Abstract
Background/Aims: The rat has been the most used experimental animal for studies of cardiovascular and kidney diseases. However, it is notable that there is increasing importance placed on the use of the mouse model to increase understanding of these pathophysiologies. The aim of the present study was to induce chronic kidney disease in a mouse model and to evaluate the resulting changes in blood pressure (BP) and in renal morphology and function. Methods: Adult male C57BL/6 mice underwent 5/6 nephrectomy (5/6 Nx) or a sham operation (Sham). Two weeks later, conscious animals were subjected to a 24-hour urine collection and to a direct measurement of BP. Results: Compared to Sham animals, 5/6 Nx mice showed reduced creatinine clearance (3-fold, p<0.01), proteinuria (1.5-fold, p<0.01) and uremia (4-fold, p<0.01), as well as high blood pressure (~20%, p<0.01). 5/6 Nx animals showed increases in the 24 h urine excretion of Na+ (2-fold, p<005), K+ (~2-fold, <0.01) and Ca2+ (~12-fold). Kidney histology of 5/6 Nx mice also demonstrated glomerular hypertrophy (1.5-fold, p<0.05), mesangial expansion (~40%, p<0.01) and increased glomerular collagen deposition (~30%, p<0.05). Conclusion: Induction of 5/6 nephrectomy in mice for two weeks leads to systemic arterial hypertension and to functional and morphological damage of the remnant kidney, which are considered the main characteristics of chronic kidney disease.
Keywords: Chronic kidney disease, renal function, 5/6 nephrectomy, mice
Introduction
Chronic kidney disease (CKD) is a worldwide public health problem associated with significant morbidity and mortality [1]. Several risk factors contribute to the development and progression of CKD, including hypertension, diabetes and dyslipidemia [2-4]. For this reason, strategies aiming to identify, prevent and treat CKD and its related risk factors could contribute to a better understanding of this disease. Therefore, the development of experimental models may contribute towards the understanding of the mechanisms underlying CKD.
Among the available experimental models for CKD, the 5/6 nephrectomy (5/6 Nx) has been a mainstay of studies of progressive renal disease and is performed by unilateral nephrectomy and either partial infarction or amputation of the poles of the remaining kidney [5,6]. The features of this experimental procedure are common to CKD observed in humans [7]. It has been used to test new therapies [8,9] and has been proven to be clinically relevant [10].
Although the rat model has been the primary choice for decades, there are many genetic manipulations currently available in mouse models that provide advantages supporting the use of this specie. However, few studies have investigated the consequences of 5/6 Nx in murine animals, and the results obtained are inconsistent [7,11]. In light of the burgeoning interest in murine pathophysiology and the importance of a better understanding of the CKD, the present investigation was designed to determine the effects of 5/6 Nx on renal morphology and function as well as on arterial pressure in the mouse model.
Material and methods
Animals
The experiments were performed in 3-month-old male C57BL/6 (C57) mice obtained from the animal facilities of the Health Sciences Center at the Federal University of Espirito Santo. The animals were housed according to the institutional guidelines for animal research, with constant room temperature, a 12-hour light/dark cycle, and 50±5% humidity, as well as standard mouse chow and water ad libitum. The procedures were previously approved by the Animal Use Ethics Committee of the Research Center of Emescam College of Health Sciences (CEUA, Protocol # 003/2009).
5/6 Nephrectomy
Animals underwent 5/6 Nx or sham surgery under ketamine/xylazine anesthesia (91.0/9.1 mg/kg, i.p.). Briefly, the left kidney was exposed, and the upper and lower poles were tied with a polyglycolic acid suture line, followed by right nephrectomy. The peritoneum and skin were then sutured, and the animals were returned to their individual cages.
Renal function analysis
Two weeks after sham or 5/6 Nx surgery, the animals were placed in metabolic cages to analyze renal function. Then, the animals were subjected to a 24-hour urine collection to determine creatinine concentration and urine volume. Creatinine was also measured in the blood obtained through the retro-orbital plexus under anesthesia (ketamine/xylazine, 91.0/9.1 mg/Kg, i.p.) to estimate the glomerular filtration rate (GFR) by calculating creatinine clearance. Plasma and urine samples were also used for the quantification of urea, Na+, K+ and Ca2+. Fractional excretion was calculated using the plasma and urine concentrations of these ions, the 24-hour urine volume and the GFR obtained from the creatinine clearance calculations. Total protein excretion in the urine was also determined using the Bradford [12] method.
Hemodynamic measurements
A separate group of animals was used for direct measurements of arterial blood pressure (BP) and heart rate (HR). Two weeks after sham or 5/6 Nx surgery, mice were anesthetized with a combination of ketamine and xylazine (91.0/9.1 mg/kg, i.p.), and a catheter (0.040 mm outer x 0.025 mm inner diameters, Micro- Renathane, Braintree Science, Massachusetts, USA) was inserted into the right carotid artery for the measurement of systolic (SBP), diastolic (DBP) and mean (MBP) arterial blood pressure as well as HR. The free catheter end was tunneled under the skin of the back to the level of the shoulder blades. Two days after catheter placement, hemodynamic measurements were performed in conscious, freely moving mice in their own cages when they were neither grooming nor eating. For BP and HR recordings, the arterial catheter was plugged into a disposable BP transducer (Cobe Laboratories, Colorado, USA) connected to a pressure processor amplifier and data-acquisition system (MP100, Biopac Systems, California, USA).
Renal histological analysis
At the end of the experiments, the animals were euthanized with an overdose of sodium thiopental and perfused via the left ventricle with phosphate-buffered saline (PBS, pH 7.4; 0.1 M), followed by a fixative solution of formaldehyde (4%); the kidney was removed, cleaned of connective tissue and embedded in paraffin. Tenmicrometer-thick sections were processed with hematoxylin-eosin (HE), periodic acid-Schiff (PAS) and Masson’s trichrome staining, and the glomeruli were photographed for later analysis. Images were captured with color video camera (VKC150; Hitachi, Tokyo, Japan) connected to a microscope (AX70; Olympus, Center Valley, PA) and analyzed with a specific image program (2100 Leica EWS; Leica, Wetzlar, Germany) by a person blinded to the experimental groups. The mean glomerular cross-sectional area was obtained by calculating the mean of 100 glomeruli individual areas using the Image J program. To determine glomerular sclerosis, at least 50 glomeruli were analyzed in PAS and Masson’s trichrome-stained sections, and the mean of the glomerular-stained areas (in %) was used to determine the mesangial expansion and collagen deposition for each animal.
Remnant kidney weight and femur weight
At the end of the experiments and hemodynamic recordings, the mice were euthanized with an overdose of sodium thiopental, and the femur and the remnant kidney were removed. The poles of the kidney were discarded, and the remnant area of the kidney and the femur were dried for 24 hours in an oven and then weighed. The kidneys of sham animals underwent the same procedure.
Statistical analysis
All data are expressed as the means ± SEM. The normality of the variables was evaluated using the Kolmogorov-Smirnov test. Statistical analysis was performed using Student’s t test for independent samples. The level of significance was set at p<0.05.
Results
Average values of body weight, chow intake, water intake, 24-hour urine volume and remnant kidney weight are summarized in Table 1. At the end of the experiments, body weight was significantly reduced in animals that underwent 5/6 Nx when compared with the sham group. Water intake and urine volume increased approximately 2-fold and 3.5-fold (p<0.01), respectively, in the 5/6 Nx animals compared with the Sham animals. As expected, 5/6 Nx led to hypertrophy of the remnant kidney.
Table 1.
Body weight, chow and water intake, 24-hour urine volume and remnant kidney weight in 5/6 nephrectomized animals
| Parameters | Groups | |
|---|---|---|
|
| ||
| Sham | 5/6 Nx | |
| Body weight (g) | 29.8 ± 1.1 | 25.7 ± 0.3** |
| Chow intake (mg/24h) | 4.7 ± 0.4 | 4.6 ± 0.4 |
| Water intake (mL/24h) | 7.5 ± 0.6 | 15.4 ± 0.9** |
| Urine volume (mL/24h) | 2.4 ± 0.1 | 8.5 ± 0.6** |
| Remnant kidney weight (mg) | 14.3 ± 0.8 | 21.4 ± 1.9** |
Values are means ± SEM (n=8 to 10 per group).
p<0.01 vs. sham.
Figure 1 summarizes the creatinine clearance, uremia and proteinuria levels in the Sham and 5/6 Nx animals. Nephrectomized animals exhibited a marked reduction of GFR, as demonstrated by the diminished creatinine clearance in comparison with the Sham group (142 ± 13 vs. 47 ± 8 μL/min, p<0.01). In addition, the 5/6 Nx mice showed greater values of uremia than the Sham animals (50.5 ± 9.0 vs. 12.5 ± 0.9 mmol/L, p<0.01). Proteinuria was also elevated in the 5/6 Nx animals (3.2 ± 0.05 vs. 2.1 ± 0.09 mg/24 h, p<0.01) in comparison with the sham group, which is indicative of glomerular injury.
Figure 1.
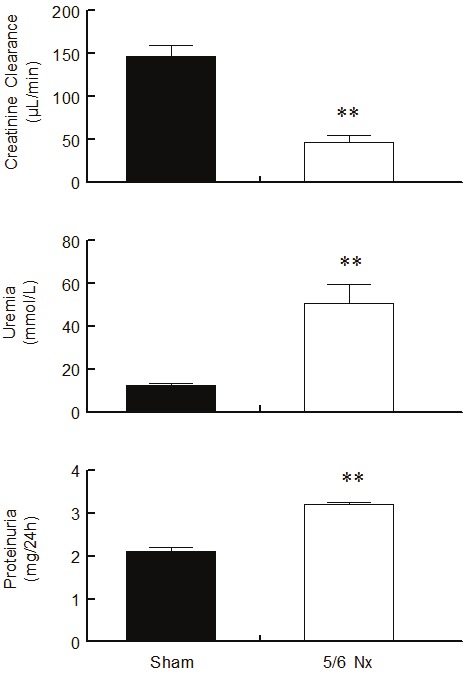
Creatinine clearance, uremia and proteinuria levels. 5/6 nephrectomized (5/6 Nx) animals presented reduced glomerular filtration rate, as indicated by reduced creatinine clearance, increased uremia and glomerular damage demonstrated by the increase in proteinuria levels compared to Sham animals. Values are means ± SEM (n= 8 to 10 per group), **p<0.01 vs. Sham group.
Table 2 shows the effects of 5/6 Nx in the 24-hour excretion and fractional excretion of Na+, K+ and Ca2+. The 5/6 Nx animals showed elevated excretion and fractional excretion of Na+ (2.2-fold and 6.5-fold, respectively, p<0.01). K+ excretion and fractional excretion were also significantly increased in the 5/6 Nx group when compared to the Sham animals (4.2-fold, p<0.01). Urine Ca2+ and the fractional excretion of Ca2+ were also markedly affected in the 5/6 Nx animals in comparison with the Sham group (~13-fold and ~24-fold, respectively, p<0.01). Moreover, plasma measurements showed increased levels of this ion in the 5/6 Nx animalsin comparison with the Sham animals (2.25 ± 0.07 vs. 1.80 ± 0.06 mmol/L, p<0.01). Corroborating this observation, femur weight was significantly lower in the 5/6 Nx animals than in the Sham animals (41 ± 2 vs. 51 ± 2 mg, p<0.01).
Table 2.
Urine and fractional excretion of Na+, K+ and Ca++ in 5/6 nephrectomized mice
| Parameters | Groups | ||
|---|---|---|---|
|
| |||
| Sham | 5/6 Nx | ||
| Na+ | |||
| Urine excretion (mmol/24h) | 0.30 ± 0.02 | 0.66 ± 0.06** | |
| Fractional Excretion (%) | 1.3 ± 0.1 | 8.4 ± 1.2** | |
| K+ | |||
| Urine excretion (mmol/24h) | 0.58 ± 0.03 | 0.93 ± 0.14** | |
| Fractional Excretion (%) | 45 ± 5 | 192 ± 28** | |
| Ca2+ | |||
| Urine excretion (mmol/24h) | 1.6 ± 0.1 | 20.6 ± 1.7** | |
| Fractional Excretion (%) | 0.68 ± 0.06 | 16.2 ± 2.5** | |
Values are means ± SEM (n=8 to 10 per group).
p<0.01 vs. sham.
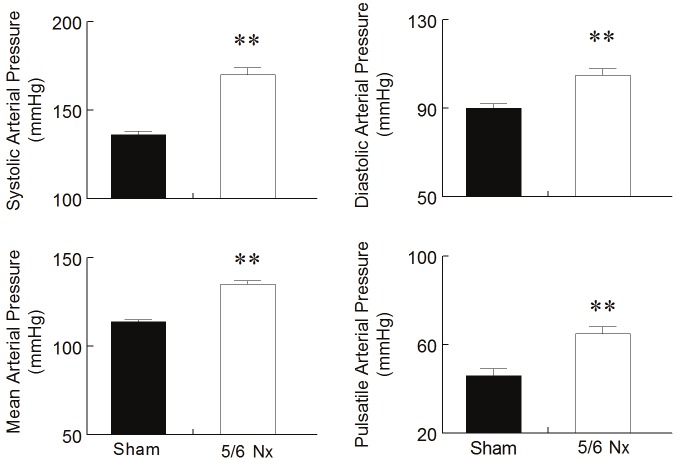
Figure 2 shows average values of BP and HR obtained by direct measurement in conscious animals. The nephrectomized animals presented severe arterial hypertension compared with the Sham animals, as shown in all the components measured: SBP (136 ± 2 vs. 170 ± 4 mmHg, p<0.01), DBP (90 ± 2 vs. 105 ± 3 mmHg, p<0.01), pulsatile pressure (46 ± 3 vs. 65 ± 3 mmHg, p<0.01) and MBP (114 ± 1 vs. 135 ± 2 mmHg, p<0.01). Resting HR values were not affected by 5/6 nephrectomy.
Figure 2.
Hemodynamic measurements. Direct measurements of systolic, diastolic and mean arterial blood pressure and heart rate show a marked arterial hypertension two weeks after 5/6 nephrectomy (5/6 Nx) compared to Sham animals. Values are means ± SEM (n= 6 to 8 per group), **p<0.01 vs. Sham group.
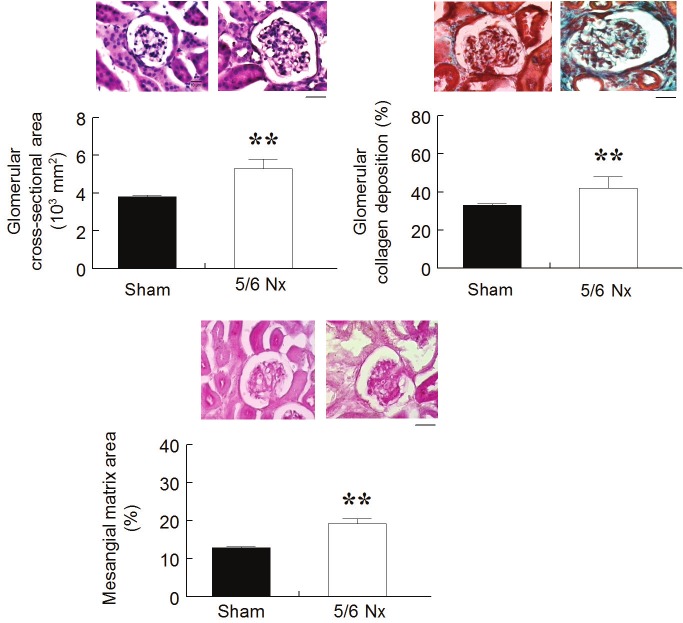
Figure 3 summarizes the kidney histology data and shows typical histological sections comparing the 5/6 Nx animals with the Sham animals. The glomerular area was significantly greater in the 5/6 Nx animals (5.3 ± 0.5 x 103 μm2, p<0.05) than in the Sham group (3.8 ± 0.1 x 103 μm2). The mesangial matrix area, indicated by the PAS stained areas, was significantly larger in the 5/6 Nx group when compared to the sham animals (19.2 ± 1.3 % vs. 12.9 ± 0.3 %, p<0.01). The nephrectomized animals also showed increased glomerular collagen deposition, as indicated by the Masson’s trichrome staining (33 ± 1 vs. 42 ± 6%, p<0.01).
Figure 3.
Renal Histological Analysis. Bar graphs and respective representative pictures show hypertrophy of remnant glomeruli, mesangial expansion and increased collagen deposition in 5/6 Nx animals compared to Sham animals. Values are the means ± SEM (n= 6 to 8 per group), **p<0.01 vs. Sham group. Scale bar: 10 μm.
Discussion
In the present study, we evaluated the effects of 5/6 Nx on renal morphology and function and on BP in a mouse model. Two weeks after induction of 5/6 Nx, mice presented reduced GFR, proteinuria, glomerular sclerosis and arterial hypertension, which are considered the main characteristics of CKD.
In humans, GFR has been estimated based on serum creatinine levels by applying different formulas [13,14]. Likewise, in experimental models of CKD, the detection of increased plasma levels of creatinine has been interpreted as being indicative of impaired GFR [7,15-17]. In the present study, we evaluated the glomerular filtration rate in 5/6 Nx mice through the creatinine clearance, which is known to be more accurate than plasma creatinine levels for assessing renal function [13,18]. We observed that GFR 2 weeks after 5/6 nephrectomy was reduced by approximately 60% in 5/6 Nx mice, which was similar to that observed by Ohashi et al. [19] after 8 weeks of nephrectomy in adiponectin-knockout mice but was more severe than that observed by Bro et al. [20] in apolipoprotein E-knockout mice. We also observed high levels of serum urea in 5/6 Nx mice, in agreement with others who have studied the model [21,22], which is considered a consequence of the reduced GFR. Conversely, in the present study, it was noted that the reduction in renal mass caused hypertrophy of the remnant glomeruli in 5/6 Nx mice, which has been interpreted as a compensatory response to the increased glomerular perfusion [23].
High BP is a hemodynamic characteristic of CKD, which could accelerate the progression of renal dysfunction by worsening glomerular injury and proteinuria [24]. The occurrence of arterial hypertension has also been demonstrated in 5/6 Nx rats [25,26]. In C57 mice, changes in BP following 5/6 Nx has been evaluated in a number of studies by the indirect tail-cuff technique, the results of which have been reported as both normal [11] and high [27]. In the present study, direct measurements of BP in conscious animals showed that 5/6 Nx animals were hypertensive, mainly due to the systolic component. Among possible mechanisms underlying this finding in this model of CKD is augmented peripheral vascular resistance as a consequence of increased vascular sympathetic activity [28] and of activation of the reninangiotensin system [27]. Based on the finding that 5/6 Nx mice develop aortic stiffness [22], we speculate that this could contribute to the severe increase in the systolic component of the BP observed in our study.
Arterial hypertension is considered a major promoter of the decline in renal function because it is transmitted within renal resistance vessels, glomerular capillaries and mesangial cells, resulting in glomerulosclerosis [24]. In our study, 5/6 Nx mice presented both mesangial expansion and increased glomerular collagen deposition, which are main features of glomerulosclerosis in CKD. These could be caused by an increase in extracellular matrix formation, diminished extracellular matrix degradation or both [29]. It is reasonable to think that arterial hypertension observed in the 5/6 Nx animals led to glomerular injury and consequently a marked proteinuria. Conversely, proteinuria is known to be tightly correlated with the decline of GFR [30], and thus, it has been considered the strongest predictor of kidney disease outcome [31].
Our data show for the first time a severe increase in urine and fractional excretions of Na+ and K+ in 5/6 Nx mice. This finding reinforces the concept that in CKD, the reduced GFR and increased filtration burden on the remnant glomeruli leads to the increased excretion of ions from and the decreased accumulation of ions in the plasma [32]. Tubular mechanisms involved in the increases in urine and fractional excretions of these ions could include alterations in the expression and activity of ion exchangers and cotransporters [33]. Considering that renal osteodystrophy is a characteristic of CKD [34], we also evaluated the Ca2+ balance. Our observation that 5/6 Nx mice presented increased calcemia and reduced femur weight is indicative of bone reabsorption, which is in agreement with other studies [15]. Notably, in the present study, we found increased urine and fractional excretions of Ca2+ in this model of CKD, contributing to a better characterization of the 5/6 Nx model in the mouse.
Perspectives
One of the limitations of our study is that the molecular mechanisms involved in the development of CKD induced by 5/6 Nx were not completely investigated and should be considered in future studies. Our hypothesis is that altered expression of inflammatory and proliferative molecules and renal transporters could be involved. Further studies will attempt to elucidate these questions.
Acknowledgments
This study was supported by the National Council for the Development of Science and Technology (CNPq), State Agency for the Development of Science and Technology (FAPES), and Funds for Science and Technology of the City of Vitoria (FACITEC).
References
- 1.Hoyert DL, Heron MP, Murphy SL, Kung HC. Deaths: final data for 2003. Natl Vital Stat Rep. 2006;54:1–120. [PubMed] [Google Scholar]
- 2.Agarwal R. Blood pressure components and the risk for end-stage renal disease and death in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:830–837. doi: 10.2215/CJN.06201208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islam TM, Fox CS, Mann D, Muntner P. Agerelated associations of hypertension and diabetes mellitus with chronic kidney disease. BMC Nephrol. 2009;10:17. doi: 10.1186/1471-2369-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C, Buring JE, Gaziano JM. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14:2084–2091. doi: 10.1681/ASN.V1482084. [DOI] [PubMed] [Google Scholar]
- 5.Kang DH, Nakagawa T, Feng L, Johnson RJ. Nitric oxide modulates vascular disease in the remnant kidney model. Am J Pathol. 2002;161:239–248. doi: 10.1016/S0002-9440(10)64175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos LS, Chin EW, Ioshii SO, Tambara Filho R. Surgical reduction of the renal mass in rats: morphologic and functional analysis on the remnant kidney. Acta Cir Bras. 2006;21:252–257. doi: 10.1590/s0102-86502006000400012. [DOI] [PubMed] [Google Scholar]
- 7.Kren S, Hostetter TH. The course of the remnant kidney model in mice. Kidney Int. 1999;56:333–337. doi: 10.1046/j.1523-1755.1999.00527.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujihara CK, Malheiros DM, Zatz R. Losartan-hydrochlorothiazide association promotes lasting blood pressure normalization and completely arrests long-term renal injury in the 5/6 ablation model. Am J Physiol Renal Physiol. 2007;292:F1810–1818. doi: 10.1152/ajprenal.00521.2006. [DOI] [PubMed] [Google Scholar]
- 9.Terzi F, Burtin M, Hekmati M, Jouanneau C, Beaufils H, Friedlander G. Sodium restriction decreases AP-1 activation after nephron reduction in the rat: role in the progression of renal lesions. Exp Nephrol. 2000;8:104–114. doi: 10.1159/000020656. [DOI] [PubMed] [Google Scholar]
- 10.Waanders F, Vaidya VS, van Goor H, Leuvenink H, Damman K, Hamming I, Bonventre JV, Vogt L, Navis G. Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: a post hoc analysis of a randomized controlled trial. Am J Kidney Dis. 2009;53:16–25. doi: 10.1053/j.ajkd.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma LJ, Fogo AB. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int. 2003;64:350–355. doi: 10.1046/j.1523-1755.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Rostoker G, Andrivet P, Pham I, Griuncelli M, Adnot S. Accuracy and limitations of equations for predicting the glomerular filtration rate during follow-up of patients with non-diabetic nephropathies. BMC Nephrol. 2009;10:16. doi: 10.1186/1471-2369-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shara NM, Resnick HE, Lu L, Xu J, Vupputuri S, Howard BV, Umans JG. Decreased GFR estimated by MDRD or Cockcroft-Gault equation predicts incident CVD: the strong heart study. J Nephrol. 2009;22:373–380. [PMC free article] [PubMed] [Google Scholar]
- 15.Gagnon RF, Duguid WP. A reproducible model for chronic renal failure in the mouse. Urol Res. 1983;11:11–14. doi: 10.1007/BF00272702. [DOI] [PubMed] [Google Scholar]
- 16.Gagnon RF, Gallimore B. Characterization of a mouse model of chronic uremia. Urol Res. 1988;16:119–126. doi: 10.1007/BF00261969. [DOI] [PubMed] [Google Scholar]
- 17.Bro S, Bentzon JF, Falk E, Andersen CB, Olgaard K, Nielsen LB. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol. 2003;14:2466–2474. doi: 10.1097/01.asn.0000088024.72216.2e. [DOI] [PubMed] [Google Scholar]
- 18.Pickering JW, Frampton CM, Walker RJ, Shaw GM, Endre ZH. Four hour creatinine clearance is better than plasma creatinine for monitoring renal function in critically ill patients. Crit Care. 2012;16:R107. doi: 10.1186/cc11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohashi K, Iwatani H, Kihara S, Nakagawa Y, Komura N, Fujita K, Maeda N, Nishida M, Katsube F, Shimomura I, Ito T, Funahashi T. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:1910–1917. doi: 10.1161/ATVBAHA.107.147645. [DOI] [PubMed] [Google Scholar]
- 20.Bro S, Bollano E, Brüel A, Olgaard K, Nielsen LB. Cardiac structure and function in a mouse model of uraemia without hypertension. Scand J Clin Lab Invest. 2008;68:660–666. doi: 10.1080/00365510802037272. [DOI] [PubMed] [Google Scholar]
- 21.Gagnon RF, Ansari M. Development and progression of uremic changes in the mouse with surgically induced renal failure. Nephron. 1990;54:70–76. doi: 10.1159/000185812. [DOI] [PubMed] [Google Scholar]
- 22.Maizel J, Six I, Slama M, Tribouilloy C, Sevestre H, Poirot S, Giummelly P, Atkinson J, Choukroun G, Andrejak M, Kamel S, Mazière JC, Massy ZA. Mechanisms of aortic and cardiac dysfunction in uremic mice with aortic calcification. Circulation. 2009;119:306–313. doi: 10.1161/CIRCULATIONAHA.108.797407. [DOI] [PubMed] [Google Scholar]
- 23.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 24.Ravera M, Re M, Deferrari L, Vettoretti S, Deferrari G. Importance of blood pressure control in chronic kidney disease. J Am Soc Nephrol. 2006;17:S98–103. doi: 10.1681/ASN.2005121319. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh SS, Krieg RJ, Sica DA, Wang R, Fakhry I, Gehr T. Cardiac hypertrophy in neonatal nephrectomized rats: the role of the sympathetic nervous system. Pediatr Nephrol. 2009;24:367–377. doi: 10.1007/s00467-008-0978-8. [DOI] [PubMed] [Google Scholar]
- 26.Podjarny E, Bernheim J, Hasdan G, Karsh D, Rashid G, Green J, Katz B, Bernheim J. Additive renoprotective effect of candesartan and tetrahydrobiopterin in rats after 5/6 nephrectomy. Nephrol Dial Transplant. 2007;22:1864–1872. doi: 10.1093/ndt/gfm129. [DOI] [PubMed] [Google Scholar]
- 27.Hobo A, Yuzawa Y, Kosugi T, Kato N, Asai N, Sato W, Maruyama S, Ito Y, Kobori H, Ikematsu S, Nishiyama A, Matsuo S, Kadomatsu K. The growth factor midkine regulates the renin-angiotensin system in mice. J Clin Invest. 2009;119:1616–1625. doi: 10.1172/JCI37249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campese VM, Mozayeni P, Ye S, Gumbard M. High salt intake inhibits nitric oxide synthase expression and aggravates hypertension in rats with chronic renal failure. J Nephrol. 2002;15:407–413. [PubMed] [Google Scholar]
- 29.Chatziantoniou C, Boffa JJ, Tharaux PL, Flamant M, Ronco P, Dussaule JC. Progression and regression in renal vascular and glomerular fibrosis. Int J Exp Pathol. 2004;85:1–11. doi: 10.1111/j.0959-9673.2004.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet. 2001;357:1601–1608. doi: 10.1016/S0140-6736(00)04728-0. [DOI] [PubMed] [Google Scholar]
- 31.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Kwon TH, Frøkiaer J, Fernández-Llama P, Maunsbach AB, Knepper MA, Nielsen S. Altered expression of Na transporters NHE-3, NaPi-II, Na-K-ATPase, BSC-1, and TSC in CRF rat kidneys. Am J Physiol. 1999;277:F257–270. doi: 10.1152/ajprenal.1999.277.2.F257. [DOI] [PubMed] [Google Scholar]
- 33.Kim EJ, Jung YW, Kwon TH. Angiotensin II AT1 receptor blockade changes expression of renal sodium transporters in rats with chronic renal failure. J Korean Med Sci. 2005;20:248–255. doi: 10.3346/jkms.2005.20.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hruska KA, Saab G, Mathew S, Lund R. Renal osteodystrophy, phosphate homeostasis, and vascular calcification. Semin Dial. 2007;20:309–315. doi: 10.1111/j.1525-139X.2007.00300.x. [DOI] [PubMed] [Google Scholar]