia. J Pediatr. 2003;142:397–401. doi: 10.1067/mpd.2003.140. [DOI] [PubMed] [Google Scholar]
- 21.Konduri GG, Solimani A, Sokol GM, et al. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics. 2004;113:559–564. doi: 10.1542/peds.113.3.559. [DOI] [PubMed] [Google Scholar]
- 22.Konduri GG, Vohr B, Robertson C, et al. Early inhaled nitric oxide therapy for term and near-term newborn infants with hypoxic respiratory failure: neurodevelopmental follow-up. J Pediatr. 2007;150:235–240. doi: 10.1016/j.jpeds.2006.11.06. 40.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez A, Fabres J, D'Apremont I, et al. Randomized controlled trial of early compared with delayed use of inhaled nitric oxide in newborns with a moderate respiratory failure and pulmonary hypertension. J Perinatol. 2010;30:420–424. doi: 10.1038/jp.2009.171. [DOI] [PubMed] [Google Scholar]
- 24.Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. New Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 25.Davidson D, Barefield ES, Kattwinkel J, et al. Safety of withdrawing inhaled nitric oxide therapy in persistent pulmonary hypertension of the newborn. Pediatrics. 1999;104:231–236. doi: 10.1542/peds.104.2.231. [DOI] [PubMed] [Google Scholar]
- 26.Black SM, Heidersbach RS, McMullan DM, et al. Inhaled nitric oxide inhibits NOS activity in lambs: potential mechanism for rebound pulmonary hypertension. Am J Physiol Heart Circ Physiol. 1999;277:H1849-H56. doi: 10.1152/ajpheart.1999.277.5.H1849. [DOI] [PubMed] [Google Scholar]
- 27.Thelitz S, Oishi P, Sanchez LS, et al. Phosphodiesterase-3 inhibition prevents the increase in pulmonary vascular resistance following inhaled nitric oxide withdrawal in lambs. Pediatr Crit Care Med. 2004;5:234–239. doi: 10.1097/01.pcc.0000124021.25393.2d. [DOI] [PubMed] [Google Scholar]
- 28.Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics. 1997;99:838–845. doi: 10.1542/peds.99.6.838. [DOI] [PubMed] [Google Scholar]
- 29.Fliman PJ, deRegnier RA, Kinsella JP, et al. Neonatal extracorporeal life support: impact of new therapies on survival. J Pediatr. 2006;148:595–599. doi: 10.1016/j.jpeds.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Afshar S, Gibson LL, Yuhanna IS, et al. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2003;284:L749–L758. doi: 10.1152/ajplung.00334.2002. [DOI] [PubMed] [Google Scholar]
- 31.Mourani PM, Ivy DD, Gao D, et al. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004;170:1006–1013. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]
- 32.Donohue PK, Gilmore MM, Cristofalo E, et al. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics. 2011;127:e414–e422. doi: 10.1542/peds.2010-3428. [DOI] [PubMed] [Google Scholar]
- 33.Steinhorn RH, Shaul PW, deRegnier RA, et al. Inhaled nitric oxide and bronchopulmonary dysplasia. Pediatrics. 2011;128:e255–e256. doi: 10.1542/peds.2011-1270A. author reply e6-7. [DOI] [PubMed] [Google Scholar]
- 34.Ballard RA, Truog WE, Cnaan A, et al. Inhaled Nitric Oxide in Preterm Infants Undergoing Mechanical Ventilation. N Engl J Med. 2006;355:343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 35.Hibbs AM, Walsh MC, Martin RJ, et al. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. J Pediatr. 2008;153:525–529. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barr FE, Macrae D. Inhaled nitric oxide and related therapies. Pediatr Crit Care Med. 2010;11:S30–S36. doi: 10.1097/PCC.0b013e3181c76b42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller OI, Tang SF, Keech A, et al. Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: a randomised double-blind study. Lancet. 2000;356:1464–1469. doi: 10.1016/S0140-6736(00)02869-5. [DOI] [PubMed] [Google Scholar]
- 38.Atz AM, Adatia I, Lock JE, et al. Combined effects of nitric oxide and oxygen during acute pulmonary vasodilator testing. J Am Coll Cardiol. 1999;33:813–819. doi: 10.1016/s0735-1097(98)00668-8. [DOI] [PubMed] [Google Scholar]
- 39.Rossaint R, Falke KJ, Lopez F, et al. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 40.Dobyns EL, Cornfield DN, Anas NG, et al. Multicenter randomized controlled trial of the effects of inhaled nitric oxide therapy on gas exchange in children with acute hypoxemic respiratory failure. J Pediatr. 1999;134:406–412. doi: 10.1016/s0022-3476(99)70196-4. [DOI] [PubMed] [Google Scholar]
- 41.Sokol J, Jacobs SE, Bohn D. Inhaled nitric oxide for acute hypoxemic respiratory failure in children and adults. Cochrane Database Syst Rev. 2003:CD002787. doi: 10.1002/14651858.CD002787. [DOI] [PubMed] [Google Scholar]
- 42.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1877–1885. doi: 10.1161/ATVBAHA.107.142943. [DOI] [PubMed] [Google Scholar]
- 43.Pearson DL, Dawling S, Walsh WF, et al. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. 2001;344:1832–1838. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- 44.Ananthakrishnan M, Barr FE, Summar ML, et al. L-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:L506-L11. doi: 10.1152/ajplung.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith HA, Canter JA, Christian KG, et al. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:56–65. doi: 10.1016/j.jtcvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Barr FE, Tirona RG, Taylor MB, et al. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery:potential therapy for postoperative pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;134:319–326. doi: 10.1016/j.jtcvs.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 47.Farrow KN, Steinhorn RH. Phosphodiesterases: emerging therapeutic targets for neonatal pulmonary hypertension. Handb Exp Pharmacol. 2011:251–277. doi: 10.1007/978-3-642-17969-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrow KN, Lakshminrusimha S, Czech L, et al. Superoxide dismutase and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299:L109–L116. doi: 10.1152/ajplung.00309.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez LS, Filippov G, Zapol WM, et al. cGMP-binding, cGMP-specific phosphodiesterase gene expression is regulated during lung development. Pediatr Res. 1995;37:348A. doi: 10.1203/00006450-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Hanson KA, Abman SH, Clarke WR. Elevation of pulmonary PDE5-specific activity in an experimental fetal ovine perinatal pulmonary hypertension model. Pediatr Res. 1996;39:334A. [Google Scholar]
- 51.Farrow KN, Wedgwood S, Lee KJ, et al. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol. 2010;174:272–281. doi: 10.1016/j.resp.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagendran J, Archer SL, Soliman D, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 53.Atz AM, Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999;91:307–310. doi: 10.1097/00000542-199907000-00041. [DOI] [PubMed] [Google Scholar]
- 54.Lee JE, Hillier SC, Knoderer CA. Use of sildenafil to facilitate weaning from inhaled nitric oxide in children with pulmonary hypertension following surgery for congenital heart disease. Journal of intensive care medicine. 2008;23:329–334. doi: 10.1177/0885066608321389. [DOI] [PubMed] [Google Scholar]
- 55.Namachivayam P, Theilen U, Butt WW, et al. Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med. 2006;174:1042–1047. doi: 10.1164/rccm.200605-694OC. [DOI] [PubMed] [Google Scholar]
- 56.Barst RJ, Ivy DD, Gaitan G, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–334. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 57.Baquero H, Soliz A, Neira F, et al. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117:1077–1083. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 58.Fraisse A, Butrous G, Taylor MB, et al. Intravenous sildenafil for postoperative pulmonary hypertension in children with congenital heart disease. Intensive Care Med. 2011;37:502–509. doi: 10.1007/s00134-010-2065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinhorn RH, Kinsella JP, Pierce C, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009;155:841–847. doi: 10.1016/j.jpeds.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Ladha F, Bonnet S, Eaton F, et al. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med. 2005;172:750–756. doi: 10.1164/rccm.200503-510OC. [DOI] [PubMed] [Google Scholar]
- 61.Mourani PM, Sontag MK, Ivy DD, et al. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154:379–384. doi: 10.1016/j.jpeds.2008.09.021. 84 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keller RL, Moore P, Teitel D, et al. Abnormal vascular tone in infants and children with lung hypoplasia: Findings from cardiac catheterization and the response to chronic therapy. Pediatr Crit Care Med. 2006;7:589–594. doi: 10.1097/01.PCC.0000244401.53189.CB. [DOI] [PubMed] [Google Scholar]
- 63.Luong C, Rey-Perra J, Vadivel A, et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation. 2011;123:2120–2131. doi: 10.1161/CIRCULATIONAHA.108.845909. [DOI] [PubMed] [Google Scholar]
- 64.Takatsuki S, Calderbank M, Ivy DD. Initial Experience With Tadalafil in Pediatric Pulmonary Arterial Hypertension. Pediatr Cardiol. 2012 doi: 10.1007/s00246-012-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brannon TS, MacRitchie AN, Jaramillo MA, et al. Ontogeny of cyclooxygenase-1 and cyclooxygenase-2 gene expression in ovine lung. Am J Physiol. 1998;274:L66–L71. doi: 10.1152/ajplung.1998.274.1.L66. [DOI] [PubMed] [Google Scholar]
- 66.Brannon TS, North AJ, Wells LB, et al. Prostacyclin synthesis in ovine pulmonary artery is developmentally regulated by changes in cyclooxygenase-1 gene expression. J Clin Invest. 1994;93:2230–2235. doi: 10.1172/JCI117220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 68.Lakshminrusimha S, Porta NF, Farrow KN, et al. Milrinone enhances relaxation to prostacyclin and iloprost in pulmonary arteries isolated from lambs with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. 2009;10:106–112. doi: 10.1097/PCC.0b013e3181936aee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones RL, Qian Y, Wong HN, et al. Prostanoid action on the human pulmonary vascular system. Clin Exp Pharmacol Physiol. 1997;24:969–972. doi: 10.1111/j.1440-1681.1997.tb02730.x. [DOI] [PubMed] [Google Scholar]
- 70.Rosenzweig EB, Kerstein D, Barst RJ. Long-term prostacyclin for pulmonary hypertension with associated congenital heart defects. Circulation. 1999;99:1858–1865. doi: 10.1161/01.cir.99.14.1858. [DOI] [PubMed] [Google Scholar]
- 71.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999;99:1197–1208. doi: 10.1161/01.cir.99.9.1197. [DOI] [PubMed] [Google Scholar]
- 72.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 73.Barst RJ, Rubin LJ, McGoon MD, et al. Survival in primary pulmonary hypertension with long-term continuous intravenous prostacyclin. Ann Intern Med. 1994;121:409–415. doi: 10.7326/0003-4819-121-6-199409150-00003. [DOI] [PubMed] [Google Scholar]
- 74.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 75.Yung D, Widlitz AC, Rosenzweig EB, et al. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–665. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]
- 76.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 77.Doran AK, Ivy DD, Barst RJ, et al. Guidelines for the prevention of central venous catheter-related blood stream infections with prostanoid therapy for pulmonary arterial hypertension. Int J Clin Pract Suppl. 2008:5–9. doi: 10.1111/j.1742-1241.2008.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zwissler B, Rank N, Jaenicke U, et al. Selective pulmonary vasodilation by inhaled prostacyclin in a newborn with congenital heart disease and cardiopulmonary bypass. Anesthesiology. 1995;82:1512–1516. doi: 10.1097/00000542-199506000-00021. [DOI] [PubMed] [Google Scholar]
- 79.Bindl L, Fahnenstich H, Peukert U. Aerosolised prostacyclin for pulmonary hypertension in neonates. Arch Dis Child Fetal Neonatal Ed. 1994;71:F214–F216. doi: 10.1136/fn.71.3.f214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soditt V, Aring C, Groneck P. Improvement of oxygenation induced by aerosolized prostacyclin in a preterm infant with persistent pulmonary hypertension of the newborn. Intensive Care Med. 1997;23:1275–1278. doi: 10.1007/s001340050498. [DOI] [PubMed] [Google Scholar]
- 81.Olmsted K, Oluola O, Parthiban A, et al. Can inhaled prostacyclin stimulate surfactant in ELBW infants? J Perinatol. 2007;27:724–726. doi: 10.1038/sj.jp.7211811. [DOI] [PubMed] [Google Scholar]
- 82.Kelly LK, Porta NF, Goodman DM, et al. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr. 2002;141:830–832. doi: 10.1067/mpd.2002.129849. [DOI] [PubMed] [Google Scholar]
- 83.Kumar VH, Swartz DD, Rashid N, et al. Prostacyclin and milrinone by aerosolization improve pulmonary hemodynamics in newborn lambs with experimental pulmonary hypertension. J Appl Physiol. 2010;109:677–684. doi: 10.1152/japplphysiol.01082.2009. [DOI] [PubMed] [Google Scholar]
- 84.Porta NF, Steinhorn RH. Pulmonary vasodilator therapy in the NICU: inhaled nitric oxide, sildenafil, and other pulmonary vasodilating agents. Clin Perinatol. 2012;39:149–164. doi: 10.1016/j.clp.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ewert R, Schaper C, Halank M, et al. Inhalative iloprost - pharmacology and clinical application. Expert Opin Pharmacother. 2009;10:2195–2207. doi: 10.1517/14656560903164228. [DOI] [PubMed] [Google Scholar]
- 86.Ehlen M, Wiebe B. Iloprost in persistent pulmonary hypertension of the newborn. Cardiol Young. 2003;13:361–363. doi: 10.1017/s1047951103000726. [DOI] [PubMed] [Google Scholar]
- 87.Eifinger F, Sreeram N, Mehler K, et al. Aerosolized iloprost in the treatment of pulmonary hypertension in extremely preterm infants: a pilot study. Klin Padiatr. 2008;220:66–69. doi: 10.1055/s-2007-984370. [DOI] [PubMed] [Google Scholar]
- 88.Hallioglu O, Dilber E, Celiker A. Comparison of acute hemodynamic effects of aerosolized and intravenous iloprost in secondary pulmonary hypertension in children with congenital heart disease. Am J Cardiol. 2003;92:1007–1009. doi: 10.1016/s0002-9149(03)00991-3. [DOI] [PubMed] [Google Scholar]
- 89.Ivy DD, Doran AK, Smith KJ, et al. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:161–169. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Limsuwan A, Wanitkul S, Khosithset A, et al. Aerosolized iloprost for postoperative pulmonary hypertensive crisis in children with congenital heart disease. Int J Cardiol. 2008;129:333–338. doi: 10.1016/j.ijcard.2007.08.084. [DOI] [PubMed] [Google Scholar]
- 91.Muller M, Scholz S, Kwapisz M, et al. Use of inhaled iloprost in a case of pulmonary hypertension during pediatric congenital heart surgery. Anesthesiology. 2003;99:743–744. doi: 10.1097/00000542-200309000-00032. [DOI] [PubMed] [Google Scholar]
- 92.Tissot C, Beghetti M. Review of inhaled iloprost for the control of pulmonary artery hypertension in children. Vasc Health Risk Manag. 2009;5:325–331. doi: 10.2147/vhrm.s3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Luca D, Zecca E, Piastra M, et al. Iloprost as 'rescue' therapy for pulmonary hypertension of the neonate. Paediatr Anaesth. 2007;17:394–395. doi: 10.1111/j.1460-9592.2006.02104.x. [DOI] [PubMed] [Google Scholar]
- 94.Chotigeat U, Jaratwashirakul S. Inhaled iloprost for severe persistent pulmonary hypertension of the newborn. J Med Assoc Thai. 2007;90:167–170. [PubMed] [Google Scholar]
- 95.Ivy DD, Claussen L, Doran A. Transition of stable pediatric patients with pulmonary arterial hypertension from intravenous epoprostenol to intravenous treprostinil. Am J Cardiol. 2007;99:696–698. doi: 10.1016/j.amjcard.2006.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levy M, Celermajer DS, Bourges-Petit E, et al. Add-on therapy with subcutaneous treprostinil for refractory pediatric pulmonary hypertension. J Pediatr. 2011;158:584–588. doi: 10.1016/j.jpeds.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 97.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary hypertension. Am J Respir Crit Care Med. 2002;165:1–5. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 98.Chen B, Lakshminrusimha S, Czech L, et al. Regulation of phosphodiesterase 3 in the pulmonary arteries during the perinatal period in sheep. Pediatr Res. 2009;66:682–687. doi: 10.1203/PDR.0b013e3181bce574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 100.Chang AC, Atz AM, Wernovsky G, et al. Milrinone: systemic and pulmonary effects in neonates after cardiac surgery. Crit Care Med. 1995;23:1907–1914. doi: 10.1097/00003246-199511000-00018. [DOI] [PubMed] [Google Scholar]
- 101.Bassler D, Choong K, McNamara P, et al. Neonatal persistent pulmonary hypertension treated with milrinone: four case reports. Biol Neonate. 2006;89:1–5. doi: 10.1159/000088192. [DOI] [PubMed] [Google Scholar]
- 102.Deb B, Bradford K, Pearl RG. Additive effects of inhaled nitric oxide and intravenous milrinone in experimental pulmonary hypertension. Crit Care Med. 2000;28:795–799. doi: 10.1097/00003246-200003000-00031. [DOI] [PubMed] [Google Scholar]
- 103.McNamara PJ, Laique F, Muang-In S, et al. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J Crit Care. 2006;21:217–222. doi: 10.1016/j.jcrc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 104.Abman SH. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu Rev Med. 2009;60:13–23. doi: 10.1146/annurev.med.59.110106.212434. [DOI] [PubMed] [Google Scholar]
- 105.Giaid A, Yanagisawa M, Lagleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 106.Hiramatsu T, Imai Y, Takanashi Y, et al. Time course of endothelin-1 and nitrate anion levels after cardiopulmonary bypass in congenital heart defects. Ann Thorac Surg. 1997;63:648–652. doi: 10.1016/s0003-4975(96)01055-7. [DOI] [PubMed] [Google Scholar]
- 107.Komai H, Adatia IT, Elliott MJ, et al. Increased plasma levels of endothelin-1 after cardiopulmonary bypass in patients with pulmonary hypertension and congenital heart disease. J Thorac Cardiovasc Surg. 1993;106:473–478. [PubMed] [Google Scholar]
- 108.Reddy MV, Hendricks-Munoz KD, Rajasinghe HA, et al. Post-cardiopulmonary bypass pulmonary hypertension in lambs with increased pulmonary blood flow: a role for endothelin 1. Circualtion. 1997;95:1054–1061. doi: 10.1161/01.cir.95.4.1054. [DOI] [PubMed] [Google Scholar]
- 109.Schulze-Neick I, Li J, Reader JA, et al. The endothelin antagonist BQ123 reduces pulmonary vascular resistance after surgical intervention for congenital heart disease. J Thorac Cardiovasc Surg. 2002;124:435–441. doi: 10.1067/mtc.2002.121492. [DOI] [PubMed] [Google Scholar]
- 110.Keller RL, Tacy TA, Hendricks-Munoz K, et al. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. Am J Respir Crit Care Med. 2010;182:555–561. doi: 10.1164/rccm.200907-1126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 112.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 113.Rosenzweig EB, Ivy DD, Widlitz A, et al. Effects of long-term bosentan in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:697–704. doi: 10.1016/j.jacc.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 114.Maiya S, Hislop AA, Flynn Y, et al. Response to bosentan in children with pulmonary hypertension. Heart. 2006;92:664–670. doi: 10.1136/hrt.2005.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barst RJ, Ivy D, Dingemanse J, et al. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Ther. 2003;73:372–382. doi: 10.1016/s0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 116.Nakwan N, Choksuchat D, Saksawad R, et al. Successful treatment of persistent pulmonary hypertension of the newborn with bosentan. Acta Paediatr. 2009;98:1683–1685. doi: 10.1111/j.1651-2227.2009.01386.x. [DOI] [PubMed] [Google Scholar]
- 117.Goissen C, Ghyselen L, Tourneux P, et al. Persistent pulmonary hypertension of the newborn with transposition of the great arteries: successful treatment with bosentan. Eur J Pediatr. 2008;167:437–440. doi: 10.1007/s00431-007-0531-y. [DOI] [PubMed] [Google Scholar]
- 118.Ivy DD, Doran A, Claussen L, et al. Weaning and discontinuation of epoprostenol in children with idiopathic pulmonary arterial hypertension receiving concomitant bosentan. Am J Cardiol. 2004;93:943–946. doi: 10.1016/j.amjcard.2003.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Loon RL, Hoendermis ES, Duffels MG, et al. Long-term effect of bosentan in adults versus children with pulmonary arterial hypertension associated with systemic-to-pulmonary shunt: does the beneficial effect persist? Am Heart J. 2007;154:776–782. doi: 10.1016/j.ahj.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 120.Beghetti M, Hoeper MM, Kiely DG, et al. Safety experience with bosentan in 146 children 2-11 years old with pulmonary arterial hypertension: results from the European Postmarketing Surveillance program. Pediatr Res. 2008;64:200–204. doi: 10.1203/PDR.0b013e318179954c. [DOI] [PubMed] [Google Scholar]
- 121.Channick RN, Sitbon O, Barst RJ, et al. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:62S–67S. doi: 10.1016/j.jacc.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 122.Galie N, Badesch D, Oudiz R, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 123.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]