Abstract
d-Fenfluramine (d-Fen) increases serotonin (5-HT) content in the synaptic cleft and exerts anorexigenic effects in animals and humans. However, the neural circuits that mediate these effects are not fully identified. To address this issue, we assessed the efficacy of d-Fen-induced hypophagia in mouse models with manipulations of several genes in selective populations of neurons. Expectedly, we found that global deletion of 5-HT 2C receptors (5-HT2CRs) significantly attenuated d-Fen-induced anorexia. These anorexigenic effects were restored in mice with 5-HT2CRs expressed only in pro-opiomelanocortin (POMC) neurons. Further, we found that deletion of melanocortin 4 receptors (MC4Rs), a downstream target of POMC neurons, abolished anorexigenic effects of d-Fen. Reexpression of MC4Rs only in SIM1 neurons in the hypothalamic paraventricular nucleus and neurons in the amygdala was sufficient to restore the hypophagic property of d-Fen. Thus, our results identify a neurochemically defined neural circuit through which d-Fen influences appetite and thereby indicate that this 5-HT2CR/POMC-MC4R/SIM1 circuit may yield a more refined target to exploit for weight loss.
Introduction
d-Fenfluramine (d-Fen), a drug that increases serotonin (5-HT) content by stimulating synaptic release of serotonin and blocking its reuptake into presynaptic terminals (Rowland and Carlton, 1986), exerts a potent anorexigenic effect in rodents and humans (McGuirk et al., 1991). In the 1990s, d-Fen was widely prescribed and was clinically effective in the treatment of obesity. However, the drug was withdrawn from clinical use due to its adverse cardiopulmonary events (Connolly et al., 1997). Due to the effectiveness of this drug, efforts have focused on understanding the mechanisms underlying the anorexigenic effects of d-Fen which may lead to the development of new pharmaceutical agents that mimic the appetite-suppressing property of d-Fen with fewer side effects.
The effects of d-Fen on food intake have been primarily attributed to serotonin action at 5-HT 2C receptors (5-HT2CRs), as the hypophagic responses induced by d-Fen are significantly blunted in 5-HT2CR knock-out mice (Vickers et al., 1999). 5-HT2CR knock-out mice also display hyperphagia and a late-onset obesity (Nonogaki et al., 1998), demonstrating that the endogenous 5-HT2CRs are physiological regulators of feeding and body weight.
Pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of hypothalamus (ARC) express 5-HT2CRs (Heisler et al., 2002) and receive inputs from serotonin-immunoreactive nerve terminals (Kiss et al., 1984). Electrophysiological studies demonstrated that serotonin and serotonergic compounds, including d-Fen, activate POMC neurons (Heisler et al., 2002; Qiu et al., 2007). In addition, 5-HT2CR agonists increase POMC expression in the ARC (Zhou et al., 2007; Lam et al., 2008). We recently reported that reexpression of 5-HT2CRs only in POMC neurons is sufficient to rescue hyperphagia and obesity seen in mice with global 5-HT2CR deficiency (Xu et al., 2008). Collectively, these observations indicate that POMC neurons are a physiologically relevant target of 5-HT2CRs in the regulation of feeding and body weight. We hypothesize that this subpopulation of 5-HT2CR/POMC-expressing neurons may also be important to the appetite-suppressing effects of d-Fen.
POMC neurons produce α-melanocyte-stimulating hormone (α-MSH), an endogenous ligand that acts at melanocortin receptors, such as the melanocortin 4 receptors (MC4Rs) (Williams and Schwartz, 2005). MC4Rs are widely expressed in the CNS (Mountjoy et al., 1994). Mutations in the Mc4r/MC4R gene lead to severe hyperphagia and obesity in mice (Huszar et al., 1997) and in humans (Vaisse et al., 1998) and an insensitivity to the anorectic effect of d-Fen (Heisler et al., 2006). Particularly, MC4Rs are abundantly expressed by SIM1 neurons in the paraventricular nucleus of the hypothalamus (PVH) and in the amygdala (Balthasar et al., 2005). SIM1 is a transcription factor that controls development of the PVH and mutations in Sim1/SIM1 gene produce obesity in mice and humans (Holder et al., 2000; Michaud et al., 2001). We previously reported that restoration of MC4Rs in SIM1 neurons is sufficient to rescue hyperphagia caused by global MC4R deficiency (Balthasar et al., 2005). Therefore, we hypothesize that d-Fen may require functional MC4Rs in SIM1 neurons to suppress feeding.
In the present study, we used several genetic mouse models to determine critical and discrete subpopulations of 5-HT2CRs and MC4Rs through which d-Fen influences appetite.
Materials and Methods
Animal care.
All mice used were group housed with food and water available ad libitum in a temperature-controlled room with 12 h light-dark cycle in the animal facility of UT Southwestern Medical Center. Most mice were weaned on regular chow (#7001, 4% chow, Harlan Teklad). Additional cohorts of mice were weaned on high fat diet (HFD, TD.88137, 42% calories from fat, Harlan Teklad).
Mouse strains.
All mice have been backcrossed (>10 generations) to the C57BL/6J background. Experiments in Figure 1, A and B, were performed with male wild-type (WT) and 2C-null littermates in which expression of 5-HT2CRs is globally disrupted by a loxP-flank transcription blocker (loxTB) inserted into the X-linked Htr2c (5-HT2CR) gene (Xu et al., 2008). Experiments in Figure 1, C and D, were performed with male WT, 2C-null and 2C/POMC mice. 2C/POMC male mice were hemizygous for 2C-null allele and carried the POMC-Cre transgene. In 2C/POMC mice, Cre-mediated recombination removed the loxTB and restored expression of 5-HT2CRs only in POMC neurons (Xu et al., 2008).
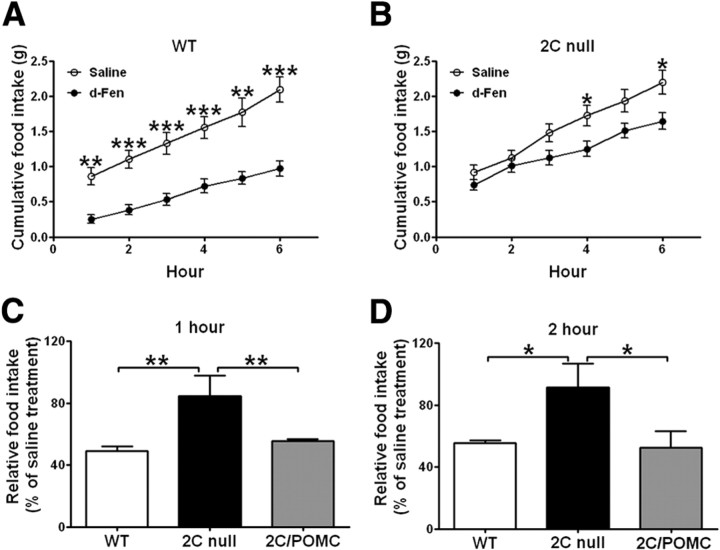
Figure 1.
A, B, Six hour chow intake in WT (A) and 2C-null (B) littermates (12 weeks) treated with d-Fen (3 mg/kg) or saline (i.p.) (n = 6–8 per genotype). Data are mean ± SEM; *p < 0.05, **p < 0.01, and ***p < 0.001 for d-Fen versus saline. C, D, One hour (C) and 2 h (D) chow intake in WT, 2C-null, and 2C/POMC littermates (12 weeks) treated with d-Fen (3 mg/kg) or saline (i.p.) (n = 7–9 per genotype). d-Fen-induced food intake was normalized by food intake after saline injections. Data are mean ± SEM, *p < 0.05 and **p < 0.01 for 2C-null versus WT or 2C/POMC.
Experiments in Figure 2 were performed with male WT, MC4R-null and MC4R/SIM1 mice. MC4R-null mice were previously generated by inserting the loxTB in the Mc4r gene (Balthasar et al., 2005). The loxTB disrupts MC4R expression globally (Balthasar et al., 2005). In the present study, MC4R-null mice were crossed with mice carrying the Sim1-Cre transgene (Balthasar et al., 2005) to generate MC4R/SIM1 mice, whose MC4Rs were selectively reexpressed in SIM1 neurons (Balthasar et al., 2005).
Figure 2.
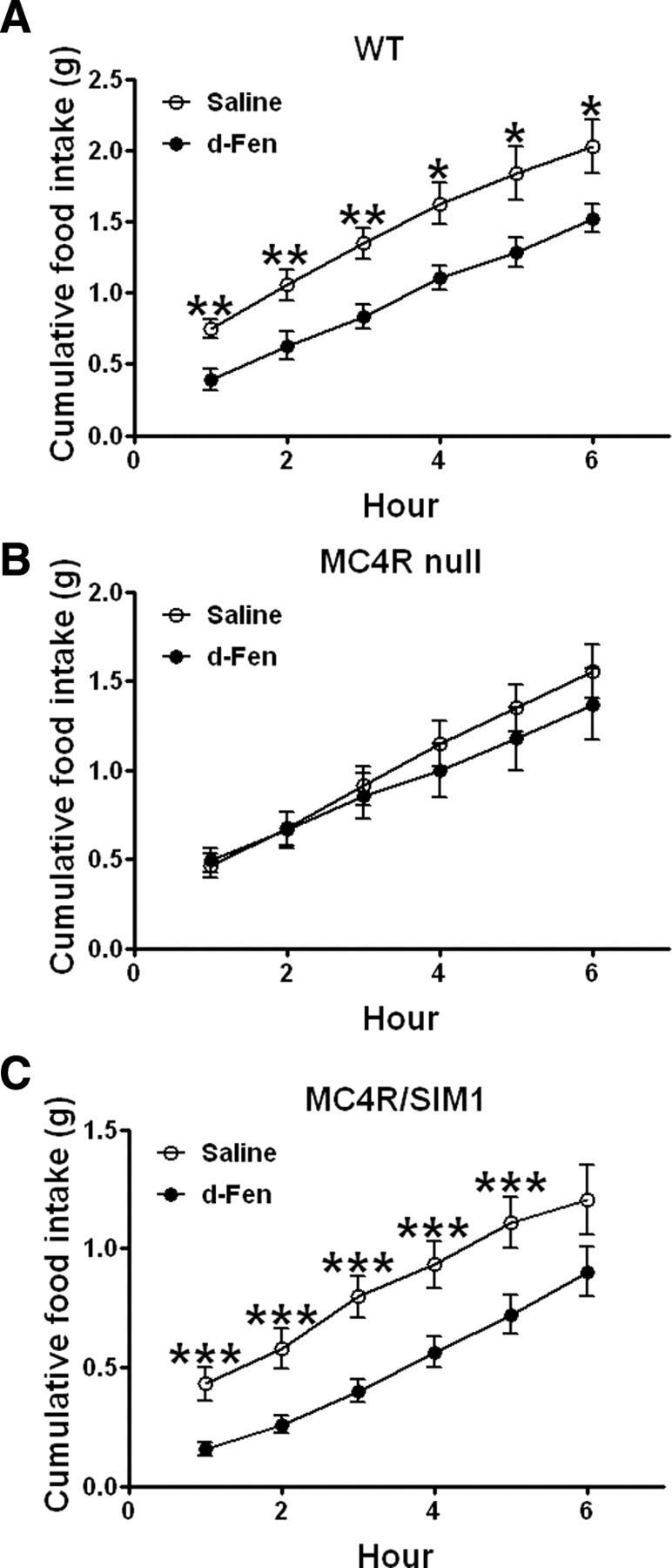
A–C, Six hour chow intake in WT (A), and MC4R-null (B) and MC4R/SIM1 (C) littermates (12 weeks) treated with d-Fen (3 mg/kg) or saline (i.p.) (n = 7–11 per genotype). Data are mean ± SEM, *p < 0.05, **p < 0.01, and ***p < 0.001 for d-Fen versus saline.
Experiments in Figure 3, A and B, were performed with male WT and heterozygous SIM1 knock-out (SIM1 HET) littermates (Holder et al., 2004).
Figure 3.
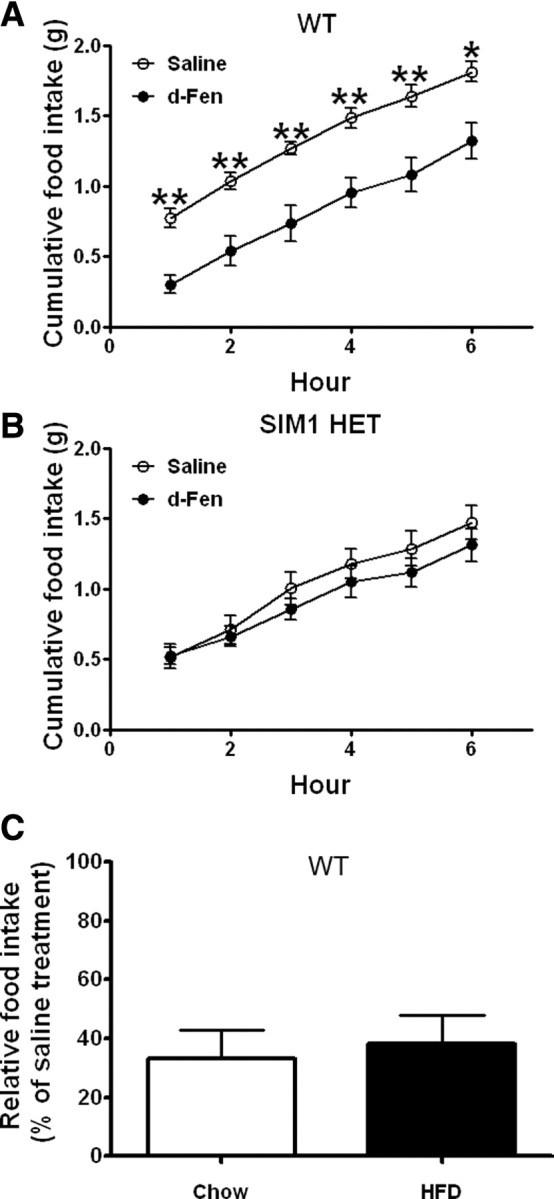
A, B, Six hour chow intake in WT (A) and SIM1 HET (B) littermates (10 weeks) treated with d-Fen (3 mg/kg) or saline (i.p.) (n = 5–7 per genotype). Data are mean ± SEM, **p < 0.01 for d-Fen versus saline. C, Chow-fed and HFD-fed WT mice (16 weeks; n = 8–9 per group) treated with d-Fen (3 mg/kg) or saline. d-Fen-induced 1 h food intake was normalized by food intake after saline. Data are mean ± SEM.
Experiments in Figure 3C were performed with C57BL/6J mice maintained on regular chow or HFD.
Acute anorexigenic responses to d-Fen.
After a 14 h fast (12 h dark cycle and 2 h light cycle), mice received intraperitoneal injections of saline or d-Fen (3 mg/kg, in a volume of 0.01 ml/g body weight). This dose has been demonstrated to induce hypophagia in mice without causing sedative responses and resting (Vickers et al., 1999). Diet (chow or HFD) was provided 30 min after injections. Food intake over the next 1–2 or 1–6 h was measured. Each mouse was tested with saline and d-Fen in a counterbalanced order with 7 d between treatments.
Statistical analysis.
Data are presented as mean ± SEM and were analyzed with t test, one-way ANOVA followed by Student–Newman–Keuls post hoc comparisons or repeated measures (RM) ANOVA followed by LSD post hoc comparisons, where appropriate. Statistical analyses were performed with SPSS or SigmaStat software. p < 0.05 indicated statistical significance.
Results
Anorexigenic effects of d-Fen are blunted in 2C-null mice
We first performed the time course experiment to examine the efficacy of d-Fen on food intake in chow-fed WT mice and 2C-null mice. As expected, d-Fen significantly reduced 6 h food intake in WT mice (Fig. 1A; RM ANOVA main effect drug: F(1,7) = 32.04, p < 0.001). In contrast, the anorexigenic effects of d-Fen were significantly attenuated in 2C-null mice, as d-Fen administration did not significantly alter food intake of 2C-null mice in the 6 h period (Fig. 1B; RM ANOVA main effect drug: F(1,5) = 5.96, NS). However, we observed a significant interaction between drug treatment and time in the 2C-null mice (Fig. 1B; RM ANOVA time × drug interaction: F(5,25) = 8.15, p < 0.001). Specifically, while d-Fen did not alter food intake at 1, 2, 3, and 5 h in 2C-null mice, food intake was significantly reduced at 4 and 6 h after d-Fen injections. The data suggest that action at the 5-HT2CRs is required to mediate the acute (within the first 3 h) anorexigenic effect of d-Fen, but the longer-term effects of d-Fen on food intake are not fully dependent on 5-HT2CR-mediated mechanisms. Importantly, it was shown that mice lacking 5-HT2CRs globally have comparable basal brain serotonin content as wild type mice and the mutant mice display potentiated serotonin release upon treatment of selective serotonin receptor inhibitors (e.g., fluoxetine) (Cremers et al., 2004). Therefore, the lack of d-Fen efficacy in 2C-null mice is not likely due to impaired serotonin release, but rather to loss of 5-HT2CR-mediated signals.
Anorexigenic effects of d-Fen are restored in 2C/POMC mice
To identify the critical 5-HT2CR-expressing sites that mediate d-Fen anorexia, we tested the efficacy of d-Fen in WT mice, 2C-null mice and 2C/POMC mice (which selectively express 5-HT2CRs only in POMC neurons). Consistent with findings in Figure 1, A and B, d-Fen significantly suppressed 1 h (Fig. 1C) and 2 h (Fig. 1D) food intake in WT mice, but these responses were significantly blunted in 2C-null mice (Fig. 1C, one-way ANOVA F(2,20) = 6.47, p < 0.01, and Fig. 1D, one-way ANOVA F(2,20) = 3.87, p < 0.05). In contrast, d-Fen significantly suppressed food intake in 2C/POMC mice, effects that were indistinguishable to those seen in WT mice (Fig. 1C,D). Therefore, these results indicate that 5-HT2CRs expressed by POMC neurons are sufficient to mediate the effects of d-Fen in inhibiting acute food intake.
Anorexigenic effects of d-Fen are restored in MC4R/SIM1 mice
Our observations that d-Fen anorexia is restored in mice expressing 5-HT2CRs in POMC neurons suggest that activation of the central melanocortin pathway underlies this compound's effect on appetite. Here we further examined this possibility by assessing whether deletion of a downstream target of α-MSH, the MC4Rs, abolishes the anorexigenic effect of d-Fen. As expected, d-Fen significantly decreased 6 h food intake in WT mice (Fig. 2A; RM ANOVA main effect drug: F(1,6) = 16.35, p < 0.01). In contrast, MC4R-null mice were unresponsive to d-Fen anorexia (Fig. 2B; RM ANOVA main effect drug: F(1,9) = 0.78, NS). We next examined the efficacy of d-Fen in MC4R/SIM1 mice to determine whether restoration of MC4Rs selectively in SIM1 neurons can rescue the hypophagic effect of d-Fen. The effects of d-Fen on food intake over a 6 h period were completely restored in MC4R/SIM1 mice (Fig. 2C; RM ANOVA main effect drug: F(1,10) = 26.96, p < 0.0001). Our results therefore indicate that MC4Rs exclusively expressed by SIM1 neurons are sufficient to mediate d-Fen anorexia.
We further established the role of CNS SIM1 neurons in d-Fen hypophagia using SIM1 HET mice that lack one SIM1 allele. While d-Fen significantly decreased 6 h food intake in WT mice (Fig. 3A; RM ANOVA main effect drug: F(1,10) = 11.99, p < 0.01), SIM1 HETS were insensitive to the anorectic effect of d-Fen (Fig. 3B; main effect drug: F(1,4) = 0.66, NS). These results indicate that intact SIM1 function is required to mediate the complete acute anorexigenic effects of d-Fen.
It is important to note that at the time of studies, MC4R-null mice, MC4R/SIM1 mice and SIM1 HET mice were significantly obese compared with their WT littermates (Table 1). To exclude the possibility that the blunted d-Fen responses in these mice were simply due to increased body weight, we compared the efficacy of d-Fen in chow-fed lean mice and mice with HFD-induced obesity. Our results indicate that d-Fen induced similar anorexia in lean and obese mice (Fig. 3C; t = 0.3607, df = 15, NS). These findings indicate that efficacy of d-Fen-induced hypophagia is not affected by body weight per se.
Table 1.
| Body weight (g) | Age (week) | Diets | |
|---|---|---|---|
| Fig. 1A,B | |||
| WT | 23.8 ± 1.1 | 12 | Chow |
| 2C null | 25.1 ± 2.2 | 12 | Chow |
| Fig. 1C,D | |||
| WT | 24.5 ± 0.9 | 12 | Chow |
| 2C null | 23.5 ± 1.2 | 12 | Chow |
| 2C/POMC | 23.7 ± 1.0 | 12 | Chow |
| Fig. 2 | |||
| WT | 19.0 ± 0.6 | 12 | Chow |
| MC4R null | 34.7 ± 3.7a | 12 | Chow |
| MC4R/SIM1 | 31.4 ± 1.9b | 12 | Chow |
| Fig. 3A,B | |||
| WT | 20.2 ± 0.5 | 10 | Chow |
| SIM1 HET | 26.9 ± 1.7c | 10 | Chow |
| Fig. 2C | |||
| Chow | 25.5 ± 0.9 | 16 | Chow |
| HFD | 35.7 ± 1.4d | 16 | HFD |
ap < 0.01 for MC4R null versus WT;
bp < 0.01 for MC4R/SIM1 versus WT;
cp < 0.01 for SIM1 HET versus WT;
dp < 0.01 for HFD versus chow.
Discussion
Understanding the mechanisms underlying the anorexigenic effects of d-Fen has been one of the priorities for many laboratories and pharmaceutical companies due to the potent appetite-suppressing and body weight-reducing benefits of this drug. Here we used multiple genetic mouse models to systematically assess the role of the 5-HT2CR-MC4R circuit in mediating the effects of d-Fen on food intake. Our results indicate that the anorexigenic effects of d-Fen involve stimulation of 5-HT2CRs on POMC neurons, which in turn activate MC4Rs on SIM1 neurons to suppress food intake.
Like the current obesity treatment sibutramine (a serotonin-norepinephrine reuptake inhibitor), d-Fen also promotes increased serotonin content in the synaptic cleft (Rowland and Carlton, 1986). Pharmacological and genetic efforts to discern which of the 14 serotonin receptors mediates serotonin's effects on appetite indicate a primary role for the 5-HT2CRs (Garfield and Heisler, 2009). Consistent with data obtained following 5-HT2CR antagonist pretreatment and with the traditional 5-HT2CR knock-out mouse (Vickers et al., 1999; Clifton et al., 2000), we observed that the efficacy of d-Fen is substantially reduced in mice with global 5-HT2CR deficiency. Together, these results indicate the necessity of the 5-HT2CRs in the acute effects of d-Fen on appetite.
The 5-HT2CRs are widely expressed in the rodent brain (Molineaux et al., 1989), including many regions associated with food intake and energy balance. Previously, we observed that a subpopulation of 5-HT2CRs are anatomically positioned to influence the activity of neurons expressing POMC, the gene precursor of the potent anorectic neuropeptide and endogenous melanocortin agonist α-MSH (Heisler et al., 2002). Further probing of this anatomical localization revealed that exogenous treatment with 5-HT2CR agonists activates POMC neurons (Heisler et al., 2002; Qiu et al., 2007) and enhances POMC expression (Zhou et al., 2007; Lam et al., 2008). Illustrating the functional relevance of this 5-HT2CR modulation of POMC activity/expression, we observed that expression of 5-HT2CRs only in POMC neurons (in 2C/POMC mice) is sufficient to rescue hyperphagia and obesity seen in mice with global 5-HT2CR deficiency (Xu et al., 2008). Therefore, 5-HT2CRs expressed by POMC neurons are physiologically important in the regulation of energy homeostasis. Here we assessed whether the specific subpopulation of POMC neurons expressing 5-HT2CRs is also critical to mediate the hypophagic effect of d-Fen. We observed that while the reduction in food intake induced by d-Fen is attenuated in 2C-null mice, the efficacy of d-Fen is fully rescued in 2C/POMC mice. These findings indicate that 5-HT2CRs specifically expressed by POMC neurons are sufficient to mediate the anorexigenic effects of d-Fen.
It is worth noting that in 2C-null mice, d-Fen-induced hypophagia is only attenuated but not completely abolished. These results suggest that other 5-HT2CR-independent mechanisms may also contribute to the anorexigenic effects of d-Fen. Since it was shown that d-Fen can potently increase noradrenalin release in the brain (Rothman et al., 2003), it is possible that effects of d-Fen on food intake may be mediated partly by noradrenergic actions. Alternatively, our results can also be interpreted to suggest that other serotonin receptors may contribute to the effect of d-Fen on feeding. Supporting this possibility, it was shown that d-Fen-induced hypophagic responses are attenuated in 5-HT1BR knock-out mice (Lucas et al., 1998). Therefore, both 5-HT2CRs and 5-HT1BRs may function in concert to mediate the anorexigenic effects of d-Fen. Like 5-HT2CRs, 5-HT1BRs also act on the central melanocortin system to influence feeding. Specifically, 5-HT1BRs are expressed in agouti-related protein (AgRP)/neuropeptide Y (NPY) neurons (Heisler et al., 2006), which produce the endogenous antagonist of MC4Rs, AgRP (Williams and Schwartz, 2005). AgRP/NPY neurons also inhibit POMC neurons via the inhibitory GABAergic projections to these neurons (Tong et al., 2008). We have previously demonstrated that 5-HT1BR agonists directly inhibit AgRP/NPY neurons and decrease the inhibitory drive onto POMC neurons (Heisler et al., 2006). In addition, the anorexigenic effects of 5-HT1BR agonists are abolished in both MC4R knock-out mice and in mice with constitutive ectopic expression of the endogenous MCR antagonist, agouti peptide (Ay mice) (Heisler et al., 2006). Collectively, these findings support the possibility that elevated 5-HT content by d-Fen may also directly act on AgRP/NPY neurons to lead to reciprocal increases in α-MSH release and decreases in AgRP release, which in turn would promote the suppression of food intake.
MC4Rs are widely expressed in the CNS, including many regions classically associated with food intake (Mountjoy et al., 1994). In particular, the MC4Rs are densely expressed by SIM1 neurons in the PVH and in the amygdala (Balthasar et al., 2005), key regions associated with appetite. Illustrating the functional importance of MC4Rs in SIM1 neurons in energy balance, restoration of MC4Rs specifically in SIM1 neurons is sufficient to rescue hyperphagia caused by global MC4R deficiency (Balthasar et al., 2005). Here we investigated whether this important subpopulation of MC4R-expressing neurons also underlies the effects of d-Fen on appetite. We observed that selective reactivation of MC4Rs only in SIM1 neurons is sufficient to restore the efficacy of d-Fen which is otherwise abolished in MC4R-null mice. The anorexigenic effects of d-Fen were also abolished in mice heterozygous for Sim1-null allele. These findings indicate that the hypophagic effects of d-Fen are mediated by MC4Rs expressed by SIM1 neurons.
In summary, we used several unique genetic mouse models to demonstrate that the 5-HT2CR-MC4R circuit in the brain is sufficient to mediate actions by d-Fen to reduce food intake. Together, the data support the following model: elevated serotonin induced by d-Fen activates POMC neurons via 5-HT2CRs, and POMC neurons secrete α-MSH, which in turn acts on MC4Rs expressed by SIM1 neurons to inhibit appetite. It is important to consider that our findings do not demonstrate that this 5-HT2CR-MC4R circuit is the only pathway that mediates the effect of d-Fen on food intake, as the genetic mouse lines used (2C/POMC and MC4R/SIM1 mice) do not determine whether this circuit is also required for the effects of d-Fen. In fact, both 5-HT2CRs (Molineaux et al., 1989) and MC4Rs (Mountjoy et al., 1994) are also expressed in other brain regions that may provide redundant pathways mediating the anorexigenic effects of d-Fen. The potential physiological relevance of these possible redundant 5-HT2CR or MC4R sites has yet to be characterized.
Here we describe a discrete pathway through which one of the most clinically effective pharmacological obesity treatments influences appetite—via 5-HT2CRs expressed with POMC neurons that influence the activity of MC4Rs expressed in SIM1 neurons. This pathway is critical for normal energy balance since restoration of 5-HT2CRs or MC4Rs in these specific subsets of chemically defined neurons is sufficient to normalize aberrant feeding behavior. Collectively, these data suggest that selective therapeutic targets to this 5-HT2CR/POMC-MC4R/SIM1 circuit may provide a discrete and effective treatment for obesity.
Footnotes
This work was supported by National Institutes of Health Grant K99 DK0853301-01 and the Canadian Institute of Health Research (Y.X.), National Institutes of Health Grant DK065171 and Wellcome Trust Grant WT081713 (L.K.H.), and National Institutes of Health Grants R01 DK79986 (A.R.Z.), PO1 DK56116 and R01 DK075632 (B.B.L.), and R01DK53301, R01MH61583, and RL1DK081185 (J.K.E.). Support also was given by the American Diabetes Association and a Smith Family Foundation Pinnacle Program Project Award. We thank the Mouse Metabolic Phenotyping Core at University of Texas Southwestern Medical Center (National Institutes of Health Grants 1PL1DK081182 and 1UL1RR024923) for technical support.
References
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Clifton PG, Lee MD, Dourish CT. Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl) 2000;152:256–267. doi: 10.1007/s002130000504. [DOI] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mørk A, Honig G, Bøgesø KP, Westerink BH, den Boer H, Wikstrom HV, Tecott LH. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol. 2009;587:49–60. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- Holder JL, Jr, Zhang L, Kublaoui BM, DiLeone RJ, Oz OK, Bair CH, Lee YH, Zinn AR. Sim1 gene dosage modulates the homeostatic feeding response to increased dietary fat in mice. Am J Physiol Endocrinol Metab. 2004;287:E105–E113. doi: 10.1152/ajpendo.00446.2003. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Kiss J, Léránth C, Halász B. Serotoninergic endings on VIP-neurons in the suprachiasmatic nucleus and on ACTH-neurons in the arcuate nucleus of the rat hypothalamus. A combination of high resolution autoradiography and electron microscopic immunocytochemistry. Neurosci Lett. 1984;44:119–124. doi: 10.1016/0304-3940(84)90068-5. [DOI] [PubMed] [Google Scholar]
- Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O'Rahilly S, Heisler LK. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JJ, Yamamoto A, Scearce-Levie K, Saudou F, Hen R. Absence of fenfluramine-induced anorexia and reduced c-Fos induction in the hypothalamus and central amygdaloid complex of serotonin 1B receptor knock-out mice. J Neurosci. 1998;18:5537–5544. doi: 10.1523/JNEUROSCI.18-14-05537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirk J, Goodall E, Silverstone T, Willner P. Differential effects of d-fenfluramine, l-fenfluramine and d-amphetamine on the microstructure of human eating behaviour. Behav Pharmacol. 1991;2:113–119. [PubMed] [Google Scholar]
- Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Lévy E, Mitchell GA, Himms-Hagen J, Fan CM. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet. 2001;10:1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- Molineaux SM, Jessell TM, Axel R, Julius D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci U S A. 1989;86:6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females. Mol Pharmacol. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Clark RD, Partilla JS, Baumann MH. (+)-Fenfluramine and its major metabolite, (+)-norfenfluramine, are potent substrates for norepinephrine transporters. J Pharmacol Exp Ther. 2003;305:1191–1199. doi: 10.1124/jpet.103.049684. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Carlton J. Neurobiology of an anorectic drug: fenfluramine. Prog Neurobiol. 1986;27:13–62. doi: 10.1016/0301-0082(86)90011-0. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Williams DL, Schwartz MW. The melanocortin system as a central integrator of direct and indirect controls of food intake. Am J Physiol Regul Integr Comp Physiol. 2005;289:R2–R3. doi: 10.1152/ajpregu.00226.2005. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, McCrimmon RJ, Elmquist JK, Butler AA, Heisler LK. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]