Abstract
Acetylcholine in the brain alters neuronal excitability, influences synaptic transmission, induces synaptic plasticity and coordinates the firing of groups of neurons. As a result, it changes the state of neuronal networks throughout the brain and modifies their response to internal and external inputs: the classical role of a neuromodulator. Here we identify actions of cholinergic signaling on cellular and synaptic properties of neurons in several brain areas and discuss the consequences of this signaling on behaviors related to drug abuse, attention, food intake, and affect. The diverse effects of acetylcholine depend on the site of release, the receptor subtypes, and the target neuronal population, however, a common theme is that acetylcholine potentiates behaviors that are adaptive to environmental stimuli and decreases responses to ongoing stimuli that do not require immediate action. The ability of acetylcholine to coordinate the response of neuronal networks in many brain areas makes cholinergic modulation an essential mechanism underlying complex behaviors.
INTRODUCTION
Acetylcholine (ACh) is a fast-acting, point-to-point neurotransmitter at the neuromuscular junction and in the autonomic ganglia; however, there are fewer demonstrations of similar actions in the brain (Changeux, 2010). Instead, central cholinergic neurotransmission predominantly changes neuronal excitability, alters presynaptic release of neurotransmitters, and coordinates the firing of groups of neurons (Kawai et al., 2007; Rice and Cragg, 2004; Wonnacott, 1997; Zhang and Sulzer, 2004). As a result, ACh appears to act as a neuromodulator in the brain, despite its role as the primary excitatory neurotransmitter in the periphery.
The definition of a neuromodulator is flexible but has evolved to describe any kind of neurotransmission that is not directly excitatory (mediated through ionotropic glutamate receptors) or inhibitory (mediated through ionotropic GABA receptors) (Ito and Schuman, 2008; Siggins, 1979). Neuromodulation can be thought of as a change in the state of a neuron, or group of neurons, that alters its response to subsequent stimulation. A number of models have been proposed to explain the actions of ACh in the central nervous system (CNS). For example, ACh has been suggested to be critical for the response to uncertainty, such that an increase in cholinergic tone predicts the unreliability of predictive cues in a known context, and improves the signal-to-noise ratio in a learning environment (Yu and Dayan, 2005). Another model has suggested that ACh reinforces neuronal loops and cortical dynamics during learning by enhancing the influence of feed-forward afferent inputs to the cortex carrying sensory information and decreasing excitatory feedback activity mediating retrieval (Hasselmo, 2006). ACh can also alter firing of neurons on a rapid time scale, as in fear-conditioning, when foot-shock results in direct cholinergic activation of interneurons in the auditory cortex that contribute to learning (Letzkus et al., 2011). All these models are consistent with a primary role of ACh as a neuromodulator that changes the state of an ensemble of neurons in response to changing environmental conditions.
In this review, we will provide further support for the idea that cholinergic neurotransmission in the brain is primarily neuromodulatory and is categorically distinct from the actions of ACh at the neuromuscular junction. We propose that the role of ACh as a neuromodulator in the brain is to increase neurotransmitter release in response to other inputs, to promote burst firing and/or suppress tonic firing, depending upon the system and the neuronal subtypes stimulated. Further, ACh contributes to synaptic plasticity in many brain areas.
CHOLINERGIC NEURONS AND ACH RECEPTORS
The two primary sources of ACh in the brain include projection neurons that innervate distal areas and local interneurons that are interspersed among their cellular targets. Cholinergic projection neurons are found in nuclei throughout the brain, such as the pedunculopontine and laterodorsal tegmental areas (PPtg and LDTg), the medial habenula (MHb) (Ren et al., 2011), and the basal forebrain (BF) complex (Mesulam, 1995; Zaborszky, 2002; Zaborszky et al., 2008), including the medial septum (MS). These cholinergic neurons project widely and diffusely, innervating neurons throughout the CNS. Cholinergic interneurons are typified by the tonically-active ACh neurons of the striatum and nucleus accumbens, and there is some indication from anatomical studies that cholinergic interneurons are present in the rodent and human neocortex, but not the non-human primate cortex (Benagiano et al., 2003; Mesulam, 1995; von Engelhardt et al., 2007). The actions of ACh released from both populations of cholinergic cells are mediated through pre- and postsynaptic receptors on a large variety of neuronal subtypes throughout the brain, and it should be noted that cholinergic inputs contribute to cortical and hippocampal function across phylogeny.
ACh signals through two classes of receptors: metabotropic muscarinic receptors (mAChRs) and ionotropic nicotinic receptors (nAChRs) (reviewed in (Picciotto et al., 2000; Wess, 2003a)). Muscarinic receptors are coupled either to Gq proteins (M1, M3, and M5 subtypes) that activate phospholipase C (PLC) or Gi/o proteins (M2 and M4 subtypes) that negatively couple to adenylate cyclase (reviewed in (Wess, 2003a)), linking ACh activity to a variety of biochemical signaling cascades. Moreover, mAChRs are located both pre- and post-synaptically throughout the brain, producing diverse consequences for brain activity (Figure 1). As examples of the heterogeneous effects of mAChR stimulation, presynaptic M2/M4 mAChRs can act as inhibitory autoreceptors on cholinergic terminals (Douglas et al., 2002; Raiteri et al., 1984) and reduce glutamate release from corticocortical and corticostriatal synapses (Higley et al 2009, Gil et al 1997). In contrast, M1/M5 receptors can stimulate dopamine (DA) release from striatal synaptosomes (Zhang et al., 2002) and postsynaptic M1/M5 receptors can increase excitability of cortical pyramidal neurons (Douglas et al., 2002; McCormick and Prince, 1985).
Figure 1. Sites of action for nicotinic and muscarinic acetylcholine receptors.
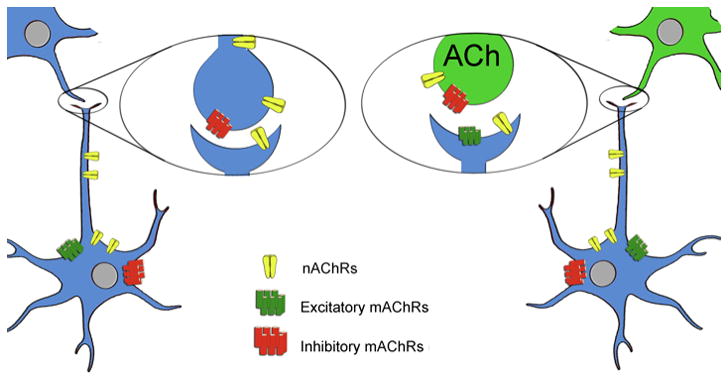
Nicotinic (nAChR) and muscarinic (mAChR) acetylcholine receptors are localized both pre- and post-synaptically. Presynaptic mAChRs (M2, M4) are largely inhibitory, and act as inhibitory autoreceptors on cholinergic terminals, with M2 the predominant autoreceptor in hippocampus and cerebral cortex, and M4 predominant in striatum (Wess, 2003b; Wess et al., 2003). Post-synaptic mAChRs can be either inhibitory (M2, M4) or excitatory (M1, M3, M5) (Wess, 2003b; Wess et al., 2003). Presynaptic nAChRs induce release of a number of neurotransmitters including GABA, glutamate, dopamine, serotonin, norepinephrine and acetylcholine (McGehee et al., 1995; Wonnacott, 1997). Postsynaptic nAChRs depolarize neurons, increase their firing rate and can contribute to long-term potentiation (Bucher and Goaillard, 2011; Ge and Dani, 2005; Ji et al., 2001; Kawai et al., 2007; Mansvelder and McGehee, 2000; Picciotto et al., 1995; Picciotto et al., 1998; Radcliffe and Dani, 1998; Wooltorton et al., 2003).
Nicotinic receptors function as non-selective, excitatory cation channels (Changeux et al., 1998; Picciotto et al., 2001) and occur as homomeric or heteromeric assemblies of a large family of α- and β-subunits (α2-α7 and β2-β4; reviewed in (Picciotto et al., 2000)). While neuromodulators are typically associated with metabotropic signaling, the role of the ionotropic nAChRs in the brain appears to be largely modulatory as well (Picciotto, 2003). For example, nAChRs are not clustered at postsynaptic membranes apposed to sites of ACh release, but are rather dispersed along the surface (and intracellular compartments) of neurons, including presynaptic terminals (McGehee et al., 1995; Vidal and Changeux, 1993), cell bodies and even axons (Arroyo-Jimenez et al., 1999; Hill Jr. et al., 1993; Kawai et al., 2007). In addition, stimulation of nAChRs can increase the release of glutamate, GABA, dopamine (DA), ACh, norepinephrine, and serotonin (McGehee et al., 1995; Wonnacott, 1997) (Figure 1). Nicotinic modulation of neurotransmitter release is often subtype-specific and this specificity can vary across brain areas, with distinct nAChRs coupling to release of glutamate (α7) vs. GABA (α4β2*) (Mansvelder et al., 2002) or DA (α4/α6β2*) vs. ACh (α3β4*) (Grady et al., 2001) in the VTA, while β2* nAChRs can modulate the release of glutamate from thalamo-cortical projections (Parikh et al., 2010). Presynaptic effects of nAChRs contribute to synaptic plasticity in the VTA (Mansvelder and McGehee, 2000; Wooltorton et al., 2003), hippocampus (Ge and Dani, 2005; Ji et al., 2001; Radcliffe and Dani, 1998), and prefrontal cortex (Couey et al., 2007). In addition, nAChRs may also be important for synchronizing neuronal activity. For example, nicotine is reported to coordinate firing of thalamocortical fibers through effects on nAChRs in white matter (Bucher and Goaillard, 2011; Kawai et al., 2007). Despite the clear effects of presynaptic nAChRs in electrophysiological studies, their relationship to the behavioral consequences of nicotine administration is not completely understood. For example, although nicotine stimulates the firing of DA neurons through actions in the ventral tegmental area (VTA) and increases release of DA from the midbrain projections to the NAc through actions on terminal nAChRs, local infusion of nicotine into the VTA has much greater effects on locomotion and self-administration than local infusion into the NAc (Ferrari et al., 2002; Ikemoto et al., 2006). Recent studies have, however, suggested that nAChRs in the NAc are important for the motivational effects of nicotine (association between stimulus and drug intake), rather than the primary reinforcing effects of the drug (desire for drug) (Brunzell et al., 2010). Additionally, it is clear that cholinergic interneurons and their regulation of muscarinic receptor signaling are also critical components in striatum-dependent decision making (see, e.g. (Goldberg et al., 2012)).
While presynaptic effects of nAChRs have been the focus of a great deal of work, effects of nicotinic stimulation are clearly not exclusively presynaptic (Figure 1). Exogenous application of nicotine can induce significant inward currents in neurons in a number of brain areas (Léna and Changeux, 1999; Picciotto et al., 1995; Picciotto et al., 1998), and there have been several examples of direct post-synaptic effects of ACh in the brain (Alkondon et al., 1998; Jones et al., 1999). Notably, recent studies using optogenetic techniques demonstrated that ACh can mediate postsynaptic responses through nAChRs in hippocampus (Bell et al., 2011; Gu and Yakel, 2011) and cortex (Arroyo et al., 2012).
MODES OF CHOLINERGIC NEUROMODULATION
Although there is considerable evidence for the actions of ACh on target neurons, the mode of cholinergic transmission has remained controversial. The debate has focused on whether cholinergic signaling occurs via traditional synapses (cellular specializations comprising closely apposed pre- and postsynaptic membranes with associated release/receptor machinery) or via volume transmission (actions of a neurotransmitter that occur at a distance from its site of release, mediated by diffusion through the extracellular space (Zoli et al., 1999). Accumulating evidence indicates that ACh can act through volume transmission in the brain. The relatively diffuse nature of brain cholinergic innervation further reinforces this idea. There is an anatomical mismatch between the sites of ACh release (Houser, 1990; Wainer et al., 1984a; Wainer et al., 1984b) and the location of cholinergic receptors (Arroyo-Jimenez et al., 1999; Hill Jr. et al., 1993; Kawai et al., 2007). There is also evidence that extracellular levels of ACh fluctuate in a manner that is not consistent with localized clearance of a synaptic transmitter (Hajnal et al., 1998; Laplante et al., 2004; Mark et al., 1996; Parikh et al., 2004; Reid et al., 1998). However, contrasting observations, including the role of ACh in fast synaptic transmission at the neuromuscular junction and the high level of expression of ACh esterase (a highly efficient degradative enzyme responsible for clearing ACh from the extracellular space), have limited the acceptance of this idea. Ultimately, it is difficult to know how far ACh can diffuse from its site of release and whether volume transmission would allow for rapid transfer of information, suggesting that this is not the only mechanism through which ACh influences neuronal function in the brain. Anatomical studies have identified cortical cholinergic synapses that are structurally similar to those of other point-to-point neurotransmitters, in both rats (Turrini et al., 2001) and humans (Smiley et al., 1997). Effects of ACh on a rapid time-scale likely underlie its role in stimulus-response tasks in which subsecond reactivity is required for appropriate behavioral responses, as in prefrontal cortex-dependent cue detection (Parikh et al., 2007a) or auditory discrimination (Letzkus et al., 2011). The data indicate that differences in sites of receptor expression, affinity of ACh effects at both mAChRs and nAChRs, as well as rates of synaptic clearance (mediated through AChE activity) and local concentration of ACh in and outside the synapse, are critical for the control and specificity of cholinergic signaling. Further, differences in the time-scale of release at the local microcircuit level further refine the action of ACh in complex behaviors (reviewed in (Hasselmo and Giocomo, 2006; Sarter et al., 2009; Yu and Dayan, 2005)).
ROLE OF ACH IN SYNAPTIC PLASTICITY AND NEURONAL DEVELOPMENT
An important role for both nAChRs and mAChRs has been defined in hippocampal synaptic plasticity (reviewed in (Giocomo and Hasselmo, 2007; McKay et al., 2007)) and these effects are mediated through intracellular signaling pathways downstream of mAChRs and nAChRs (reviewed in (Berg and Conroy, 2002; Cancela, 2001; Lanzafame et al., 2003; Rathouz et al., 1996)). Recent studies suggest that the timing of ACh release and the subtype of receptor is critical for the type of plasticity induced (Gu and Yakel, 2011); however, it is clear that nAChRs and mAChRs on both GABAergic and glutamatergic neurons in the hippocampus can alter the subsequent response to excitatory inputs (Drever et al., 2011). Similarly, stimulation of nAChRs on glutamatergic terminals in the VTA can induce long-term potentiation (LTP) of excitatory inputs onto DA neurons (Mansvelder and McGehee, 2000), whereas differential effects of nAChRs on glutamatergic and GABAergic terminals in this area appears to be important for changes in dopaminergic firing following prolonged exposure to nicotine (Mansvelder et al., 2002; Wooltorton et al., 2003).
The ability of ACh to influence synaptic plasticity and dynamics of local circuits can also occur through astrocytic control of synaptic Ca2+ concentration following nAChR stimulation (Takata et al., 2011). Astrocytic signaling can lead to LTP as a result of the temporal coincidence of the postsynaptic activity and the astrocyte Ca2+ signal simultaneously evoked by cholinergic stimulation (Navarrete et al., 2012).
In contrast to the ability of nAChR stimulation to promote LTP in a number of brain areas, nAChR-mediated facilitation of GABA release reduces calcium levels in prefronto-cortical dendrites (Couey et al., 2007). In addition, activation of nAChRs can also decrease subsequent stimulation of calcium entry into cortical neurons in response to glutamate (Stevens et al., 2003). The decrease in glutamate-mediated calcium entry is mediated through activation of high affinity nAChRs, subsequent activation of the protein phosphatase calcineurin and inactivation of L-type calcium channels. If this mechanism is also recruited as a result of ACh signaling in vivo, it would suggest that one consequence of cholinergic activity in cortical neurons would be a significant decrease in subsequent calcium-mediated glutamate responses.
Finally, in addition to the ability of ACh to modulate neuronal activity acutely in adulthood, ACh can also alter a number of processes in neuronal development, and the molecular basis for a number of these developmental effects of ACh signaling have been elucidated recently. For example, one fundamental role for ACh signaling through nAChRs is to regulate the timing of expression of the chloride transporter that is necessary for the ability of GABA to hyperpolarize, and therefore inhibit, central neurons (Liu et al., 2006). Disrupting nAChR signaling delays the switch from GABA-mediated excitation to inhibition. Recent studies have also shown that nAChRs contribute to the maturation of GABAergic (Kawai et al., 2002; Zago et al., 2006) and glutamatergic (Lozada et al., 2012a, b) synapses, highlighting an important role for ACh signaling in synaptic development, as well as neuronal pathfinding and target selection (reviewed in (Role and Berg, 1996). In addition, signaling through nAChRs is also important for establishing critical periods for activity-dependent shaping of visual cortical function (Morishita et al., 2010) and maturation of thalamocortical (Aramakis and Metherate, 1998; Aramakis et al., 2000; Hsieh et al., 2002) and corticothalamic (Heath et al., 2010; Horst et al., 2012; King et al., 2003; Picciotto et al., 1995) glutamatergic synapses. It appears likely that ACh release, potentially in response to salient stimuli, potentiates glutamatergic synapses during development through an LTP-like mechanism (Aramakis and Metherate, 1998), highlighting another important role for cholinergic signaling in synaptic plasticity. Several neurotrophic factors are also involved in the development and maturation cholinergic neurons, but the dependence on neurotrophins is not homogenous throughout the CNS (for reviews, see (Angelucci et al., 2005; Schindowski et al., 2008)). Although a comprehensive review of the developmental effects of ACh is beyond the scope of this article, it is important to note that various developmental processes can be affected by ACh signaling (for more comprehensive reviews, see (Heath and Picciotto, 2009; Liu et al., 2007; Metherate and Hsieh, 2003; Role and Berg, 1996)).
BRAIN SYSTEMS MODULATED BY ACH SIGNALING
Mesolimbic DA system, addiction and reward
A great deal of research has focused on the effects of cholinergic agents on the mesolimbic DA system and its short- and long-term modulation (for reviews see (Fagen et al., 2003; Mansvelder et al., 2003), particularly because the addictive effects of nicotine are mediated primarily through stimulation of nAChRs in the VTA (Drenan et al., 2008; Maskos et al., 2005; McGranahan et al., 2011; Picciotto et al., 1998). Cholinergic input from the PPTg and LDTg acting through both mAChRs and nAChRs is critical for modulating the function of the VTA. Stimulation of nAChR and M5-type mAChRs increases the tonic excitability of these DA neurons (Corrigall et al., 2002; Miller and Blaha, 2005; Yeomans and Baptista, 1997). ACh released in the VTA is likely to potentiate glutamatergic synaptic transmission onto DA neurons through α7 nAChRs, and may therefore increase the likelihood of burst firing of these neurons (Grenhoff et al., 1986; Maskos, 2008; McGehee et al., 1995).
Extracellular ACh levels are increased in the VTA during drug self-administration (You et al., 2008), that could result from an increase in ACh release from PPTg and LDTg afferents (Futami et al., 1995; Omelchenko and Sesack, 2006). Cholinergic neurons within PPTg neurons do not exhibit burst firing, and they are more active during wakefulness and REM sleep versus slow wave sleep, but show more activity during REM sleep than slow wave sleep (Datta and Siwek, 2002); however, there is currently no evidence that VTA DA neurons show circadian variations in activity, suggesting that the diurnally regulated neurons may not project to VTA. In addition, PPTg neurons change their firing rate in response to both locomotion and acquisition of reward (Datta and Siwek, 2002). These observations have led to the idea that the PPtg acts as a gate for salient sensory information associated with reward and/or requiring movement (Norton et al., 2011).
In contrast to the increased firing rate of cholinergic neurons in the PPTg in response to contextual information related to reward, tonically active cholinergic interneurons in the striatum pause their firing following exposure to cues associated with reward (Goldberg and Reynolds, 2011). The pause is thought to be mediated by interactions between the cells’ intrinsic membrane properties and strong feed-forward excitation from the thalamus (Ding et al., 2010). These cholinergic interneurons can regulate the duration, magnitude, and spatial pattern of activity of striatal neurons, potentially creating an attentional gate that facilitates movement toward salient stimuli (Oldenburg and Ding, 2011). Function of striatal cholinergic interneurons is also impaired in patients with movement disorders that are dependent on function of the dopaminergic system such as Parkinson’s and Huntington’s disease and in animal models of these diseases (Ding et al., 2011). Cholinergic signaling in striatum and NAc is also thought to be critical for mediating the association between drugs of abuse and cues in the environment that drive drug craving and relapse to drug use after abstinence (Exley and Cragg, 2008). The effects of striatal ACh are mediated in part through activation of nAChRs on dopaminergic terminals, leading to tonic, low level DA release when cholinergic interneurons are firing. The pause results in decreased tonic DA release but maintained phasic DA release (Exley and Cragg, 2008). In contrast, mAChRs reduce the probability of glutamate release from excitatory afferents to the striatum, negatively regulating the ability of these inputs to drive striatal activity (Barral et al., 1999; Higley et al., 2009; Pakhotin and Bracci, 2007). Reduced concentration of glutamate in the synaptic cleft results in diminished activation of voltage-dependent NMDA-type glutamate receptors, shortening excitatory response duration and limiting temporal integration of inputs (Higley 2009). Thus, the pause in cholinergic interneuron firing would be predicted to enhance the efficacy and summation of glutamatergic inputs arriving during this period.
These findings suggest that salient sensory stimuli in the environment, such as those associated with rewards or drugs of abuse, would increase activity of PPTg cholinergic neurons, leading to increased phasic firing of DA neurons in the VTA (Maskos, 2008), while at the same time, decreasing the firing of tonically active cholinergic neurons in the NAc and striatum leading to a larger differential in DA release in response to phasic firing as compared to tonic firing (Exley and Cragg, 2008) (Figure 2). At the behavioral level, this conclusion is consistent with the finding that disruption of PPTg activity decreases the rewarding and locomotor effects of drugs of abuse such as cocaine and nicotine (Champtiaux et al., 2006; Corrigall et al., 1994; Corrigall et al., 2002), while lesion of NAc cholinergic neurons increases cocaine self-administration, as might be expected if a pause in cholinergic interneuron firing in NAc signals salience (Smith et al., 2004).
Figure 2. Effects of acetylcholine on activity of dopamine neurons in the mesolimbic circuit.
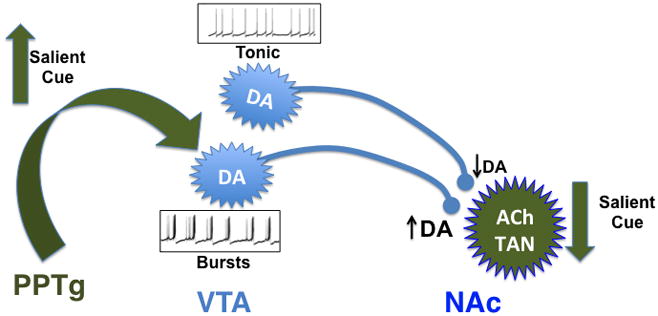
Salient cues associated with primary rewards increase activity of pedunculopontine tegmental area (PPTg) neurons, inducing acetylcholine release in the ventral tegmental area (VTA) (Futami et al., 1995; Omelchenko and Sesack, 2006). Acetylcholine increases firing of dopamine (DA) neurons in the VTA and is likely to be important for burst firing of these neurons (Maskos, 2008). Salient cues associated with rewards also induce a pause in firing of tonically active cholinergic neurons (ACh TAN) in the nucleus accumbens (NAc) (Goldberg and Reynolds, 2011). Decreased release of ACh onto terminals in NAc attenuates DA release due to tonic firing of DA neurons, while preserving DA release in response to phasic firing (Exley and Cragg, 2008).
The behavioral role of individual ACh receptor subtypes in NAc is more complex, however. Consistent with a role for the pause in NAc cholinergic neurons in behaviors related to drug reward, antagonism of α7-type nAChRs in NAc increases motivation to lever press for nicotine (Brunzell and McIntosh, 2012). Less intuitively, blockade of mAChRs using scopolamine decreases reinstatement of cocaine seeking (Yee et al., 2011), but this may be due to increased ACh release through blockade of inhibitory autoreceptors (Douglas et al., 2001). Since neuromodulation can be complex, it has also been shown that antagonism of the α6/β2 class of nAChRs expressed on DA terminals in NAc decreases the breakpoint for progressive ratio responding in rats self-administering nicotine, suggesting that there is also a role for ACh signaling through this class of receptors for mediating the motivational value of nicotine (Brunzell et al., 2010).
A number of studies have focused on the ability of the habenula, particularly the MHb to oppose the behavioral processes mediated through the VTA (for reviews, see (Fowler and Kenny, 2012; Hikosaka, 2010)). The MHb-interpeduncular pathway is cholinergic, and it has been proposed that its effects on VTA neuron firing are mediated indirectly through inhibition of the PPTg (Maskos, 2008). Decreasing the expression of nAChRs containing the α5 subunit in the MHb results in increased nicotine self-administration (Fowler et al., 2011), suggesting that this cholinergic system normally acts as a brake on drug reward.
Taken together, these studies suggest that point-to-point ACh signaling could have opposing behavioral consequences, depending on the receptor subtypes, neuronal populations and brain areas stimulated, and that effects of ACh mediated through volume transmission could be distinct from those mediated locally.
Cortex and attention
Numerous studies indicate that ACh plays an important and diverse role in the regulation of cortical activity over multiple timescales. The precise function of ACh on any given circuit also greatly depends on the specific expression patterns of nAChRs and mAChRs, as well as the temporal dynamics of ACh concentration in the extracellular space. Neocortical ACh function has been linked to control of circuits underlying attention, cue detection, and memory (Hasselmo and Sarter, 2011). The primary cholinergic input to the cerebral cortex comes from the BF complex, and particularly from substantia innominata of the the nucleus basalis of Meynert (Mesulam, 1995) though the latter remains debated (Zaborszky et al., 1999). Cholinergic terminals are distributed throughout the cortex, with more dense projections in superficial layers (Mesulam, 1995).
The cellular mechanisms underlying the effects of ACh on cortical circuits have been investigated at many levels. Seminal studies revealed that ACh can produce biphasic changes in the activity of pyramidal neurons, the principal excitatory cells in the neocortex, comprising fast inhibition followed by a slow depolarization (McCormick and Prince, 1985, 1986). The fast inhibition is at least partially mediated by the actions of both nAChRs and mAChRs that increase the excitability and firing rates of dendrite-targeting GABAergic interneurons (Arroyo et al., 2012; Couey et al., 2007; Fanselow et al., 2008; Ferezou et al., 2002; Gulledge et al., 2007; Kawaguchi and Kubota, 1997). The slow depolarization is mediated by M1 mAChR-mediated closure of M-type (KCNQ) potassium channels in pyramidal neurons (Delmas and Brown, 2005) enhancing their excitability and reducing their spike frequency adaptation (Gulledge et al., 2007; Hasselmo and Giocomo, 2006). In addition, nAChRs expressed in deep layer pyramidal neurons may contribute to direct excitation of these cells (Bailey et al., 2010; Kassam et al., 2008; Poorthuis et al., 2012).
ACh also modulates synaptic transmission in cortical circuits (Figure 3). Activation of α4β2 nAChRs on thalamocortical terminals enhances glutamate release in both sensory and association cortex (Gil et al., 1997; Lambe et al., 2003; Oldford and Castro-Alamancos, 2003), whereas activation of mAChRs on terminals of parvalbumin-expressing interneurons decreases the probability of GABA release onto the perisynaptic compartment of pyramidal neurons, and therefore reduces post-synaptic inhibition of pyramidal neurons (Kruglikov and Rudy, 2008). These interneurons normally decrease the response of cortical neurons to feed-forward excitation (Gabernet et al., 2005; Higley and Contreras, 2006), and the reduction of GABA release from these interneurons by ACh therefore enhances the ability of thalamocortical inputs to stimulate pyramidal neuron firing (Kruglikov and Rudy, 2008).
Figure 3. Effects of acetylcholine on activity of cortical neurons.
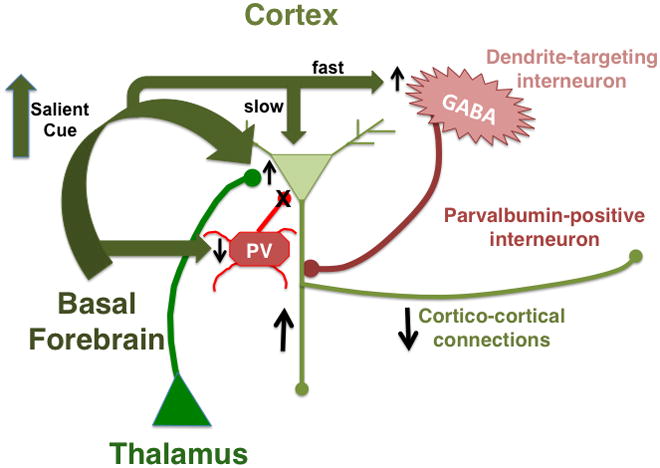
Salient cues induce acetylcholine release onto interneurons targeting the apical dendrites of cortical pyramidal neurons, resulting in rapid inhibition of pyramidal cells (Arroyo et al., 2012; Couey et al., 2007; Fanselow et al., 2008; Ferezou et al., 2002; Gulledge et al., 2007; Kawaguchi and Kubota, 1997). Acetylcholine subsequently depolarizes pyramidal neurons through M1 mAChRs (Delmas and Brown, 2005; McCormick and Prince, 1985, 1986). Acetylcholine also activates stimulatory α4β2 nAChRs on glutamatergic thalamocortical terminals (Gil et al., 1997; Lambe et al., 2003; Oldford and Castro-Alamancos, 2003) and inhibitory M2 mAChRs on GABAergic terminals of parvalbumin-expressing (PV) interneurons (Kruglikov and Rudy, 2008). Activation of PV interneurons enhances stimulation of pyramidal neuron firing by thalamocortical inputs (Gabernet et al., 2005; Higley and Contreras, 2006; Kruglikov and Rudy, 2008). Acetylcholine also suppresses cortico-cortical transmission through inhibitory M2 mAChRs on pyramidal cell axon terminals (Gil et al., 1997; Hsieh et al., 2000; Kimura and Baughman, 1997; Oldford and Castro-Alamancos, 2003), reducing intra-cortical communication while preserving responses to thalamic inputs (Kimura et al., 1999).
In contrast, mAChRs located on pyramidal cell axon terminals suppress cortico-cortical transmission (Gil et al., 1997; Hsieh et al., 2000; Kimura and Baughman, 1997; Oldford and Castro-Alamancos, 2003). Moreover, the ACh-mediated increased excitability of dendrite-targeting interneurons described above likely contributes to reduced efficacy of intra-cortical communication. The simultaneous enhancement of feed-forward inputs from the thalamus through cholinergic actions on parvalbumin-positive interneurons, and suppression of intra-cortical feed-back inputs through effects on dendrite-targeting interneurons, may increase the “signal-to-noise” ratio in cortical networks, making neurons more sensitive to external stimuli. In keeping with this view, mAChR activation strongly suppresses the spread of intra-cortical activity, leaving responses to thalamic inputs relatively intact (Kimura et al., 1999). Intriguingly, in the prefrontal cortex, the expression of nicotinic receptors in deep pyramidal cells may produce layer-specific cholinergic modulation, selectively enhancing activity of output neurons (Poorthuis et al., 2012).
Although the cellular and synaptic effects of ACh described above provide a potential mechanism for the ability of ACh to increase signal detection and modulate sensory attention, a number of observations suggest that this simple model is incomplete. ACh directly inhibits spiny stellate cells in somatosensory cortex receiving thalamic input via M4 mAChRs (Eggermann and Feldmeyer, 2009). Furthermore, activation of M1 mAChRs hyperpolarizes pyramidal neurons via a mechanism dependent on fully-loaded internal calcium stores that occurs more quickly than the closure of M-type potassium channels (Gulledge et al., 2007; Gulledge and Stuart, 2005). Thus, the effect of ACh on the activity of cortical neurons clearly depends critically on the state of the neuron and the timing of ACh release. Neurons with depleted calcium stores would be more susceptible to ACh-induced depolarization via M4 mAChRs, whereas rapid inhibitory effects of ACh through M1 mAChRs would dominate in neurons with fully-replenished stores. Furthermore, studies showing that mAChR activation reduces cortico-cortical transmission have relied on electrical stimulation to evoke glutamate release, leaving the identity of the activated presynaptic terminals ambiguous. It is possible that distinct populations of intra-cortical synapses, such as those comprising local recurrent networks versus long-range intra-areal projections, might be differentially modulated by ACh. Indeed, in the CA1 region of the hippocampus, long-range perforant inputs from the entorhinal cortex are less inhibited by ACh than the Schaeffer collaterals arising from CA3 (Hasselmo and Schnell, 1994). The advent of optogenetic tools for selectively targeted difference populations of excitatory inputs (Gradinaru et al., 2007) will be a key development for elucidating the precise role of ACh on various circuit elements.
ACh also modulates cortical circuits over longer time scales by influencing the plasticity of cortical circuits. In the auditory cortex, pairing sensory stimulation with stimulation of the basal forebrain results in a long-term reorganization of cortical receptive field structure, including a persistent shift in the receptive field towards the paired stimulus (Froemke et al., 2007). In the visual system, ACh facilitates ocular dominance plasticity in kittens via M1 mAChRs (Gu and Singer, 1993) and in rodents, the protein Lynx1 suppresses nicotinic signaling in primary visual cortex, and its removal promotes ocular dominance plasticity in older animals (Morishita et al., 2010).
At the cellular level, cholinergic agonists enhance LTP of glutamatergic association fibers in the piriform cortex and Schaeffer collaterals in the CA1 region of the hippocampus (Huerta and Lisman, 1993). In contrast, M3 mAChRs facilitate long term depression (LTD) of synapses in the monocular area of superficial visual cortex (Kirkwood et al., 1999; McCoy and McMahon, 2007). Surprisingly, the same authors observed enhanced LTP in binocular cortex (McCoy et al., 2008). These regional differences indicate that cell-specific expression of different receptor subtypes is critical for the varied actions of ACh.
The pleiotropic effects of ACh on cortical circuits described above are likely to underlie its ability to modulate cognitive behaviors. In rodents, lesions of cholinergic inputs to the cortex impair tests of sustained attention, particularly across sensory modalities (McGaughy et al., 2002; McGaughy et al., 1996; Turchi and Sarter, 1997). In addition, stimulation of α4β2 nicotinic receptors in the medial prefrontal cortex enhances performance in a visual attention task (Howe et al., 2010), while genetic deletion of these receptors in the mPFC impairs visual attention (Guillem et al., 2011) and auditory discrimination (Horst et al., 2012). Notably, transient rises in prefrontal ACh are significantly correlated with cue detection, suggesting that the temporal dynamics of cholinergic signaling are also critical for normal behavior (Parikh et al., 2007b). In primates, locally applied ACh enhances the attentional modulation of neuronal activity in the primary visual cortex, while the muscarinic antagonist scopolamine reduces the effects of attention (Herrero et al., 2008). Taken together, these findings suggest that cholinergic actions across both ionotropic and metabotropic receptors and diverse brain areas contribute to cognitive processing.
Hypothalamus and food intake
The role of ACh in control of autonomic functions is well known, but it is likely that actions of ACh in the brain also modulate adaptive responses to environmental and metabolic conditions. Cholinergic signaling can alter thermoregulation (Myers and Waller, 1973), sleep patterns (Steriade, 2004), food intake (Grunberg et al., 1988; Mineur et al., 2011) and endocrine functions such as pancreatic release of insulin and glucagon (Ishikawa et al., 1982). The hypothalamus is essential for homeostatic responses regulating metabolism, and consequently, modulation of hypothalamic function by ACh is likely to be an important component of adaptation to peripheral autonomic signals to the brain.
A small number of studies have investigated the role of ACh signaling in the hypothalamus, which receives input from the PPTg and LDTg (Hallanger and Wainer, 1988; Jones and Beaudet, 1987). Activity in both these areas adapts quickly to environmental changes (Majkutewicz et al., 2010; Woolf, 1991) and is linked to peripheral control of feeding behavior (Phillis, 2005). There are also intrinsic neurons within the hypothalamus that express cholinergic markers (Tago et al., 1987) as well as the pro-opiomelanocortin (POMC) peptide (Meister et al., 2006), and nAChRs in the hypothalamus are critical for feeding behavior (Jo et al., 2002). It has also been suggested that neurons in the median eminence could project to the hypothalamus (Schafer et al., 1998). Corticotropin-releasing hormone-expressing neurons in this area can affect metabolism. In non-human primates, neurons in the substantia innominata and LH, most of which express cholinergic markers, were activated in response to presentation of food when the animals were hungry (Rolls et al., 1979). Consistent with a potential role for ACh in coordinating caloric need with food-seeking behaviors, long-term maintenance on a high-fat/high-sugar diet significantly down-regulated levels of ACh-esterase (AChE) in a number of brain areas that was particularly pronounced in the hypothalamus (Kaizer et al., 2004). One possibility is that the role of ACh in the hypothalamus is to integrate the interoceptive cues related to hunger with exteroceptive cues of food availability, threat or other salient conditions, a function consistent with the role of the hypothalamus in integration of interoceptive and exteroceptive conditions (Craig, 2002, 2003), but this remains to be tested.
At the cellular level, stimulation of nAChRs and mAChRs on lateral hypothalamic (LH) neurons increases and decreases GABA release, respectively (Jo and Role, 2002). The data suggest that the nAChRs and mAChRs may be localized to different populations of GABAergic terminals, but from these studies it is difficult to determine what the effects of synaptically evoked ACh on LH GABA release might be. Optogenetic stimulation of cholinergic transmission in the LH and hypothalamus will be useful in identifying the source of ACh input to these areas, the role of intrinsic ACh in hypothalamic function, and the differential role of mAChRs and nAChRs in shaping responses to ACh in these brain regions. In the arcuate nucleus of the hypothalamus, nicotine increases the firing rate of both POMC- and neuropeptide Y (NPY)-positive neurons, although the increase in POMC neuron activity predominates in vitro due to more rapid desensitization of nAChR responses in NPY neurons, and in vivo, as evidenced by an increase in c-fos immunoreactivity predominantly in POMC-positive cells (Huang et al., 2011; Mineur et al., 2011). Thus, as in the mesolimbic system and the cortex, distinct actions of ACh appear to converge through effects on receptor populations with different electrophysiological properties expressed on distinct subsets of neurons to promote a coordinated output, in this case, activation of POMC neurons.
ACh also regulates glutamatergic transmission in other neuronal subtypes involved in food intake. Stimulation of nAChRs on orexin-positive neurons in the LH induces concurrent release of glutamate and ACh, which could lead to feed-forward stimulation of this circuit once activated (Pasumarthi and Fadel, 2010). There is also some indication from studies of hypothalamic neurons in culture that ACh signaling can be upregulated to compensate for prolonged blockade of glutamatergic signaling (Belousov et al., 2001). Thus, ACh acting through nAChRs may also potentiate glutamate signaling in particular neuronal subtypes of the hypothalamus, although the functional consequences of this regulation are not yet known.
As might be expected from the complex regulation of hypothalamic neuronal activity by ACh, cholinergic modulation of feeding behavior is multifactorial and state-dependent. In rats, the mAChR competitive antagonist atropine modestly altered the frequency and choice of meals but not their size (Nissenbaum and Sclafani, 1988). Consistent with the ability of nicotine in tobacco smoke to decrease body weight in humans and food intake in rats (Grunberg et al., 1988), β4-containing nAChRs on POMC neurons are critical for the ability of nicotine to reduce food intake in mice (Mineur et al., 2011). These observations underscore a potential role for ACh in metabolic regulation involving POMC neurons; however, very little is known about the role of endogenous ACh-mediated modulation of the arcuate nucleus.
ACH and stress-related systems
Increasing evidence suggests that ACh signaling in a number of brain areas is important for stress responses (Figure 4). In addition to the well-documented role of the hippocampus in learning and memory, the amygdala in mediating fear responses and the PFC in attention, these brain areas are critical nodes in adaptation and responses to stress (Belujon and Grace, 2011; Gozzi et al., 2010; McGaugh, 2004; Sapolsky, 2000; Tottenham and Sheridan, 2009). Dysfunction in the activity of these regions is strongly implicated in major depressive disorder (Sheline et al., 1998; Videbech and Ravnkilde, 2004). The hippocampus, amygdala and PFC receive a very high level of cholinergic input that come from the BF complex, and in particular, from the medial septum and nucleus basalis, respectively (Mesulam, 1995). Several studies have shown that stress increases ACh release in a brain region-specific manner (Mark et al., 1996). For instance, hippocampal and cortical ACh levels can increase following restraint stress in rats, while ACh levels in the amygdala are unchanged, although an increase in amygdalar cholinergic tone can also reduce BLA activity though activation of mAChRs (Power and Sah, 2008). Conversely, acute activation of presynaptic α7 nAChRs in the BLA can also favor the release of glutamate from impinging cortical projections, which is critical for aversive memory and fear (Klein and Yakel, 2006). Stimulation of this pathway during development blunts paired facilitation due to subsequent stimulation, however, which would be expected to decrease BLA reactivity (Jiang and Role, 2008), further highlighting the role of cholinergic signaling in plasticity of this system. The hippocampus provides inhibitory feedback to the amygdala through inhibition of the hypothalamic-pituitary-adrenal (HPA) axis (Tasker and Herman, 2011). Interestingly, relief from stress leads to an increase in cholinergic signaling in the amygdala and PFC (Mark et al., 1996), indicating that the valence of ACh varies by brain area. The effect of increased cortical ACh levels on amygdala signaling has not been studied, but stress impairs PFC output (Arnsten, 2009), and PFC can normally decrease basolateral amygdala activity through projections to the intercalated nucleus (Manko et al., 2011; Pinard et al., 2012).
Figure 4. Effects of acetylcholine on hippocampal-amygdala stress response.
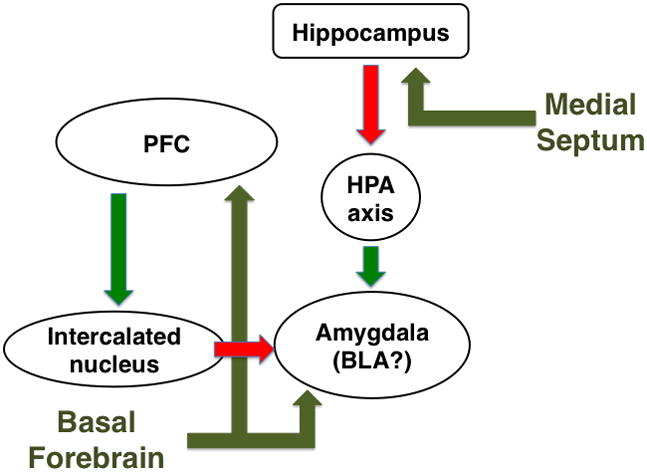
Stress increases acetylcholine release in the hippocampus and frontal cortex (Mark et al., 1996) and impairs signaling in the prefrontal cortex (PFC) (Arnsten, 2009). The hippocampus provides inhibitory feedback to the amygdala through inhibition of the hypothalamic-pituitary-adrenal (HPA) axis (Tasker and Herman, 2011) whereas the PFC can normally decrease basolateral amygdala activity through projections to the intercalated nucleus (Manko et al., 2011; Pinard et al., 2012). The effects of stress-induced acetylcholine release on output of hippocampus and cortex is unknown, but cholinergic modulation of cortico-amygdala glutamatergic connections strengthens associations between environmental stimuli and stressful events (Mansvelder et al., 2009).
At the cellular level, neuronal activity in the hippocampus is strongly modulated by both nAChRs and mAChRs. Cholinergic inputs to the hippocampus from the medial septum and the diagonal band of Broca impinge on both glutamatergic and GABAergic neurons throughout the structure, and a comprehensive review of the effects of ACh on synaptic plasticity in the hippocampus has been published recently (Drever et al., 2011). The ability of ACh to induce synaptic plasticity through actions on pre- and post-synaptic nAChRs and mAChRs is likely to modulate learning and memory, including memory of stressful events (Nijholt et al., 2004), and a role for ACh in regulation of hippocampal excitability through presynaptic release of glutamate and GABA has also been well-characterized (Alkondon et al., 1997; Freund et al., 1988; Radcliffe et al., 1999). Stress also induces alternative splicing of the AChE mRNA in the hippocampus leading to altered ACh signaling in this structure (Nijholt et al., 2004). There is currently no consensus on how these cholinergic actions converge to regulate the output of the hippocampus in response to stress, although one possibility is that ACh is critical for regulating theta oscillations, and the concurrent effects of mAChRs and nAChRs on excitatory and inhibitory transmission serve to regulate rhythmic activity (Drever et al., 2011; Fisahn et al., 1998). Although theta rhythms are thought to be critical for memory encoding, disturbance of hippocampal rhythms may also contribute to mood disorders (Femenia et al., 2012).
The amygdala also receives cholinergic inputs from the basal forebrain complex (Mesulam, 1995) and is consistently hyperactivated in fMRI studies of patients with mood disorders (Drevets, 2001). In rodents, decreasing ACh signaling through nAChRs depresses neuronal activity in the basolateral amygdala as measured by c-fos immunoreactivity (Mineur et al., 2007). As discussed above, ACh shapes the output of cortical neurons, and cortico-amygdala glutamatergic connections are also strongly and persistently potentiated by nAChR stimulation (Mansvelder et al., 2009). Thus, ACh release in the amygdala is thought to strengthen associations between environmental stimuli and stressful events, potentially contributing to maladaptive learning underlying affective disorders (Mansvelder et al., 2009).
There is strong evidence that increasing ACh signaling in humans results in increased symptoms of depression (Janowsky et al., 1972; Risch et al., 1980). This has been observed with administration of the AChE blocker physostigmine to patients with a history of depression, individuals with Tourette’s syndrome and normal volunteers (Risch et al., 1980; Risch et al., 1981; Shytle et al., 2000). A similar effect has also been described with organophosphate inhibitors of AChE (Rosenstock et al., 1991). More recently, human imaging and post mortem studies suggested that there is increased occupancy of nAChRs by ACh that is highest in individuals who are actively depressed and intermediate in those who have a history of depression with no change in overall nAChR number (Saricicek et al., 2012). In rodent studies, the Flinders rat model was selected for its sensitivity to challenge with an AChE inhibitor, and sensitive rats also display a constellation of depression-like endophenotypes, supporting the idea that increasing ACh levels increases symptoms of depression (Overstreet, 1993).
Consistent with an increase in ACh leading to symptoms of depression, antagonism of mAChRs or nAChRs, or blockade of ACh signaling through nAChRs with partial agonists, can decrease depression-like behavior in rodents (Caldarone et al., 2004; De Pablo et al., 1991; Mineur et al., 2007; Picciotto et al., 2002; Rabenstein et al., 2006). Consistent with a role for increased ACh signaling in affective disorders in humans, clinical trials have suggested that blockade of either mAChRs (Furey and Drevets, 2006; Furey et al., 2010) or nAChRs (George et al., 2008; Shytle et al., 2002) can decrease symptoms of depression. While an increase in cholinergic tone appears to be sufficient to induce depression-like symptoms in humans, a recent study has shown that decreasing striatal cholinergic tone in the mouse can lead to depression-like symptoms, likely through interneuron-dependent disinhibition of striatal neurons (Warner-Schmidt et al., 2012), highlighting the fact that ACh can induce heterogeneous effects in different brain areas that appear to have opposite behavioral consequences. The behavioral effect of ACh signaling in vivo likely depends on the baseline conditions in the particular circuit of interest at the time of ACh release, and is the result of integration of its, sometimes conflicting, effects in different circuits. More studies are necessary to determine whether preclinical studies of cholinergic signaling in hippocampus, PFC and/or amygdala can be linked to the effects of ACh in human subjects, and to identify physiological mechanisms that are essential for these effects on behaviors related to mood and affect.
CONCLUSIONS
A comprehensive explanation of cholinergic neuromodulation is not yet possible, given the large number of behaviors, circuits, neuronal subtypes and cholinergic receptors in the brain. Despite that complexity, some unifying themes have emerged. The well-defined temporal association between firing of cholinergic projection neurons in the brain stem and the pause in firing of tonically active cholinergic interneurons in the striatum can facilitate the association of salient rewarding events with cues in the environment, contributing to reward prediction and promoting orienting behaviors toward potentially rewarding stimuli. This likely occurs through coordinated increases in glutamatergic drive that facilitate DA neuron burst firing, and decreases in response to subthreshold, tonic signals from DA terminals. Similarly, salient signals that require focused attention for correct performance of behavioral tasks, increase feed-forward activation of principal cortical neurons and decrease inhibition through specific classes of interneurons. The promotion of coordinated firing of adjacent axons and the promotion of rhythmic activity in structures such as the hippocampus when ACh is released and levels are high may provide an increase in the baseline excitability of neurons that are then available for robust responses to glutamate, and this state dependent facilitation of neurotransmission in pathways activated in response to ACh release is likely to be maintained due to facilitated neuronal plasticity. This organization is echoed in the hypothalamus where, despite the ubiquitous expression of nAChRs on multiple neuronal subtypes with reciprocal functions, the kinetics of activation of one set of receptors may bias the output in one direction, based on the starting conditions. This is obviously a gross oversimplification that will be sensitive to the timing, duration and localization of ACh signaling, but may provide a framework for generation of hypotheses. Finally, increases in ACh signaling appear to contribute to stress-related illnesses such as major depressive disorder, although the specific neuronal substrates and cellular mechanisms responsible for these effects are only beginning to be studied.
Despite a great deal of progress, there are still critical gaps in our understanding of the dynamics of ACh release from different neuronal populations, how that changes in response to environmental conditions such as metabolic need or stress, and how far from the site of release ACh can diffuse in different brain areas. While novel tools will allow more precise stimulation of ACh release, the patterns of release will not be optimal unless there is a better understanding of the physiological patterns of firing. The ability to mimic patterns of ACh release in vivo will be critical for identifying the physiological effects of cholinergic neuromodulation, and distinguishing the actual, from the possible, effects of ACh in the brain.
Acknowledgments
This work was supported by NIH grants DA014241 and MH077681 (MRP), a Smith Family Award for Excellence in Neuroscience (MJH) and a Sloan Research Fellowship (MJH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Molecular psychiatry. 2005;10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- Aramakis V, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–8495. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20:6106–6116. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Rev Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo S, Bennett C, Aziz D, Brown SP, Hestrin S. Prolonged disynaptic inhibition in the cortex mediated by slow, non-alpha7 nicotinic excitation of a specific subset of cortical interneurons. J Neurosci. 2012;32:3859–3864. doi: 10.1523/JNEUROSCI.0115-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-Jimenez MM, Bourgeois JP, Marubio LM, Le Sourd AM, Ottersen OP, Rinvik E, Fairen A, Changeux JP. Ultrastructural localization of the alpha4-subunit of the neuronal acetylcholine nicotinic receptor in the rat substantia nigra. J Neurosci. 1999;19:6475–6487. doi: 10.1523/JNEUROSCI.19-15-06475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CDC, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30:9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral J, Galarraga E, Bargas J. Muscarinic presynaptic inhibition of neostriatal glutamatergic afferents is mediated by Q-type Ca2+ channels. Brain Res Bull. 1999;49:285–289. doi: 10.1016/s0361-9230(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Bell KA, Shim H, Chen CK, McQuiston AR. Nicotinic excitatory postsynaptic potentials in hippocampal CA1 interneurons are predominantly mediated by nicotinic receptors that contain alpha4 and beta2 subunits. Neuropharmacology. 2011;61:1379–1388. doi: 10.1016/j.neuropharm.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov AB, O’Hara BF, Denisova JV. Acetylcholine becomes the major excitatory neurotransmitter in the hypothalamus in vitro in the absence of glutamate excitation. J Neurosci. 2001;21:2015–2027. doi: 10.1523/JNEUROSCI.21-06-02015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–121. doi: 10.1111/j.1749-6632.2010.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano V, Virgintino D, Flace P, Girolamo F, Errede M, Roncali L, Ambrosi G. Choline acetyltransferase-containing neurons in the human parietal neocortex. Eur J Histochem. 2003;47:253–256. doi: 10.4081/835. [DOI] [PubMed] [Google Scholar]
- Berg DK, Conroy WG. Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol. 2002;53:512–523. doi: 10.1002/neu.10116. [DOI] [PubMed] [Google Scholar]
- Brunzell D, Boschen K, Hendrick E, Beardsley P, McIntosh J. alpha-Conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–1143. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D, Goaillard JM. Beyond faithful conduction: short-term dynamics, neuromodulation, and long-term regulation of spike propagation in the axon. Prog Neurobiol. 2011;94:307–346. doi: 10.1016/j.pneurobio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Cancela JM. Specific Ca2+ signaling evoked by cholecystokinin and acetylcholine: the roles of NAADP, cADPR, and IP3. Ann Rev Physiol. 2001;63:99–117. doi: 10.1146/annurev.physiol.63.1.99. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Kalivas P, Bardo M. Contribution of dihydro-beta-erythroidine sensitive nicotinic acetylcholine receptors in the ventral tegmental area to cocaine-induced behavioral sensitization in rats. Behav Brain Res. 2006;168:120–126. doi: 10.1016/j.bbr.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Changeux JP. Allosteric receptors: from electric organ to cognition. Ann Rev Pharmacol Toxicol. 2010;50:1–38. doi: 10.1146/annurev.pharmtox.010909.105741. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Bertrand D, Corringer PJ, Dehaene S, Edelstein S, Léna C, Le Novère N, Marubio L, Picciotto M, Zoli M. Brain nicotinic receptors - structure and regulation, role in learning and reinforcement. Brain Res Rev. 1998;26:198–216. doi: 10.1016/s0165-0173(97)00040-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson L. Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology. 2002;160:198–205. doi: 10.1007/s00213-001-0965-2. [DOI] [PubMed] [Google Scholar]
- Couey J, Meredith R, Spijker S, Poorthuis R, Smit A, Brussaard A, Mansvelder H. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- De Pablo JM, Ortiz-Caro J, Sanchez-Santed F, Guillamon A. Effects of diazepam, pentobarbital, scopolamine and the timing of saline injection on learned immobility in rats. Physiol Behav. 1991;50:895–899. doi: 10.1016/0031-9384(91)90411-g. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nature Rev Neuroscience. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Won L, Britt JP, Lim SA, McGehee DS, Kang UJ. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci U S A. 2011;108:840–845. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas CL, Baghdoyan HA, Lydic R. M2 muscarinic autoreceptors modulate acetylcholine release in prefrontal cortex of C57BL/6J mouse. J Pharmacol Exp Ther. 2001;299:960–966. [PubMed] [Google Scholar]
- Douglas CL, Baghdoyan HA, Lydic R. Postsynaptic muscarinic M1 receptors activate prefrontal cortical EEG of C57BL/6J mouse. J Neurophysiol. 2002;88:3003–3009. doi: 10.1152/jn.00318.2002. [DOI] [PubMed] [Google Scholar]
- Drenan R, Grady S, Whiteaker P, McClure-Begley T, McKinney S, Miwa J, Bupp S, Heintz N, McIntosh J, Bencherif M, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha6* nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drever BD, Riedel G, Platt B. The cholinergic system and hippocampal plasticity. Behav Brain Res. 2011;221:505–514. doi: 10.1016/j.bbr.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Feldmeyer D. Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proc Natl Acad Sci U S A. 2009;106:11753–11758. doi: 10.1073/pnas.0810062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagen ZM, Mansvelder HD, Keath JR, McGehee DS. Short- and long-term modulation of synaptic inputs to brain reward areas by nicotine. Ann NY Acad Sci. 2003;1003:185–195. doi: 10.1196/annals.1300.011. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenia T, Gomez-Galan M, Lindskog M, Magara S. Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res. 2012 doi: 10.1016/j.brainres.2012.03.053. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R, Le Novere N, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Habenular signaling in nicotine reinforcement. Neuropsychopharmacology. 2012;37:306–307. doi: 10.1038/npp.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund RK, Jungschaffer DA, Collins AC, Wehner JM. Evidence for modulation of GABAergic neurotransmission by nicotine. Brain Res. 1988;453:215–220. doi: 10.1016/0006-8993(88)90160-6. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35:2479–2488. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futami T, Takakusaki K, Kitai ST. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res. 1995;21:331–342. doi: 10.1016/0168-0102(94)00869-h. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Ge S, Dani J. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25:6084–6091. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: A preliminary study. J Clin Psychopharm. 2008;28:340–344. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol. 2012:223–241. doi: 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Reynolds JN. Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum. Neuroscience. 2011;198:27–43. doi: 10.1016/j.neuroscience.2011.08.067. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Jain A, Giovannelli A, Bertollini C, Crestan V, Schwarz AJ, Tsetsenis T, Ragozzino D, Gross CT, Bifone A. A neural switch for active and passive fear. Neuron. 2010;67:656–666. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Meinerz NM, Cao J, Reynolds A, Picciotto MR, Changeux JP, McIntosh MJ, Marks MJ, Collins AC. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. J Neurochem. 2001;76:258–268. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Popp KA, Winders SE. Effects of nicotine on body weight in rats with access to “junk” foods. Psychopharmacology. 1988;94:536–539. doi: 10.1007/BF00212851. [DOI] [PubMed] [Google Scholar]
- Gu Q, Singer W. Effects of intracortical infusion of anticholinergic drugs on neuronal plasticity in kitten striate cortex. Eur J Neurosci. 1993;5:475–485. doi: 10.1111/j.1460-9568.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spijker S, Mansvelder HD. Nicotinic acetylcholine receptor beta2 subunits in the medial prefrontal cortex control attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signaling in neocortical neurons. J Neurophysiol. 2007;97:2215–2229. doi: 10.1152/jn.00493.2006. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci. 2005;25:10308–10320. doi: 10.1523/JNEUROSCI.2697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Pothos EN, Lenard L, Hoebel BG. Effects of feeding and insulin on extracellular acetylcholine in the amygdala of freely moving rats. Brain Res. 1998;785:41–48. doi: 10.1016/s0006-8993(97)01291-2. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Wainer BH. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;274:483–515. doi: 10.1002/cne.902740403. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM. Cholinergic modulation of cortical function. J Mol Neurosci. 2006;30:133–135. doi: 10.1385/JMN:30:1:133. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J Neurosci. 1994;14:3898–3914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, King SL, Gotti C, Marks MJ, Picciotto MR. Cortico-thalamic connectivity is vulnerable to nicotine exposure during early postnatal development through alpha4/beta2/alpha5 nicotinic acetylcholine receptors. Neuropsychopharmacology. 2010;35:2324–2338. doi: 10.1038/npp.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56:254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci. 2009;12:1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nature Rev Neuroscience. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Jr, Zoli M, Bourgeois JP, Changeux JP. Immunocytochemical localization of a neuronal nicotinic receptor: the β2 subunit. J Neurosci. 1993;13:1551–1568. doi: 10.1523/JNEUROSCI.13-04-01551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NK, Heath CJ, Neugebauer NM, Kimchi EY, Laubach M, Picciotto MR. Impaired auditory discrimination learning following perinatal nicotine exposure or beta2 nicotinic acetylcholine receptor subunit deletion. Behav Brain Res. 2012;231:170–180. doi: 10.1016/j.bbr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR. Cholinergic synapses in the central nervous system: studies of the immunocytochemical localization of choline acetyltransferase. J Electron Microscopy Technique. 1990;15:2–19. doi: 10.1002/jemt.1060150103. [DOI] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35:1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CY, Cruikshank SJ, Metherate R. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Research. 2000;880:51–64. doi: 10.1016/s0006-8993(00)02766-9. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Leslie FM, Metherate R. Nicotine exposure during a postnatal critical period alters NR2A and NR2B mRNA expression in rat auditory forebrain. Dev Brain Res. 2002;133:19–25. doi: 10.1016/s0165-3806(01)00314-5. [DOI] [PubMed] [Google Scholar]
- Huang H, Xu Y, van den Pol A. Nicotine excites hypothalamic arcuate anorexigenic proopiomelanocortin neurons and orexigenic neuropeptide Y neurons: similarities and differences. J Neurophysiol. 2011;106:1191–1202. doi: 10.1152/jn.00740.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364:723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Suzuki M, Shimazu T. Effects of acetylcholine injection into the hypothalamus on the insulin and glucagon release. Neuroendocrinology. 1982;34:310–314. doi: 10.1159/000123319. [DOI] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. Frequency-dependent signal transmission and modulation by neuromodulators. Frontiers Neurosci. 2008;2:138–144. doi: 10.3389/neuro.01.027.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Jiang L, Role LW. Facilitation of cortico-amygdala synapses by nicotine: activity-dependent modulation of glutamatergic transmission. J Neurophysiol. 2008;99:1988–1999. doi: 10.1152/jn.00933.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Role LW. Cholinergic modulation of purinergic and GABAergic co-transmission at in vitro hypothalamic synapses. J Neurophysiol. 2002;88:2501–2508. doi: 10.1152/jn.00352.2002. [DOI] [PubMed] [Google Scholar]
- Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol. 2002;53:618–632. doi: 10.1002/neu.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Beaudet A. Retrograde labeling of neurones in the brain stem following injections of [3H]choline into the forebrain of the rat. Exp Brain Res. 1987;65:437–448. doi: 10.1007/BF00236317. [DOI] [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Tr Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- Kaizer RR, da Silva AC, Morsch VM, Correa MC, Schetinger MR. Diet-induced changes in AChE activity after long-term exposure. Neurochem Res. 2004;29:2251–2255. doi: 10.1007/s11064-004-7033-3. [DOI] [PubMed] [Google Scholar]
- Kassam S, Herman P, Goodfellow N, Alves N, Lambe E. Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors. J Neurosci. 2008;28:8756–8764. doi: 10.1523/JNEUROSCI.2645-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nature Neurosci. 2007;10:1168–1175. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- Kawai H, Zago W, Berg DK. Nicotinic alpha 7 receptor clusters on hippocampal GABAergic neurons: regulation by synaptic activity and neurotrophins. J Neurosci. 2002;22:7903–7912. doi: 10.1523/JNEUROSCI.22-18-07903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura F, Baughman RW. Distinct muscarinic receptor subtypes suppress excitatory and inhibitory synaptic responses in cortical neurons. J Neurophysiol. 1997;77:709–716. doi: 10.1152/jn.1997.77.2.709. [DOI] [PubMed] [Google Scholar]
- Kimura F, Fukuda M, Tsumoto T. Acetylcholine suppresses the spread of excitation in the visual cortex revealed by optical recording: possible differential effect depending on the source of input. Eur J Neurosci. 1999;11:3597–3609. doi: 10.1046/j.1460-9568.1999.00779.x. [DOI] [PubMed] [Google Scholar]
- King SL, Marks MJ, Grady SR, Caldarone BJ, Koren AO, Mukhin AG, Collins AC, Picciotto MR. Conditional expression in corticothalamic efferents reveals a developmental role for nicotinic acetylcholine receptors in modulation of passive avoidance behavior. J Neurosci. 2003;23:3837–3843. doi: 10.1523/JNEUROSCI.23-09-03837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RC, Yakel JL. Functional somato-dendritic alpha7-containing nicotinic acetylcholine receptors in the rat basolateral amygdala complex. J Physiol. 2006;576:865–872. doi: 10.1113/jphysiol.2006.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Lanzafame AA, Christopoulos A, Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors & channels. 2003;9:241–260. [PubMed] [Google Scholar]
- Laplante F, Stevenson CW, Gratton A, Srivastava LK, Quirion R. Effects of neonatal ventral hippocampal lesion in rats on stress-induced acetylcholine release in the prefrontal cortex. J Neurochem. 2004;91:1473–1482. doi: 10.1111/j.1471-4159.2004.02831.x. [DOI] [PubMed] [Google Scholar]
- Léna C, Changeux JP. The role of beta 2-subunit-containing nicotinic acetylcholine receptors in the brain explored with a mutant mouse. Ann N Y Acad Sci. 1999;868:611–616. doi: 10.1111/j.1749-6632.1999.tb11333.x. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang J, Berg DK. Role of endogenous nicotinic signaling in guiding neuronal development. Biochem Pharmacol. 2007;74:1112–1119. doi: 10.1016/j.bcp.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK. Glutamatergic synapse formation is promoted by alpha7-containing nicotinic acetylcholine receptors. J Neurosci. 2012a;32:7651–7661. doi: 10.1523/JNEUROSCI.6246-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK. Induction of Dendritic Spines by beta2-Containing Nicotinic Receptors. J Neurosci. 2012b;32:8391–8400. doi: 10.1523/JNEUROSCI.6247-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manko M, Geracitano R, Capogna M. Functional connectivity of the main intercalated nucleus of the mouse amygdala. J Physiol. 2011;589:1911–1925. doi: 10.1113/jphysiol.2010.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, De Rover M, McGehee DS, Brussaard AB. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur J Pharmacol. 2003;480:117–123. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Mertz M, Role LW. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Sem Cell Devel Biol. 2009;20:432–440. doi: 10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark GP, Rada PV, Shors TJ. Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience. 1996;74:767–774. doi: 10.1016/0306-4522(96)00211-4. [DOI] [PubMed] [Google Scholar]
- Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153(Suppl 1):S438–445. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci U S A. 1985;82:6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. Nature. 1986;319:402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- McCoy P, Norton TT, McMahon LL. Layer 2/3 synapses in monocular and binocular regions of tree shrew visual cortex express mAChR-dependent long-term depression and long-term potentiation. J Neurophysiol. 2008;100:336–345. doi: 10.1152/jn.01134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy PA, McMahon LL. Muscarinic receptor dependent long-term depression in rat visual cortex is PKC independent but requires ERK1/2 activation and protein synthesis. J Neurophysiol. 2007;98:1862–1870. doi: 10.1152/jn.00510.2007. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. alpha4beta2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1120–1133. doi: 10.1016/j.bcp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B, Gomuc B, Suarez E, Ishii Y, Durr K, Gillberg L. Hypothalamic proopiomelanocortin (POMC) neurons have a cholinergic phenotype. Eur J Neurosci. 2006;24:2731–2740. doi: 10.1111/j.1460-9568.2006.05157.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Structure and Function of Cholinergic Pathways in the Cerebral Cortex, Limbic System, Basal Ganglia, and Thalamus of the Human Brain. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. New York, New York: Raven Press; 1995. [Google Scholar]
- Metherate R, Hsieh CY. Regulation of glutamate synapses by nicotinic acetylcholine receptors in auditory cortex. Neurobiol Learning & Mem. 2003;80:285–290. doi: 10.1016/s1074-7427(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Miller AD, Blaha CD. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur J Neurosci. 2005;21:1837–1846. doi: 10.1111/j.1460-9568.2005.04017.x. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, Diano S, De Biasi M, Horvath TL, Gao XB, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RD, Waller MB. Differential release of acetylcholine from the hypothalamus and mesencephalon of the monkey during regulation. J Physiol. 1973;230:273–293. doi: 10.1113/jphysiol.1973.sp010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Maglio L, Pastor J, Garcia de Sola R, Araque A. Astrocyte Calcium Signal and Gliotransmission in Human Brain Tissue. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs122. [DOI] [PubMed] [Google Scholar]
- Nijholt I, Farchi N, Kye M, Sklan EH, Shoham S, Verbeure B, Owen D, Hochner B, Spiess J, Soreq H, et al. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol Psychiatry. 2004;9:174–183. doi: 10.1038/sj.mp.4001446. [DOI] [PubMed] [Google Scholar]
- Nissenbaum JW, Sclafani A. A comparison of the effects of atropine on real-feeding and sham-feeding of sucrose in rats. Pharmacol Biochem Behav. 1988;29:231–238. doi: 10.1016/0091-3057(88)90150-5. [DOI] [PubMed] [Google Scholar]
- Norton AB, Jo YS, Clark EW, Taylor CA, Mizumori SJ. Independent neural coding of reward and movement by pedunculopontine tegmental nucleus neurons in freely navigating rats. Eur J Neurosci. 2011;33:1885–1896. doi: 10.1111/j.1460-9568.2011.07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg IA, Ding JB. Cholinergic modulation of synaptic integration and dendritic excitability in the striatum. Curr Opin Neurobiol. 2011;21:425–432. doi: 10.1016/j.conb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldford E, Castro-Alamancos MA. Input-specific effects of acetylcholine on sensory and intracortical evoked responses in the “barrel cortex” in vivo. Neuroscience. 2003;117:769–778. doi: 10.1016/s0306-4522(02)00663-2. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev. 1993;17:51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. J Neurosci. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30:3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007a;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M, Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007b;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004;20:1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Stimulation of lateral hypothalamic glutamate and acetylcholine efflux by nicotine: implications for mechanisms of nicotine-induced activation of orexin neurons. J Neurochem. 2010;113:1023–1035. doi: 10.1111/j.1471-4159.2010.06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW. Acetylcholine release from the central nervous system: a 50-year retrospective. Crit Rev Neurobiol. 2005;17:161–217. doi: 10.1615/critrevneurobiol.v17.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Nicotine as a modulator of behavior: beyond the inverted U. Tr Pharmacol Sci. 2003;24:493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, King SL, Zachariou V. Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology. 2000;22:451–465. doi: 10.1016/S0893-133X(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Léna C, Bessis A, Lallemand Y, Le Novère N, Vincent P, Merlo Pich E, Brulet P, Changeux JP. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Merlo Pich E, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta-2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pinard CR, Mascagni F, McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience. 2012;205:112–124. doi: 10.1016/j.neuroscience.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis RB, Bloem B, Schak B, Wester J, de Kock CP, Mansvelder HD. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr390. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Sah P. Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J Neurosci. 2008;28:3209–3220. doi: 10.1523/JNEUROSCI.4310-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild type but not β2 or α7 nicotinic acetylcholine receptor knockout mice. Psychopharmacology. 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe KA, Fisher JL, Gray R, Dani JA. Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann N Y Acad Sci. 1999;868:591–610. doi: 10.1111/j.1749-6632.1999.tb11332.x. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Leardi R, Marchi M. Heterogeneity of presynaptic muscarinic receptors regulating neurotransmitter release in the rat brain. J Pharmacol Exp Ther. 1984;228:209–214. [PubMed] [Google Scholar]
- Rathouz MM, Vijayaraghavan S, Berg DK. Elevation of intracellular calcium levels in neurons by nicotinic acetylcholine receptors. Mol Neurobiol. 1996;12:117–131. doi: 10.1007/BF02740649. [DOI] [PubMed] [Google Scholar]
- Reid MS, Nishino S, Tafti M, Siegel JM, Dement WC, Mignot E. Neuropharmacological characterization of basal forebrain cholinergic stimulated cataplexy in narcoleptic canines. Exp Neurol. 1998;151:89–104. doi: 10.1006/exnr.1998.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M. Habenula “cholinergic” neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, Kalin NH, Murphy DL. Mood and behavioral effects of physostigmine on humans are accompanied by elevations in plasma beta-endorphin and cortisol. Science. 1980;209:1545–1546. doi: 10.1126/science.7433977. [DOI] [PubMed] [Google Scholar]
- Risch SC, Cohen RM, Janowsky DS, Kalin NH, Sitaram N, Gillin JC, Murphy DL. Physostigmine induction of depressive symptomatology in normal human subjects. Psychiatry Res. 1981;4:89–94. doi: 10.1016/0165-1781(81)90012-3. [DOI] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sanghera MK, Roper-Hall A. The latency of activation of neurones in the lateral hypothalamus and substantia innominata during feeding in the monkey. Brain Res. 1979;164:121–135. doi: 10.1016/0006-8993(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Rosenstock L, Keifer M, Daniell WE, McConnell R, Claypoole K. Chronic central nervous system effects of acute organophosphate pesticide intoxication. The Pesticide Health Effects Study Group. Lancet. 1991;338:223–227. doi: 10.1016/0140-6736(91)90356-t. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, Chen JI, Cosgrove KP, Kerestes R, Ghose S, et al. Persistent beta2*-Nicotinic Acetylcholinergic Receptor Dysfunction in Major Depressive Disorder. Am J Psychiatry. 2012;169:851–859. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nature Rev Neuroscience. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindowski K, Belarbi K, Buee L. Neurotrophic factors in Alzheimer’s disease: role of axonal transport. Genes, brain, and behavior. 2008;7(Suppl 1):43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Sanberg PR. Comorbid bipolar disorder in Tourette’s syndrome responds to the nicotinic receptor antagonist mecamylamine (Inversine) Biological Psychiatry. 2000;48:1028–1031. doi: 10.1016/s0006-3223(00)00945-8. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Sheehan KH, Sheehan DV, Sanberg PR. Neuronal nicotinic receptor inhibition for treating mood disorders: preliminary controlled evidence with mecamylamine. Depression & Anxiety. 2002;16:89–92. doi: 10.1002/da.10035. [DOI] [PubMed] [Google Scholar]
- Siggins GR. Neurotransmitters and neuromodulators and their mediation by cyclic nucleotides. Adv Exp Med Biol. 1979;116:41–64. doi: 10.1007/978-1-4684-3503-0_3. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Morrell F, Mesulam MM. Cholinergic synapses in human cerebral cortex: an ultrastructural study in serial sections. Exp Neurol. 1997;144:361–368. doi: 10.1006/exnr.1997.6413. [DOI] [PubMed] [Google Scholar]
- Smith J, Co C, Yin X, Sizemore G, Liguori A, Johnson W, Martin T. Involvement of cholinergic neuronal systems in intravenous cocaine self-administration. Neurosci Biobehav Rev. 2004;27:841–850. doi: 10.1016/j.neubiorev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Steriade M. Acetylcholine systems and rhythmic activities during the waking--sleep cycle. Prog Brain Res. 2004;145:179–196. doi: 10.1016/S0079-6123(03)45013-9. [DOI] [PubMed] [Google Scholar]
- Stevens TR, Krueger SR, Fitzsimonds RM, Picciotto MR. Neuroprotection by nicotine in mouse primary cortical cultures involves activation of calcineurin and L-type calcium channel inactivation. J Neurosci. 2003;23:10093–10099. doi: 10.1523/JNEUROSCI.23-31-10093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago H, McGeer PL, Bruce G, Hersh LB. Distribution of choline acetyltransferase-containing neurons of the hypothalamus. Brain Res. 1987;415:49–62. doi: 10.1016/0006-8993(87)90268-x. [DOI] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14:398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers Human Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Cortical acetylcholine and processing capacity: effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Cogn Brain Res. 1997;6:147–158. doi: 10.1016/s0926-6410(97)00027-x. [DOI] [PubMed] [Google Scholar]
- Turrini P, Casu MA, Wong TP, De Koninck Y, Ribeiro-da-Silva A, Cuello AC. Cholinergic nerve terminals establish classical synapses in the rat cerebral cortex: synaptic pattern and age-related atrophy. Neuroscience. 2001;105:277–285. doi: 10.1016/s0306-4522(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Vidal C, Changeux JP. Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neurosci. 1993;56:23–32. doi: 10.1016/0306-4522(93)90558-w. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer BH, Bolam JP, Freund TF, Henderson Z, Totterdell S, Smith AD. Cholinergic synapses in the rat brain: a correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res. 1984a;308:69–76. doi: 10.1016/0006-8993(84)90918-1. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Levey AI, Mufson EJ, Mesulam MM. Cholinergic systems in mammalian brain identified with antibodies against choline acetyltransferase. Neurochem Int. 1984b;6:163–182. doi: 10.1016/0197-0186(84)90089-5. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, Ibanez-Tallon I, Heintz N, Greengard P. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Tr Pharmacol Sci. 2003a;24:414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Tr Pharmacol Sci. 2003b;24:414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- Wess J, Duttaroy A, Zhang W, Gomeza J, Cui Y, Miyakawa T, Bymaster FP, McKinzie L, Felder CC, Lamping KG, et al. M1-M5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system. Receptors & Channels. 2003;9:279–290. [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Tr Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Wooltorton JRA, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J, Famous KR, Hopkins TJ, McMullen MC, Pierce RC, Schmidt HD. Muscarinic acetylcholine receptors in the nucleus accumbens core and shell contribute to cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2011;650:596–604. doi: 10.1016/j.ejphar.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans J, Baptista M. Both nicotinic and muscarinic receptors in ventral tegmental area contribute to brain-stimulation reward. Pharmacol Biochem Behav. 1997;57:915–921. doi: 10.1016/s0091-3057(96)00467-4. [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Wise RA. Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci. 2008;28:9021–9029. doi: 10.1523/JNEUROSCI.0694-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zaborszky L. The modular organization of brain systems. Basal forebrain: the last frontier. Prog Brain Res. 2002;136:359–372. doi: 10.1016/s0079-6123(02)36030-8. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann NY Acad Sci. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Zago WM, Massey KA, Berg DK. Nicotinic activity stabilizes convergence of nicotinic and GABAergic synapses on filopodia of hippocampal interneurons. Mol Cell Neurosci. 2006;31:549–559. doi: 10.1016/j.mcn.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Tr Pharmacol Sci. 1999;20:142–150. doi: 10.1016/s0165-6147(99)01343-7. [DOI] [PubMed] [Google Scholar]


