Abstract
Optogenetics – the use of optically-activated proteins to control neuronal function – is a recent development in neuroscience methodology. Optogenetic techniques provide a means of activating or inhibiting distinct populations of neurons with an unprecedented degree of spatial, temporal, and neurochemical precision. Channelrhodopsin-2 (ChR2), an algal protein from Chlamydomonas reinhardtii, is a light-activated cation channel capable of inducing depolarization and action potentials in neurons. Three protocols are presented in this unit for the use of ChR2, with emphasis on technical aspects of fiber optics. The first describes the use of ChR2 in electrophysiological recordings from brain slices. The second and third involve the use of ChR2 in vivo, with light delivered through chronic fiber implants or guide cannula.
Keywords: Optogenetics, channelrhodopsin, ChR2, optical, light, laser, LED
UNIT INTRODUCTION
Optogenetics – the use of optically-activated proteins to control neuronal function – is a recent development in neuroscience methodology. Optogenetic techniques provide a means of activating or inhibiting distinct populations of neurons with an unprecedented degree of spatial, temporal, and neurochemical precision. It is the only available method capable of activating specific neuronal populations embedded in dense, heterogeneous structures on a millisecond timescale. Optogenetic approaches have been successfully used in vitro to study basic synaptic properties of specific neural circuits as well as in vivo to study the role of such circuits in physiology and behavior. The most common light-sensitive protein in use today is channelrhodopsin-2 (ChR2), an algal protein from Chlamydomonas reinhardtii. It is a light-activated cation channel capable of transducing millisecond long flashes of blue light into defined spike trains as fast as 50 Hz. Although this article is written for ChR2, the basic principles of fiber optics outlined in this unit are also applicable to the rapidly-evolving array of inhibitory opsin genes. Contained within this unit are protocols for preparation and use of fiber optic cables and connectors for slice physiology and for use in awake, behaving mice via chronic implants or guide cannula.
STRATEGIC PLANNING
The principal advantage of optogenetics is the ability to activate specific neurons or processes that are embedded among heterogeneous cells. To achieve this specificity it is necessary to limit the expression of ChR2 to specific groups of neurons. Careful consideration needs to be given to the method of gene delivery, ChR2 variant, light source, type of optical fiber, ferrule size, and method of tissue implantation (for in vivo experiments).
Opsin Gene Delivery and Expression
A prerequisite for any optogenetic experiment is the delivery of DNA encoding the optogenetic gene(s) into the cells of interest. The three most commonly used methods to do so are virus, in vivo electroporation, and generation of transgenic animals. Viral approaches have become the most popular due to their rapid generation and ease of use. A simple way of restricting viral expression to particular subtypes of neurons is by inserting a selective promoter into the viral DNA sequence (Adamantidis et al., 2007). Because viruses have limited capacity for exogenous DNA, these promoter regions must be very small, typically less than 3 kilobases. Consequently, it must be evaluated on a gene-by-gene basis whether it is possible to isolate a fragment of DNA this size that is both sufficient to induce strong expression in relevant neurons and selective enough to prevent off-target expression. This approach is best suited to viral systems that tolerate larger inserts, such as lentivirus (Unit 4.21) and herpes simplex virus (Units 4.12 – 4.14).
An alternative strategy for restricting viral-mediated expression of optogenetic genes is to take advantage of cre/lox genetic technology (Unit 4.31). In this approach, viruses are designed such that they only express functional opsin genes in the presence of cre recombinase. The wide variety of cre-expressing mouse lines thus allow for selective expression in neurons that only express particular genes. These viruses are typically designed with either a floxed stop cassette preceding the gene of interest, or an inverted open reading frame that is reversed into sense orientation when recombination occurs at two sets of tandem non-complimentary lox sites flanking the gene. This approach allows for use of small, highly active promoters such as cytomegalovirus (CMV). If the total size of the insert DNA – including promoter, gene, polyA, and modification sequences – is less than approximately 4.5 kilobases, adeno-associated virus may be used (Unit 4.17). This virus has many advantages, particularly safety, ease of production of high-titer preparations, and low level of tissue inflammation.
In vivo electroporation can also be used to deliver ChR2 DNA to CNS neurons (Saito and Nakatsuji, 2001). While this method may not have the spatial precision of viral injections in adult animals, expression can be restricted via cre/lox recombination. A unique advantage of this approach is that cell types can be targeted based on their developmental timing. One last approach for introducing ChR2 DNA is mouse genetics, via generation of a line carrying a transgene or targeted insert. The slow generation time has hindered progress of such mice.
Channelrhodopsin Variants
Mutagenesis, chimeragenesis and bioinformatic approaches are being used to increase the variety and effectiveness of light-activated channels. Several factors influence the effectiveness of opsin proteins, including their conductance, selectivity, kinetics, desensitization, light sensitivity, spectral response, membrane trafficking and expression. This topic has been thoroughly reviewed elsewhere (Lin, 2011), but the field is rapidly progressing and channelrhodopsin variants are being made at a rapid pace. What is currently true, and will most likely remain true, is that the specific application will dictate which channelrhodopsin variant is the most appropriate. Presently, the best channelrhodopsin variant for high-frequency, repetitive stimulation is a chimeric protein of ChR1 and ChR2 called ChIEF (chimera EF with I170V mutation). There are also Channelrhodopsin variants that activate various G-protein coupled pathways (Airan et al., 2009; Gutierrez et al., 2011). Unfortunately, there are currently no two channelrhodopsin variants that allow for simultaneously use without cross-excitation.
Choice of Light Source
There are three critical parameters in selecting a light source: source type, wavelength, and intensity. The two options for source type include diode-pumped solid state (DPSS) lasers and light-emitting diodes (LEDs). DPSS lasers are easy to use and powerful enough to ensure maximal activation of opsin proteins. They are characterized by the wavelength and intensity of light they emit as well as whether they can driven by both digital and analog signals. Since a laser emits light at a specific wavelength, it is important to use the appropriate laser to activate a specific opsin protein. ChR2 is maximally activated by 473 nm blue light. The inhibitory protein halorhodopsin is maximally activated by 593 nm yellow light; however it can be activated by a 532 nm green laser, which is significantly less expensive than a yellow laser. Lasers can be driven by digital computer signals (TTL pulses) or analog signals. TTL driven lasers are easy to use and they often come with power knobs that allow the laser intensity to be adjusted. Analog controlled lasers require more complex electronics to activate them, but they allow for the delivery of more complex waveforms, such as sinusoids. When using a laser in an analog controlled mode, be careful to limit the supplied voltage to what the laser can accept (often 5 V). It is possible to damage the laser driver by activating it with too large of a voltage step.
LEDs represent an alternative to lasers for activating optogenetic proteins in brain slice preparations. LEDs are smaller, less expensive, and available in a greater variety of colors, with the drawback of providing less intense light. LEDs are typically attached through a dual lamphouse adapter on the back of the electrophysiology microscope. The LED light shines through the optics of the microscope and illuminates the tissue immediately underneath the objective. The diameter of the light beam can be adjusted using the field stop diaphragm on the microscope. For in vivo applications, the LED light must be collimated into an optical fiber and it is presently difficult to accomplish this while maintaining a sufficient intensity of light. There is promising work dedicated to overcoming this technical hurdle, but it is presently not practical to use LEDs for most in vivo applications.
It is important that the light source be appropriately powered, giving enough light to provide activation of opsin proteins while minimizing tissue heat. Typical opsin proteins like ChR2 are maximally activated by less than 1 mW of wavelength-specific light. To achieve this level of light at the opsin protein it is safe to use a laser capable of producing much more intense light. Light losses occur at any junction (collimators, connectors, splitters) as well as when the light passes through tissue. 5–30 mW lasers are sufficient for in vitro preparations. 50–200 mW lasers are typically used for in vivo work to enable maximal opsin protein activation. The light that comes out of DPSS lasers has to be collimated into optical fibers. The collimating devices are called fiber couplers and they can be purchased as a package with the lasers.
Optical Fiber
Optical fibers are classified as single- or multi-mode. Single mode fibers are less than ten microns in diameter and too delicate for typical optogenetic applications. Multimode fibers have a core diameter larger than 10 um and can be relatively easy to handle. The size of the core alters the width of the light beam but does not impact how much total light can travel through the fiber. The geometry of the tissue that one hopes to illuminate should influence the choice of fiber core size: smaller fibers can cause less tissue damage if implanted in vivo, but break more easily – particularly when smaller than 100 um.
The core of an optical fiber is surrounded by a transparent cladding material. This cladding keeps light in the core by total internal reflection and cannot be stripped off the fiber. A buffer material surrounds the cladding of an optical fiber and this can be stripped off with a fiber stripper for purposes of fiber termination, splicing or brain implantation.
Laser beams diverge as they propagate in space and the spread is roughly linear with distance. The extent of this spread is a property of the optical fiber referred to as numerical aperture, a dimensionless number that describes the range of angles over which the fiber can accept or emit light. The larger the numerical aperture of a fiber, the more beam divergence there is once the light leaves the fiber. Again, the geometry of the tissue that needs to be illuminated should be a factor when considering which numerical aperture to use. There is an online application to calculate light transmission through brain tissue on the Diesseroth lab website (http://www.stanford.edu/group/dlab/cgi-bin/graph/chart.php). It calculates light spread and depth of penetration using several factors, including the wavelength of light, fiber aperture, light intensity and fiber core radius.
Tissue Implantation
For in vivo experiments, the tip of the optical fiber must be placed in brain tissue. There are two methods of fiber placement, which involve chronic implantation of a guide cannula or a ferrule-attached optical fiber. Guide cannula implantation (Appendix 4A) allows for a simple fiber optic cable design, with a single cable attached directly to the light source. The major disadvantage of this approach is that optical fibers may be damaged during the threading in and out of the guide cannula. An alternative approach is to chronically implant a fiber in the brain, which is connected to a ferrule affixed to the skull of the animal. This ferrule is the connection point where the fiber coming from the light source will attach to the fiber implanted in the head of the animal. Ferrules are typically made of a stainless alloy or zirconia and come in two sizes: 2.5 or 1.25 mm diameter. The smaller 1.25 mm wide ferrules are 6.4 mm long and can accommodate optical fibers less than 330 um wide (core size equal to or less than 300 um). Due to their light weight and small size, these ferrules are particularly good for in vivo mouse work. The larger 2.5 mm wide fibers are 10.5 mm long and can accommodate fibers less than 500 um wide. These are the ferrules used in standard FC/PC fiber optic connections and are practical for in vivo rat experiments.
BASIC PROTOCOL 1: CHANNELRHODOPSIN USE IN BRAIN SLICE PREPARATIONS
There are two popular methods to activate ChR2-containing neurons in brain slices. The first uses a DPSS laser to focus light through an optical fiber positioned in a micromanipulator. This technique is the most common and is detailed here. The second method utilizes LEDs and is briefly discussed above in the Choice of Light Source section.
Prior to an experiment ChR2 must be expressed in excitable cells. The first part of this protocol, fabricating the patch cable, should be completed before the day of the experiment. Once the laser and optical equipment is in place, using an optical fiber to activate excitable tissue is comparable to using electrical stimulating electrodes.
Materials
Optical fiber (e.g., multimode, 0.22 numerical aperture, visible to near infrared, low OH, 105 um core fiber, Thorlabs, Newton, NJ)
Fiber stripping tool (e.g., clad/coat: 125 um / 250 um fiber stripping tool, Thorlabs)
FC fiber optic connector (e.g., FC simplex connector, 900 um fiber-multimode, 128 um, Fiber Instrument Sales, Oriskany, NY)
Heat-cured epoxy (e.g., blue dye epoxy, Fiber Instrument Sales)
3 cc luer-lock syringe with blunt 22 gauge needle
Diamond wedge scribe (Thorlabs)
FC/PC connector polishing disc (Thorlabs)
Fiber polishing/lapping film diamond sheets (6” × 6” sheets with grit sizes of 1, 3 or 6 um, Thorlabs)
-
200X Fiber scope (Thorlabs)
Fiber optic power meter (e.g., 400 nm – 1100 nm, 1 nW – 40 mW, Thorlabs)
Blue light laser with fiber coupler (e.g., 10 mW, 473 nm diode pumped solid state continuous wave laser system with FC/PC multimode fiber coupler, OEM Laser Systems, Salt Lake City, UT)
Fabricate fiber optic patch cable
Cut off a strand of optical fiber to desired length (typically about one meter long). The ends will either be polished or cut again so it is fine to use any scissor for this cut.
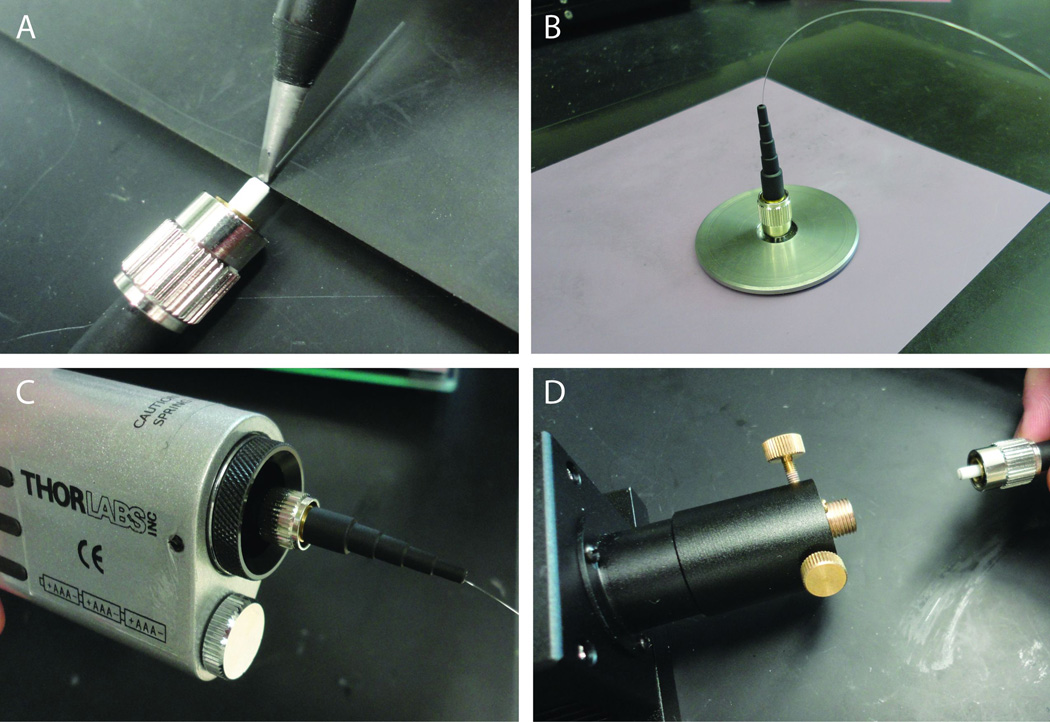
- Use the fiber stripping tool to strip about 3 cm of the buffer off both ends of the optical fiber (Fig. 1A). There is no danger of stripping off the cladding that surrounds the core of the fiber.One end of the fiber will be affixed into a fiber optic connector, which will attach to the fiber coupler on the laser. The other end of the optical fiber will remain bare. This is the end that will be held by a micromanipulator and be positioned above the brain slice.
Hold the FC fiber optic connector, ferrule side down, with a hemostat or place it in a vice (Fig. 1B). Place the boot which accompanies the connector aside for now.
- Practice threading the fiber through the back (top) of the connector.The diameter of the stripped optical fiber should be equal to or slightly smaller than the hole in the connector that will accommodate the fiber. The optical fiber used here has core diameter of 105 um. The cladding increases the diameter of the fiber to 125 um, and this will fit snugly into the 128 um internal diameter connector. It is helpful to practice threading the fiber through the connector because if the fiber does not slide smoothly through the ferrule in the connector it may be necessary to discard the connector and try another one. The fiber should be threaded through the back of the connector, the side with a large opening. The stripped end of the fiber protrudes out of the side of the connector that attaches to the laser coupler.
Mix together the two parts of the epoxy and draw it up into a 3 cc luer-lock syringe.
Attach a 22 gauge blunt needle to the syringe and inject a little epoxy into the center hole on the back of the connector, just enough so the epoxy fills the opening (Fig. 1B).
Thread the 3 cm of stripped optical fiber through the epoxy-filled connector until it is stopped by the unstripped buffer (Fig. 1C).
Give the epoxy time to harden. At room temperature it will cure over 24 hours. The use of a heat gun will speed up the curing time considerably (Fig. 1D).
Thread the boot of the connector over the other end of the fiber until it is flush against the connector (Fig. 1E).
Use the diamond wedge scribe to score the stripped end of the optical fiber just as it leaves the connector (Fig. 2A). Flick this 3 cm long loose end of the stripped fiber so it breaks where it was scored.
- Polish the fiber and connector at this new end so it can form a robust connection to the fiber coupler (Fig. 2B).Thorlabs has published a manual available freely online entitled “Guide to Connectorization and Polishing Optical Fibers” (http://thorlabs.com/Thorcat/1100/1166-D02.pdf). Sections of this manual can be very helpful when learning about fiber polishing. In brief, place the FC fiber optic connector into a FC/PC connector polishing disc. Place this disc on a 6 um fiber polishing/lapping film diamond sheet. Gently press down on the fiber connector and polishing disc and make approximately 20 figure eight patterns on the lapping film sheet. Repeat this process, first with the 3 um and then with the 1 um lapping sheets. Thorlabs sells a glass polishing plate and rubber polishing pad which help ensure a smooth surface for the lapping sheet. This can help prevent the cracks from forming in the core of the optical fiber during the polishing process.The quality of the polishing job can be inspected using a fiber scope (Fig. 2C). The core of polished fiber should be smooth and free of epoxy. If excess epoxy is on the fiber after using all the lapping sheets, repeat the polishing steps on the 3 and 1 um lapping sheets. If there is a crack in the core of the optical fiber, it may be necessary to restart the polishing process from the 6 um lapping sheet.
At the other end of the optical fiber, the loose end, use the diamond edge scribe to lightly score the fiber in the middle of the 5 cm long stripped end. Flick or pull on the tip of the fiber to create a clean break where the fiber was scored.
- Attach the completed fiber to the laser (Fig. 2D) and test its wattage output (Fig. 3A,B).Attach the fiber cord to the laser by inserting the end of the fiber cord with the FC connector into the fiber coupler on the laser. Turn the laser on full power and direct the light coming out of the loose end of the fiber cord towards the power meter. Place the end of the fiber cord within a few millimeters of the light sensor on the power meter. It may be necessary set the power meter to the 473 nm light setting for an accurate reading. For in vitro work, a light intensity of 5 mW or greater is sufficient to maximally activate ChR2 and other opsin proteins.If at least 5 mW of light output is not registering on the power meter, first check the light output of the laser itself. Another section to inspect is the fiber coupler on the laser. Slight turns on the three set screws on the laser coupler will change how it directs the light into the fiber cord. It may be easiest to adjust these set screws using a store bought (pre-tested) fiber optic cord with an identical fiber core size. If the laser is producing enough light and adjusting the set screws on the fiber coupler does not maximize the light output to at least 80% efficiency, there may be a problem with the patch cord. Inspect the cord to see if the laser light suddenly stops at a discrete spot in the cord. This would suggest a crack in the cord. The other areas to check are the two ends of the cord. The side with the FC connector can be examined with a fiber scope. Additional polishing may help if epoxy or cracks in the core are visible. The free end of the fiber cord can be re-cut with the help of the diamond wedge scribe to score the fiber.
Connect the laser driver to the digitizer or pulse generator that will be used to activate the laser driver.
Attach the loose end of the fiber cord to a micromanipulator on an electrophysiology rig (Fig. 3C).
Figure 1.

Steps to epoxy optical fiber into an FC/PC connector. (A) The fiber stripping tool clamps onto the optical fiber and strips off the buffer material that surrounds the core and cladding of the fiber. (B) A vice holds the FC/PC connector so epoxy can easily be injected into the ferrule inside the connector. (C) The fiber is threaded through the epoxy-filled connector. (D) The use of a heat gun will considerably speed up the curing time of the epoxy. (E) The boot of the connector is threaded onto the optical fiber to protect the connection.
Figure 2.
Steps to polish and examine the patch cord. (A) A diamond wedge scribe is used to score the optical fiber so that a light flick to the end of the optical fiber will cause it to break off immediately as it leaves the connector. (B) The connector is inserted into a polishing disc. The connector is gently pressed against the lapping film sheet during the polishing process. (C) The quality of the polishing job is assessed by attaching the FC/PC connector to a magnification scope. (D) The fiber patch cord is connected to the laser coupler that is attached to the laser.
Figure 3.
Testing and using the fiber patch cord. (A) The light beam created from an unpolished fiber. Excess epoxy is covering the ferrule and preventing light to enter the patch cord from the laser coupler. (B) The light beam created from a well polished patch cord has a uniform light intensity and crisp circular shape. (C) The bare fiber end of the patch cord is placed in a micromanipulator that is positioned on an electrophysiology rig.
Pulse laser to activate ChR2-containing tissue
-
16Prepare the tissue containing ChR2 proteins and place it on the electrophysiology rig. Use the micromanipulator to position the optical fiber within a few millimeters of the tissue.It is not necessary to touch the tissue with the optical fiber. Place the optical fiber in the bath of recording solution, just above the tissue and aim it so the light beam is directed at the targeted tissue. Maximal ChR2 activation can be obtained with the tip of the optical fiber several millimeters above the tissue.
-
17Pulse the laser.ChR2 protein embedded in 300 um thick tissue can be maximally activated using 1 to 5 mW of 0.1 to 5 ms long pulses of 473 nm light. Other opsin proteins have different sensitivities to these parameters. In many preparations, the ability of light-induced ChR2 activation to induce transmitter release from axon terminals is blocked with tetrodotoxin, meaning it is dependent on voltage-gated sodium channels. Also, the probability of neurotransmitter release tends to be higher when evoked with light and ChR2 than it is when evoked with electrical stimulation. One effect of this is that the second pulse of a paired-pulse of light with an inter-pulse interval less than 40 ms tends to produce significantly blunted responses.
BASIC PROTOCOL 2: CHANNELRHODOPSIN USE IN AWAKE BEHAVING ANIMALS
This protocol details how to make and use small implantable optical fibers. The small size of the components used here help lower the impact of the optical connection on the behaving animal. Larger components can be used for animals such as rats, since their strength and skull size can accommodate larger connectors. A different approach to in vivo optogenetics experiments is to thread a fresh optical fiber down an implanted guide cannula for each experiment. This method allows for the possibility to infuse drugs into the brain structure prior to optical activation and is described in Support Protocol 1. The commentary section at the end of this protocol addresses many of the considerations raised with in vivo experiments, including the use of rotary joints (also known as commutators) and bilateral stimulations.
Materials
Optical fiber (e.g., multimode, 0.22 numerical aperture, visible to near infrared, low OH, 105 um core fiber, Thorlabs)
Fiber stripping tool (e.g., clad/coat: 125 um / 250 um fiber stripping tool, Thorlabs)
Ferrule (LC 1.25 mm OD multimode ceramic zirconia ferrule with 126 um OD bore, Precision Fiber Products)
Hemostat
Heat-cured epoxy (e.g., blue dye epoxy, Fiber Instrument Sales)
3 cc luer-lock syringe with blunt 25 gauge needle
Diamond wedge scribe (Thorlabs)
1.25 mm polishing disc (e.g., Universal 1.25 mm Polish Disc, Fiber Instrument Sales)
Heat gun
Fiber polishing/lapping film diamond sheets (6” × 6” sheets with grit sizes of 1, 3 or 6 um, Thorlabs)
200X Fiber scope (Thorlabs)
FC fiber optic connector (e.g., FC simplex connector, 900 um fiber-multimode, 128 um, Fiber Instrument Sales)
FC/PC connector polishing disc (Thorlabs)
Furcation tubing (e.g., furcation tubing, 900 um OD, for 250 um fiber, Precision Fiber Products, Milpitas, CA)
Heat shrink tubing (both 3/32 inch and 1/8 inch diameter)
Blue light laser with fiber coupler (e.g., 150 mW, 473 nm diode pumped solid state continuous wave laser system with FC/PC multimode fiber coupler, OEM Laser Systems)
Fiber optic power meter (e.g., 400 nm – 1100 nm, 1 nW – 40 mW, Thorlabs)
1.25 mm internal diameter ceramic split sleeve (Precision Fiber Products)
Fabricate implantable optical fiber
- Take the entire roll of optical fiber and use the fiber stripping tool to strip 5 cm of buffer off the loose end of the optical fiber (Fig. 4A).It is easier to strip the buffer off of the fiber while it is still attached to the roll than it is to try and work with a small piece of fiber.
Use a generic scissor to cut off a 10 cm long piece (5 cm of which is now stripped of buffer) of optical fiber (Fig. 4B).
- Use a hemostat or small vice to hold the ferrule convex side pointing down. The side with the larger opening should face the ceiling (Fig. 4C).The center piece of FC fiber optic connectors, the part where the optical fiber is threaded through, is called a ferrule. Ferrules are sold as part of FC fiber optic connectors or on their own. The ferrule used here is much smaller than the ones used in FC/PC connectors (1.25 versus 2.5 mm wide). The smaller ferrules are preferable because they are less obtrusive for mice.
- Practice threading the stripped side of the fiber through the top of the ferrule, the side with the larger opening.If the fiber does not slide smoothly through the ferrule, it may be necessary to discard the ferrule and try another one.
Mix together the two parts of the epoxy and draw it up into a 3 cc luer-lock syringe.
Attach a 22 gauge blunt needle to the syringe and inject one drop of epoxy onto the top of the ferrule (Fig. 4C).
Thread about 2 cm of stripped optical fiber through the drop of epoxy and through the ferrule (Fig. 4D). There will be 2 cm of unstripped fiber on either end of the ferrule (Fig. 4E).
Give the epoxy time to harden. At room temperature it will cure over 24 hours. The use of a heat gun will speed up the curing time considerably.
Use the diamond wedge scribe to score the stripped end of the optical fiber just as it leaves the ferrule (Fig. 5A). This is the side of the ferrule that does not have epoxy on it. Flick this end of the stripped fiber so it breaks where it was scored.
- Polish the fiber and ferrule at this new end so it can form a robust connection to a matching ferrule on a patch cord (Fig. 5B,C).Hold the back end of the ferrule with a hemostat. Using the hemostat, insert the ferrule into the 1.25 mm polishing disc (Fig. 5B). Hold the polishing disc with one hand and the hemostat with another. Apply a slight even downwards pressure on the ferrule via the hemostat and polish the ferrule on the polishing/lapping film sheets. Make approximately 20 figure eight patterns on each of the three grades of the lapping sheets, starting with the 6 um grade sheet and working downwards. Some people find it easier to polish the ferrule without the aid of the polishing disc. In that case, use the hemostat to press the ferrule directly against the polishing sheets and try to hold the ferrule as vertical as possible during the polishing process (Fig. 5C). The quality of the polishing job can be inspected using a fiber scope.
Lay the ferrule down on a flat surface. Stick a piece of tape over about one centimeter of unstripped fiber on the end opposite the ferrule (Fig. 5D).
- Use the diamond wedge scribe to score the exposed fiber at the distance from the ferrule that corresponds to the depth of the targeted brain structure from the top of the skull (Fig. 5D).This fiber will be inserted directly into the skull of the animal. The length of the fiber is determined by the distance from the top of the skull to the top of the targeted brain structure.
Pull lightly on the ferrule or bend it over towards the tape so the fiber snaps off where it was scored. Put aside this implantable optic fiber until step 22.
Figure 4.
Steps to epoxy optical fiber into a loose ferrule. (A) When working with a small piece of optical fiber, it is best to strip off the buffer material surrounding the optical fiber while the fiber is still attached to the roll. (B) A small ferrule and piece of optical fiber are used to make an implantable optical fiber. (C) The ferrule is held in a hemostat while epoxy is injected into its center. (D) The piece of optical fiber is threaded through the epoxy-filled ferrule. (E) The epoxy must be completely hardened before polishing the ferrule.
Figure 5.
Steps to polish and finalize an implantable optical fiber. (A) A diamond wedge scribe is used to score the optical fiber so that a light flick to the end of the optical fiber will cause it to break off immediately as it leaves the ferrule. (B) The fragility of the optical fiber necessitates the use of a hemostat to hold and polish the ferrule. One option to polish a loose ferrule is to use a polishing disc. In this case, use the hemostat to hold and position the ferrule in the polishing disc throughout the polishing process. (C) A second option to polish a loose ferrule is to forgo the polishing disc and simply press the ferrule against the lapping sheets with the help of the hemostat alone. (D) A diamond wedge scribe is used to score the optical fiber at the distance from the ferrule that corresponds to the depth of the targeted brain structure.
Fabricate fiber optic patch cable
-
14To fabricate the end of the patch cable that will connect to the laser coupler, complete steps 1 through 11 in Basic Protocol 1.In brief, cut the desired length of optical fiber (about one meter). Strip 5 cm of the buffer off each end of the fiber. Epoxy one end of the fiber into an FC/PC connector. Score and break off the excess fiber coming out off the connector. Polish this end so it can form a smooth connection with the fiber coupler.
-
15Cut a piece of furcation tubing that is about 5 cm shorter in length than the optical fiber. Thread this over the optical fiber (Fig. 6A).Furcation tubing covers the fiber optic cable and prevents the laser light from becoming a visual cue for the animal. It also strengthens the cable and helps prevent the optical fiber from cracking.
-
16Thread a 5 cm long piece of 1/8 inch diameter heat shrink tubing over the loose end of the fiber and bring it flush against the connector (Fig. 6B).The heat shrink tubing should cover the tail end of the connector as well as the furcation tubing. The purpose of this tubing is to hold the furcation tubing in place as well as to strengthen the cord at this junction.
-
17
Use a heat gun to activate the heat shrink tubing so it tightly grips both the connector and the furcation tubing (Fig. 6B).
-
18
Mix together another helping of epoxy and prepare another zirconia ferrule as described in steps 3 through 6 of this protocol (Fig. 4C).
-
19
Take the loose end of the optical fiber and thread it through the epoxy until it is stopped by the unstripped buffer. Allow epoxy time to harden, as described in step 8 of this protocol (Fig. 4D).
-
20
Use the diamond wedge scribe to score the stripped end of the optical fiber just as it leaves the ferrule (Fig. 5A). Flick or pull this excess fiber so it breaks off where it was scored.
-
21
Polish the fiber and ferrule at this new end as described in step 10 of this protocol (Fig. 5B,C).
-
22
Attach the completed fiber to the laser. Turn on the laser and test its wattage output using the fiber optic power meter. Refer to the troubleshooting section below if wattage output is not sufficient.
-
23
Insert the ferrule three quarters of the way into a 1.25 mm ID ceramic split sleeve (Fig. 6C).
-
24Thread a 5 cm long piece of 3/32 inch heat shrink tubing over the split sleeve and ferrule (Fig. 6D).Align the heat shrink tubing so it covers the furcation tubing on one end and most of the split sleeve on the other end. The purpose of this tubing is to hold both the furcation tubing and the split sleeve in place as well as to strengthen the cord at this junction.
-
25Use a heat gun to constrict the heat-shrink tubing in place.A schematic of a completed fiber optic patch cable is presented in figure 6F.
Figure 6.
Steps to fabricate a fiber optic patch cable that can attach to an implantable optical fiber. (A) Furcation tubing covers the exposed optical fiber to prevent visualization of the light from becoming a cue to the animal. (B) Heat shrink tubing is used to both strengthen this junction and hold the furcation tubing in place. (C) A zirconia split sleeve is placed over three quarters of the ferrule. The remaining area of the split sleeve will accommodate the loose ferrule that will be implanted in the skull of an animal. (D) Heat shrink tubing is again used to both strengthen this junction and hold the furcation tubing in place. (E) The ferrule of the implantable optical fiber is inserted into the split sleeve on the patch cable. A mark is made on the loose ferrule to indicate how much of it must remain exposed to form a solid connection to the cable. Cement can be applied below this mark when affixing the ferrule to the skull of the animal. (F) Illustration of a completed fiber optic patch cable. Heatshink is indicated by translucent green sheathing over the connector boot and ferrule + ceramic split sleeve. Fiber optic cable is indicated by thin bright pink line, while furcation tubing is represented by yellow covering running along the length of exposed fiber optic cable. Drawing is not to scale, and should be viewed digitally for optimal clarity.
Test wattage output of combined fibers
-
26Insert the ferrule of the implantable optical fiber into the ceramic split sleeve of the patch cable until the two ferrules are flush against each other in the split sleeve.During the surgical implantation of the optic fiber, it will be helpful to know how much of the ferrule on the implantable optic fiber needs to be available to connect with the patch cord. To indicate this, use a permanent fine tip marker to put a small mark on the implantable fiber optic ferrule immediately where it leaves the ceramic split sleeve (Fig. 6E). When affixing the ferrule to the skull during stereotaxic surgery, do not put adhesive material over this line.
-
27Turn on the laser and test the wattage output of the two cords together using the fiber optic power meter.The difference in wattage output between that obtained in step 22 and that obtained here is the light loss that can be attributed to the implantable optic fiber. If the light loss is so great that an adequate light level will not be obtained in the brain, it may be possible to improve the fiber by polishing it again. Otherwise, a new implantable optic fiber will have to be fabricated.
-
28
Disconnect implantable optic fiber so it is available to implant in the animal during the stereotaxic surgery.
Inject virus and implant optical fiber into skull of animal
-
29Perform stereotaxic surgery to inject virus and implant optical fiber.Detailed instructions for mouse stereotaxic surgery can be found in the Appendix 4A. The Basic Protocol in that chapter describes how to microinject solutions, such as AAV virus, into the brain. Additional instruction specific to AAV virus injections in rats can be found in Unit 4.24, Basic Protocol 1.The Appendix 4A alternate protocol describes how to cement a cannula to the skull of an animal. To implant the optical fiber in the brain, follow those instructions and simply use the optical fiber in place of the cannula. Take care not to add adhesive over the indicator line on the ferrule, the section of it that is needed to mate with the patch cord.
Activate ChR2 containing tissue
-
30
Wait a sufficient amount of time following surgery to ensure ample ChR2 expression. This will depend on the strength of the promoter used and distance between injection and stimulation sites.
-
31Hold the animal tightly in one hand and mate the ferrule with split sleeve on the patch cable to the ferrule atop the head of the animal.Make sure the two ferrules are flush against each other. An air gap in the fiber optic connection will result is an unacceptable amount of light loss due to reduced coupling efficiency between the two ferrules.
-
32Place the animal into the test chamber and pulse the laser.Patch cords will coil up as the animal rotates more in one direction than the other. During short experiments where minimal rotations are expected from the animal, the coiling of the cord may not be an issue. If turning behavior is an issue, it may be necessary to put a rotary joint (commutator) in the patch cord to allow it to spin as the animal turns (see commentary section).
SUPPORT PROTOCOL 1: CHANNELRHODOPSIN USE WITH GUIDE CANNULA
A guide cannula can be used in place of a permanently implanted optical fiber for in vivo experiments. Using this approach, the fiber is inserted through a guide cannula immediately before the start of a behavioral experiment. The disadvantages to the guide cannula method include the potential breakage of an optical fiber in the guide cannula and the danger of excess tissue damage due to the repeated insertion of optical fibers. The principal benefit of this method is that it allows for an infusion of drugs through the same guide cannula, either before or after behavioral experiments. For this reason, the guide cannula method is routinely used for in vivo optogenetic behavioral experiments. This protocol details how to fabricate the components necessary to use optical fibers with a guide cannula.
Materials
Optical fiber (e.g., multimode, 0.22 numerical aperture, visible to near infrared, low OH, 105 um core fiber, Thorlabs)
Fiber stripping tool (e.g., clad/coat: 125 um / 250 um fiber stripping tool, Thorlabs)
FC fiber optic connector (e.g., FC simplex connector, 900 um fiber-multimode, 128 um, Fiber Instrument Sales)
Heat-cured epoxy (e.g., blue dye epoxy, Fiber Instrument Sales)
3 cc luer-lock syringe with blunt 22 gauge needle
Diamond wedge scribe (Thorlabs)
FC/PC connector polishing disc (Thorlabs)
Fiber polishing/lapping film diamond sheets (6” × 6” sheets with grit sizes of 1, 3 or 6 um, Thorlabs)
200X Fiber scope (Thorlabs)
Cannula connector assembly (C313C/SP without inside tubing, Plastics One,Roanoke, VA)
Infusion cannula (C312IS-4/SP without the stainless steel tubing, Plastics One)
Hemostat
Short cannula pedestal (C312GS-4/SP, Plastics One)
Fiber optic power meter (e.g., 400 nm – 1100 nm, 1 nW – 40 mW, Thorlabs)
Blue light laser with fiber coupler (e.g., 150 mW, 473 nm diode pumped solid state continuous wave laser system with FC/PC multimode fiber coupler, OEM Laser Systems)
Fabricate fiber optic cable
- To fabricate the end of the fiber cable that will connect to the laser coupler, complete steps 1 through 11 in Basic Protocol 1.In brief, cut a piece of optical fiber that is 5 cm longer than the cannula connector assembly. Strip 5 cm of the buffer off each end of the fiber. Epoxy one end of the fiber into a connector. Score and break off the excess fiber coming out off the connector. Polish this end so it can form a smooth connection with the fiber coupler.
- Cut a piece of furcation tubing that is as long as the cannula connector assembly. Thread this over the optical fiber (Fig. 6A).Furcation tubing covers the fiber optic cable and prevents the laser light from becoming a visual cue for the animal. It also strengthens the cable and helps prevent the optical fiber from cracking.
- Thread the fiber optic cord into the back side of the cannula connector assembly, the side without the captive collar.The back end of the connector assembly will be flush against the FC fiber optic connector. The end of the connector assembly with the captive collar will have stripped optical fiber protruding out of it.
Hold the hollow infusion cannula in a hemostat or vice with the large opening pointing downwards.
Mix together the two parts of the epoxy and draw it up into a 3 cc luer-lock syringe.
Attach a 22 gauge blunt needle to the syringe and inject one drop of epoxy onto the top of the infusion cannula.
Thread the stripped optical fiber through the top (small opening) of the infusion cannula until the infusion cannula reaches the captive collar of the cannula connector assembly.
Give the epoxy time to harden. At room temperature it will cure over 24 hours. The use of a heat gun will speed up the curing time considerably.
- Thread the unstripped fiber that is protruding out of the connector assembly through the short cannula pedestal. The infusion cannula snaps onto the cannula pedestal. For a more secure connection, screw the captive collar onto the cannula pedestal.The cannula pedestal will eventually be affixed to the skull of the animal during stereotaxic surgery. The length of the cannula pedestal is determined by the distance from the top of the skull to the top of the targeted brain structure. It should be 1 mm less than this distance because the optical fiber will stick out 1 mm from the cannula pedestal.
Lay the connector assembly down on a flat surface. Use the diamond wedge scribe to score the exposed fiber about 1 mm from where it leaves the cannula pedestal.
Pull lightly on the loose end of the optical fiber or bend it over towards the cannula pedestal so the fiber snaps off where it was scored. Remove the cannula pedestal so it is available to use during the stereotaxic surgery.
Attach the completed fiber to the laser and test its wattage output.
Inject virus and implant optical fiber into skull of animal
-
13Perform stereotaxic surgery to inject virus and implant cannula pedestal.Detailed instructions for mouse stereotaxic surgery can be found in Appendix 4A. The Basic Protocol in that chapter describes how to microinject solutions, such as AAV virus, into the brain. Additional instruction specific to AAV virus injections in rats can be found in Unit 4.24, Basic Protocol 1.The Appendix 4A alternate protocol describes how to cement a cannula to the skull of an animal. To implant the cannula pedestal in the brain, follow those instructions.
Activate ChR2 containing tissue
-
14
Wait a sufficient amount of time following surgery to ensure ample ChR2 expression. This will depend on the strength of the promoter used and distance between injection and stimulation sites.
-
15
Hold the animal tightly in one and thread the optical fiber that protrudes out of the infusion cannula through the cannula pedestal on the head of the animal. The infusion cannula will snap onto the cannula pedestal. For a more secure connection, screw the captive collar onto the cannula pedestal.
-
16Place the animal into the test chamber and pulse the laser.Patch cords will coil up as the animal rotates more in one direction than the other. During short experiments where minimal rotations are expected from the animal, the coiling of the cord may not be an issue. If turning behavior is an issue, it may be necessary to put a rotary joint (commutator) in the patch cord to allow it to spin as the animal turns (see commentary section).
COMMENTARY
Additional Considerations
Splitters and Commutators
Fiber splitters, also known as couplers, can combine or split light beams. Splitters are routinely used for in vivo behavioral experiments where bilateral activation is required. Precision Fiber Products is a private company that sells custom made fiber splitters to match any fiber and connector size (Fig. 7A). One consideration when using bilateral light stimulation is getting equal amounts of light on both sides of the brain. To achieve this, the implantable optical fibers have to be tested for coupling efficiency and comparable optical fibers should be used in the same animal.
Figure 7.
Accessories used for in vivo applications. (A) A fiber splitter from Precision Fiber Products with bare fiber on all ends. It splits light evenly and is customizable. (B) A fiber optic rotary joint (commutator) from Doric Lenses with FC/PC connections on either end. It has minimal torque and can be easily rotated by mice. (C) A fiber optic patch cable with rotary joint and fiber splitter can be used for bilateral optical stimulation in vivo.
Commutators, also known as rotary joints, create a junction that allows for free rotation of fiber while maintaining light intensity. This is necessary for in vivo experiments where the animal may rotate repeatedly in one direction. Optical commutators are available that have extremely low torque and allow for mice to rotate unencumbered. Doric Lenses is a private company that sells optogenetic components. They make low torque commutators that accommodate the FC/PC connectors used in this protocol (Fig. 7B). They have also combined commutators and fiber splitters, such that one or two fibers feed into a commutator that has two or four outputs, respectively.
Figure 7C depicts a fiber optic patch cable used for in vivo behavioral experiments that require both bilateral stimulation and a rotary joint. Coming from the top of the photo is a store-bought FC/PC patch cable that connects the laser to the Doric Lenses single channel commutator. The cord beneath the commutator is a Precision Fiber Products fiber splitter that was altered in house to have an FC/PC connection on one end and two ferrules on the other end. The two ferrules will connect through a split sleeve to ferrules affixed to the skull of an animal and allow for bilateral stimulation. The commutator, which will be attached to the top of the behavioral chamber, allows the animal to rotate freely.
Pathway Specificity
AAV viruses are thought to selectively infect cell bodies. After infection, cells will immediately start producing ChR2 protein. Within the cell, ChR2 is preferentially targeted to the cell membrane. It will diffuse throughout the membrane and over time it will be found in distal processes. Researchers have taken advantage of this phenomenon to activate axons that express ChR2. In brain slice preparations, axons expressing ChR2 are viable, even if the connections to the cell bodies have been severed. Researchers have also used ChR2 to study specific pathways in vivo. A common approach is to inject virus in one location and place the optical fiber in a different location, thereby allowing the selective activation of neuronal processes that project from the site of viral injection to the site of light stimulation. Because ChR2-induced action potentials can travel in both anterograde and retrograde directions, collateral pathways of ChR2-expressing soma may also be activated, and care must be taken when interpreting the results. This can be experimentally addressed by demonstrating that the effects of light stimulation persist with chemical inactivation of the cell bodies where the virus was injected and/or that the effects of light stimulation are blocked by pharmacological or genetic inhibition of receptors at the terminal site.
Potential Side-Effects
High intensity light produces a significant amount of heat. This does not appear to be an issue with millisecond-long flashes of light, but constant illumination may produce enough heat to alter physiological processes. The amount of heat produced in brain tissue by optogenetic stimulations has not been thoroughly studied. To control for nonspecific effects of light delivery, the experimental design should include a group of animals that receive light stimulation but do not have functional ChR2.
The overexpression of any foreign protein may have detrimental side effects. This is particularly true for ChR2 since it fills the cell membrane. The relationship between ChR2 expression levels and cell viability has not been thoroughly studied. To control for this, experiments should include a control group that receives ChR2 expression without light stimulation.
Critical Parameters and Troubleshooting
Laser coupler
Not obtaining a sufficient intensity of light is a typical problem. The coupling efficiency at each junction should be around 80% or greater and sufficient light intensities should be easy to obtain. Common sources of problems are the laser itself or the laser coupler. It is helpful to have a fiber optic power meter as well as a store-bought, standard FC/PC fiber optic patch cable to help with troubleshooting. The first step is to use the power meter to test that the laser itself is emitting a sufficient intensity of light, which should reflect with the specifications of the laser. If this is not the case, either the laser is defective or the attached laser coupler is not aligned properly. The laser coupler collimates the light. If not aligned properly, it will either occlude or misdirect the light. Laser couplers are typically held in place by three set screws. Gross adjustments to these set screws should be done with a standard FC patch cable attached to the coupler. Position the patch cable so the light beam shines against a wall. Adjust the set screws until the laser beam emitted from the patch cable is both bright and focused. These steps ensure that the laser is working properly and the coupler is aligned. Minor adjustments to these set screws are sometimes needed to accommodate different patch cords.
If the light intensity is not strong enough only when home-made patch cables are used, then the patch cables themselves or the connections between them are the issue. The first step is to slightly adjust the set screws on the laser coupler. If there is little to no light coming through the patch cable, this suggests there is a break in the optical fiber and a new patch cord must be made. If the emitted light is definitely present but inadequate or simply too diffuse, it may be necessary to re-polish the connectors and ferrules on the cord. If one end of the fiber is bare, try to re-cut this end with the diamond wedge scribe.
Anticipated Results
Slice electrophysiology is the most direct method to test if ChR2 proteins are expressed in a sufficient amount and responsive to light. ChR2 proteins are typically conjugated to fluorescent proteins so it is possible to identify the cells that express the protein. This fluorescence can be used to patch onto ChR2-expressing cell bodies to verify that light stimulation induces inward currents large enough to reliably induce action potentials. The fluorescence can also be used to patch in a region with ChR2-expressing terminals. If axons express ChR2 in quantities sufficient to induce an action potential, optically-stimulated post synaptic currents can be recorded in the downstream neurons.
Time Considerations
Fabricating fiber optic patch cables typically take anywhere from twenty minutes to two hours per cable, depending on the type of terminations. Getting the DNA that encodes optogenetic gene(s) into the cells of interest and expressed in sufficient quantities, however, is the first step in preparing optogenetic experiments as well as the most time consuming. Light-sensitive ion channels (e.g. ChR2) and pumps (e.g. Halorhodopsin) are typically only effective if they are trafficked to the cell membrane. Additional tags can be attached to the protein to direct it to various portions of the cell membrane, but current ChR2 variants simply diffuse within the membrane in an undirected manner. The soma of the cell and proximal processes will fill up with ChR2 before distal process will. The speed of this process will depend on the strength of the promoter used to drive ChR2. Strong promoters will drive expression of enough protein to fill the soma and make light-induced currents large enough to generate action potentials in a day or two after gene translation commences. Weak promoters can take over a week generate sufficient expression, if they do at all. Over time ChR2 will diffuse throughout the cell and populate the membrane of distal processes. It can take one to two months to see adequate expression in the terminals of axons that are several millimeters long.
Acknowledgments
This work is supported by the NIDA IRP.
Footnotes
Internet resources
http://www.stanford.edu/group/dlab/
Web site for the laboratory of Dr. Karl Deisseroth; contains information on ChR2 (and variants) DNA sequences, hardware, and protocols.
http://syntheticneurobiology.org/
Web site for the laboratory of Dr. Ed Boyden; contains information on inhibitory optogenetic proteins.
Web site for the laboratory of Dr. Garret Stuber; contains protocols for optical fiber construction and optogenetics hardware.
http://www.stanford.edu/group/dlab/cgi-bin/graph/chart.php
Calculator for the spread of light in brain tissue, courtesy of the lab of Dr. Karl Deisseroth
http://thorlabs.com/Thorcat/1100/1166-D02.pdf
“Guide to Connectorization and Polishing Optical Fibers,” courtesy of Thorlabs
Literature Cited
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Gutierrez DV, Mark MD, Masseck O, Maejima T, Kuckelsberg D, Hyde RA, Krause M, Kruse W, Herlitze S. Optogenetic control of motor coordination by Gi/o protein-coupled vertebrate rhodopsin in cerebellar Purkinje cells. J Biol Chem. 2011 doi: 10.1074/jbc.M111.253674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY. A user's guide to channelrhodopsin variants: features, limitations and future developments. Exp Physiol. 2011;96:19–25. doi: 10.1113/expphysiol.2009.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]