Abstract
Advance therapies which effectively reduce mortality in lung cancer patients become a global health challenge nowadays. Sufficient knowledge regarding cellular and molecular basis of cancer progression and metastasis would possibly help in the development of novel and effective strategies for the treatment of this disease. This review focuses on the information regarding the role and mechanism of caveolin-1 (Cav-1) protein on anoikis in Non Small Cell Lung Cancer (NSCLC). NSCLC accounts of approximately 80% of all diagnosed lung cancer cases which patients frequently presented with metastatic disease. Like other cancers, caveolin-1 (Cav-1) has been shown to play an important role in regulating cancer-cells behaviors including apoptosis resistance and metastasis. Even though further investigations as well as in vivo experiments are required, these data are of great interest and may be beneficial to the development of lung cancer therapy.
Keywords: Caveolin-1 (Cav-1), anoikis resistance, Non Small Cell Lung Cancer (NSCLC), cancer therapy, apoptosis resistance, metastasis
Non small cell lung cancer
Lung cancer is a leading cause of cancer-related death in the United States and throughout the world. Among them, Non-small-cell lung cancer (NSCLC) is accounted for approximately 80% of overall lung cancer patients that have been diagnosed and the rest is small cell lung cancer (SCLC). Although the rate of growth and spreading of NSCLC is fewer than that of SCLC, as the majority type, NSCLC has garnered more pronounced attention in the cancer research field. Many patients with an earlier stage of NSCLC are potentially curable; however, NSCLC oftentimes relapse at distant metastatic sites, resulting in the dismal 5-year survival rate of only 15% for all patients. NSCLC most commonly metastasize to the adrenal glands, pancreas, liver, brain, and bone [1]. Like other cancers, malignant lung cancer cells have an ability to dissociate from the primary tumor, to invade adjacent tissue and to survive in the blood or lymphatic systems that allow them to travel to distant sites, settle, and to form new colonies. This phenomenon is termed as metastasis, which is the cause of 90% of human cancer deaths [2].
Caveolin-1
Caveolin-1 is a 22-kDa scaffold protein essential constituent of caveolae, a flask-shaped (50–100 nm) invagination that occupies up to 20% of the cell membrane [3]. Caveolin-1 belongs to a highly conserved gene family and is coexpressed with caveolin-2 in the cells and tissues of various origins including mesenchymal, endo/epithelial, and neuronal/glial [4]. The caveolin-1 gene composes of three exons which translate into the endoplasmic reticulum (ER) as a full-length 178 amino acids α-isoform and a β-isoform lacking the first 32 amino acids. The structure of caveolin-1 protein is membranespanning, where both N-termini and C-termini are exposed to the cytoplasm. A central membrane-spanning domain (TMD), C- and N-terminal membrane attachment domains (MAD) and three palmitoyl groups at the C-terminus enable its insertion into the inner leaflet of the membrane [5]. Caveolin-1 binds to cholesterol and sphingolipids within “lipid rafts” which are considered as specialized “detergent-insoluble cholesterol- and glycolipid-rich” (DIG) membrane microdomains [4]. When Cav-1 proteins are completely translated, they move out from endoplasmic reticulum as monomers, and assemble into higher molecular weight to become homo or hetero oligomers by using their oligomerization domain (amino acid 61-101), resulting in the forming of striated caveolar coat structure named caveolae [5]. This particular structure is of importance in regulating versatile cellular-transport processes such as cholesterol efflux, clathrin-independent endocytosis, lipid and protein sorting [6]. Moreover, Cav-1 is found in various vesicle-like compartments inside the cell including caveosome, exocytic vesicle, and cytoplasmic/lipid-droplet associated form.
Cav-1 protein has been shown to function as a scaffolding protein. The domain that exerts the interaction between Cav-1 and another partner molecule is caveolin-1 scaffolding domain (CSD) (residues 82-101) [3]. Interestingly, an evidence indicates that only a selected group of peptides showed high binding affinity to this CSD. The studies show that the preferable sequences matched to the CSD of Cav-1 are the following motifs: ΦXΦXXXXΦ, ΦXXXXΦXXΦ, ΦXΦXXXXΦXXΦ, where Φ is an aromatic residue (Phe, Tyr or Trp) [7]. Furthermore, a study of known cav-1 interacting molecules demonstrated that at least such a motif could be found in their sequence, indicating that these regions are particularly important for a direct interaction with the cav-1 [8]. Such interactions are mostly found to inhibit the interacting protein’s function. The examples of this inhibition effect of Cav-1 scaffolding activity are tyrosine and serine kinases [9].
Caveolin-1 and cancer
Sufficient lines of evidences including the in vivo studies have been shown that Cav-1 expression may attribute to the aggressiveness of cancer. Evidences regarding the role of Cav-1 in promoting drug resistance were demonstrated in human lung carcinoma, ovarian caricinoma, colon adenocarcinoma and breast adenocarcinoma cell lines. All of these drug resistant variants were found overexpression of Cav-1 [10]. Morover, highly invasive phenotype of lung adenocarcinoma had elevated Cav-1 levels [11]. Strikingly, secreting Cav-1 from prostate cancer cells were taken up by tumor cells and endothelial cells was shown to promote tumor angiogenesis [12]. The studies in lung metastases of mice with prostate cancer were found to have increased Cav-1 expression compared with the primary tumor [13]. In human, the lymph node metastases of human prostate and breast cancers were shown to have a higher Cav-1 level than those of normal epithelial tissues from prostate and breast [14]. These in vivo studies highlighted the role of Cav-1 as an oncogenic and pre-metastatic potential. Indeed, several types of cancer in human patients having increased in Cav-1 level were found decreasing in a survival rate [15]. However, contradictory results have also been obtained from mice with breast cancer. Genetic deletion of Cav-1 in vivo was shown to enhance tumorigenesis and lung metastasis [16]. To reconcile these contradictory findings, researchers proposed that the Cav-1 levels may vary during the course of tumor progression and metastasis [17]. In an early stage of cancer development, the absence or down-regulation of Cav-1 is necessary for facilitating oncogenic transformation; however, the re-expression or up-regulation of Cav-1 at later stages possibly confers the cancer cells’ potential to become drug resistance and metastatic cancer [13,17,18].
The role of Cav-1 in regulating cell survival and metastasis may be distinguishable between SCLC and NSCLC. Reduced or absent Cav-1 expression is found in approximately 95% of SCLC cases. On the other hand, the Cav-1 expression in NSCLC is exceeding 76% of overall NSCLC cases. In addition, Cav-1 expression in NSCLC was found well-correlated with the increased cell proliferation and metastatic potential assessed by liquid colony formation assay [19]. Clinicopathologic profiles of pulmonary squamous cell carcinomas, which are NSCLC subtypes, were shown that Cav-1 expression was associated with poorer prognosis than those in Cav-1 negative group [20]. The additional studies on the role of Cav-1 also extend the role of Cav-1 protein to the drug resistance capacity in NSCLC. In advanced NSCLC patients treated with gemcitabine-based chemotherapy, Cav-1 expression was found correlated with drug resistance and poor prognosis [21].
Role of caveolin-1 on anoikis resistance
As anoikis-resistant capability is the key step that enables cancer cells to succeed in their colonization at the secondary site, an intensive investigation to explore the mechanisms of anoikis resistance has been conducted. Most explanations involving anoikis resistance are dealing with the integrins, which sense the mechanical forces between cells and extracellular matrix (ECM). When the cell detaches from their ECM, the unligated integrins will act as a cell-death starter through the integrin-mediated death (IMD) process [22]. However, in certain cancer cells, anoikis resistance occurrs when the cells obtaining signals from constitutively activated downstream pro-survival pathways, such as PI3K, Ras-Erk, NF-kB and Rho GTPase, such death mediating caused by the loss of integrin signaling can be ignored [23]. The study that highlighted the role of Cav-1 in sustain Akt activation by inhibiting serine/threonine protein phosphatase PP1 and PP2A suggested that Cav-1 has a role in a survival pathway [24]. Recently, our colleague have demonstrated that Cav-1 can directly confer the anoikis resistance in NSCLC by the interaction with its antiapoptotic partner, Mcl-1 protein, and prevent the latter protein from the degradation by ubiquitin-proteasomal system [25]. Whether the direct effect of Cav-1 in stabilizing anti-apoptotic molecule or sustain the pro-survival signal, Cav-1 is an interesting candidate for further study on its role in controlling anoikis resistance by its pro-survival characteristic in this particular type of cancer.
The relationship of Cav-1 and Mcl-1 that has been investigated by our group leading us to take Mcl-1 into account that Mcl-1 may be the downstream target of Cav-1 and function dependently with Cav-1 in contributing to anoikis resistance. Based-on clinical and in vitro study of NSCLC, two main types of protein that frequently found overexpressed in this type of tumor are Cav-1 and Myeloid cell leukemia -1 (Mcl-1) proteins [26,27,32]. Mcl-1 is a prosurvival member of the Bcl-2 family that was initially identified as an immediate-early gene expressed during PMA-induced differentiation of ML-1 myeloid leukemia cells [28]. The ability of Mcl-1 that can inhibit apoptosis is involved with its ability to sequester proapoptotic proteins Bcl-2 homologous antagonist killer (Bak) and Bcl-2-associated protein X (Bax) which localizes at the mitochondrial membrane and in the cytoplasm, respectively. Mcl-1 is strikingly linked with poor prognosis of human breast cancer, in which the high level of Mcl-1 was shown to relate to high tumor grade and poor survival of breast cancer patients [29]. Moreover, in various kinds of cancer cell line, Mcl-1 stabilization by GSK-3β inactivation allows Mcl-1 to be more pronounced effects in tumorigenesis [30]. The evidence in the role of Mcl-1 on NSCLC tumorigenesis stem from the study of NSCLC resected from the patients. In the group of specimens that were evaluated, Mcl-1 seems to be overexpressed in larger proportion compared to a Bcl-2 overexpressed subgroup [31]. NSCLC cell lines including A549, H460 and H1299 that are abundant in Mcl-1 protein were found dramatically increased in an apoptotic rate when using antisense targeting Mcl-1 oligonucleotide [27]. Interestingly, Mcl-1 has been shown by sufficient studies to be necessary in inhibiting anoikis in cancers [33,34]. The overexpression of Mcl-1 in human lung cancer H460 cells strongly reduce cell apoptosis after detachment, while the short hair pin RNA-mediating Mcl-1 reduction dramatically enhances anoikis response in such cells [25]. These results align with the response from the differential expression of Cav-1 affecting the cell viability after the detachment that we previously demonstrated in H460 cells [35], then supporting our recent study that Cav-1 and Mcl-1 may work dependently on anoikis regulation.
During anoikis, the decrease of Cav-1 was reported in many studies [25,35,37]. This reduction was significant in the anoikis initiation. When properly formed, Cav-1 resides in the caveolae with a slow turnover rate and may be reduced via endosomal and ubiquitin-proteasomal pathways [36]. However, the study by Mundy et al. showed that targeting Cav-1 to the lysosome is not necessary to alter the degradation rate of Cav-1 [38]. Also, we performed the experiment in detached NSCLC cells and found that Cav-1 degradation during anoikis involved the ubiquitin proteasomal system but not transcription-related mechanism [25,37], suggesting that the ubiquitin-proteasomal pathway was the primary mode of Cav-1 downregulation after cell detachment in NSCLC.
Role of reactive oxygen species and nitric oxide on caveolin-1
Since mediators presenting in the microenvironment of the primary tumor are likely to impact anoikis behaviors of the cancer cells, studies regarding the effect of species frequently found in cancer environments on Cav-1 and cell behaviors like anoikis is of interest. Nitric oxide (NO), reactive nitrogen species often found in cancer environments, has been shown to be elevated in many human metastatic lung cancers [39-41]. Endogenous NO could be synthesized from amino acid L-arginine by NO synthases [44,45]. The evidences indicate the role of NO in a regulation of several physiological and pathological processes [44,45]. In certain cases, endogenous NO in appropriate amounts has been reported to inhibit apoptosis in several cell types [46,47]. Likewise, the study found that NSCLC cells treated with NO donors resulted in an increasing rate of anoikis resistance and an opposite effect could be found with NO inhibitors [37]. NO was shown to inhibit Cav-1 reduction after cell detachment by interfering with Cav-1 ubiquitination through the process regarding with protein S-nitrosylation [37]. As the cancer cells transfected with Cav-1-overexpressing plasmid showed a sufficient increase in Cav-1 and correlated resistance to anoikis, NO inhibited Cav-1 down-regulation in such study was shown to responsible for NOmediated anoikis resistance [37] (Figure 1).
Figure 1.
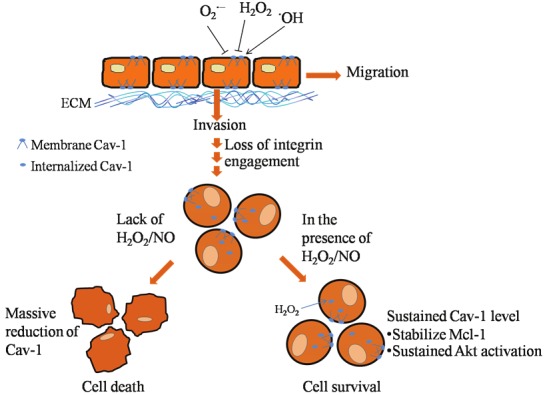
The scheme representing effects of reactive oxygen species and nitric oxide in regulation of cancer cell anoikis through a caveolin-1-dependent pathway. In the attached condition, hydroxyl radical enhances cancer cell migration and invasion by increasing the level of caveolin-1 protein, while hydrogen peroxide and superoxide anion play an opposite effect. Under cell detachment condition, the loss of cell-extracellular matrix interaction mediates caveolin-1 down-regulation and anoikis. Endogenous hydrogen peroxide functions in maintaining caveolin-1 level which subsequently suppressed anoikis. Also, exogenous hydrogen peroxide and nitric oxide sustains such caveolin-1 level and inhibited anoikis response of the cells. During anoikis, caveolin-1 inhibits anoikis via Mcl-1 and/or Akt-dependent pathways.
In terms of reactive oxygen species (ROS), ROS such as superoxide anion, hydrogen peroxide, and hydroxyl radical have been shown to be associated with several cancer cell behaviors. Indeed, ROS were found in a surrounded area of cancer cells since a high level of such mediators are produced by immune cells and cancer cells in response to inflammation [48,49]. A level or ROS has been shown to be dramatically up-regulated in the cancer-related tissue in comparison to that of normal tissue [50]. In attached lung cancer cells, cellular hydroxyl radical up-regulates the Cav-1 expression and promotes cell migration and invasion in NSCLC cells while superoxide anion and hydrogen peroxide played a negative role on cell migration and invasion by decreasing cellular Cav-1 level [51].
The evidence also highlights the role of endogenous hydrogen peroxide in reducing Cav-1 ubiquitination after cell detachment [35]. Cellular hydrogen peroxide and hydroxyl radical were shown to increase during NSCLC cell detachment. However, only hydrogen peroxide played a role in sustaining Cav-1 level during anoikis process and contributed anoikis resistance [35]. The transcriptional regulation should also take into account in the anoikis context; however, cav-1 mRNA level was shown to be unchanged during cell detachment [37,52]. Taken together, the mode of Cav-1 reduction during cell detachment was mainly due to the ubiquitin -proteasomal degradation pathway. Also, the detached lung cancer cells treated with exogenous hydrogen peroxide rendered cells resistant to anoikis by sustained Cav-1 level [35].
A distinct role of hydrogen peroxide in NSCLC is of interest since in detached condition, hydrogen peroxide sustained Cav-1 level [35] while in attached cell condition, hydrogen peroxide played an opposite effect [51]. These phenomenons fit with the explanation that detachment of the cells caused signal triggering Cav-1 degradation through the ubiquitn-proteasomal pathway and hydrogen peroxide could attenuate the process. While in the absence of detached signaling, the Cav-1 protein is quite stable in cellular caveolae [36] and could be degraded in response to oxidative stress mediated by superoxide anion and hydrogen peroxide [51] (Figure 1).
Summary
Understanding functions and underlying mechanisms which protein regulates cancer progression and metastasis are prerequisite to the development of novel cancer therapies. Even though clinical and in vivo data are of necessity, this knowledge based on cellular and molecular investigations offered a potential opportunity to develop a new strategy in treating NSCLC by targeting Cav-1 protein. Indeed, the present review indicated the possible role of Cav-1 in regulation anoikis resistance in NSCLC cells. Also, the impact of cancer microenvironment-derived mediators on cancer cell behaviors described in this review might draw an interest toward investigating those reactive species as a possible target in supporting lung cancer treatments.
Acknowledgement
The authors would like to thank postdoctoral fellowship (Ratchadaphiseksompot Endowment Fund, Chulalongkorn University) and Mr. Krich Rajprasit, a proofreader.
References
- 1.Vaporciyan AA, Nesbitt JC, Lee JS. Cancer of the Lung. In: Bast RC, Kufe DW, Pollock RE, Weichselbaum RR, Holland JF, Frie E, editors. Cancer Medicine. 5th ed. Ontario: BC Decker; 2000. pp. 1227–1292. [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmark of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Couet J, Lisanti MP. Src Tyrosine Kinases, Gα Subunits, and H-Ras Share a Common Membrane-anchored Scaffolding Protein, Caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinase. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 5.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 6.Sanna E, Miotti S, Mazzi M, Santis GD, Canevari S, Tomassetti A. Binding of nuclear caveolin-1 to promoter elements of growth-associated genes in ovarian carcinoma cells. Exp Cell Res. 2007;313:1307–1317. doi: 10.1016/j.yexcr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Razani B. Caveolae: From Cell Biology to Animal Physiology. Pharm Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 8.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 9.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 10.Lavie Y, Fiucci G, Liscovitch M. Upregulation of caveolae and caveolar constituents in multidrug-resistant cancer cells. J Biol Chem. 1998;273:32380–32383. doi: 10.1074/jbc.273.49.32380. [DOI] [PubMed] [Google Scholar]
- 11.Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol. 2002;161:1647–1656. doi: 10.1016/S0002-9440(10)64442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahir SA, Yang G, Ebara S, Timme TL, Satoh T, Li L, Goltsov A, Ittmann M, Morrisett JD, Thompson TC. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen insensitive prostate cancer. Cancer Res. 2001;61:3882–3885. [PubMed] [Google Scholar]
- 13.Lloyd PG, Hardin CD. Caveolae in cancer: two sides of the same coin? Focus on “Hydrogen peroxide inhibits non-small cell lung cancer cell anoikis through the inhibition of caveolin-1 degradation”. Am J Physiol Cell Physiol. 2011;300:C232–C234. doi: 10.1152/ajpcell.00483.2010. [DOI] [PubMed] [Google Scholar]
- 14.Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, Nasu Y, Bangma CH, Kattan MW, Scardino PT, Thompson TC. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4:1873–1880. [PubMed] [Google Scholar]
- 15.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27:715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 16.Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 17.Ravid D, Maor S, Werner H, Liscovitch M. Caveolin-1 inhibits anoikis and promotes survival signaling in cancer cells. Adv Enzyme Regul. 2006;46:163–175. doi: 10.1016/j.advenzreg.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 19.Sunaga N, Miyajima K, Suzuki M, Sato M, White MA, Ramirez RD. Different Roles for Caveolin-1 in the Development of Non-Small Cell Lung Cancer versus Small Cell Lung Cancer. Cancer Res. 2004;64:4277–4285. doi: 10.1158/0008-5472.CAN-03-3941. [DOI] [PubMed] [Google Scholar]
- 20.Yoo SH, Park YS, Kim HR, Sung SW, Kim JH, Shim YS. Expression of caveolin-1 is associated with poor prognosis of patients with squamous cell carcinoma of the lung. Lung Cancer. 2003;42:195–202. doi: 10.1016/s0169-5002(03)00287-3. [DOI] [PubMed] [Google Scholar]
- 21.Ho CC, Kuo SH, Huang PH, Huang HY, Yang CH, Yang PC. Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer. 2007;59:105–110. doi: 10.1016/j.lungcan.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:459–470. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji T, Ibaragi S, Hu GF. Epithelialmesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69:7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 Maintains Activated Akt in Prostate Cancer Cells through Scaffolding Domain Binding Site Interactions with and Inhibition of Serine/Threonine Protein Phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23:9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chunhacha P, Pongrakhananon V, Rojanasakul Y, Chanvorachote P. Caveolin-1 Regulates Mcl-1 Stability and Anoikis in Lung Carcinoma Cells. Am J Physiol Cell Physiol. 2012;302:C1284–C1292. doi: 10.1152/ajpcell.00318.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho CC, Kuo SH, Huang PH, Huang HY, Yang CH, Yang PC. Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer. 2007;59:105–110. doi: 10.1016/j.lungcan.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 Regulates Survival and Sensitivity to Diverse Apoptotic Stimuli in Human Non-Small Cell Lung Cancer Cells. Cancer Biol Ther. 2005;4:267–276. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- 28.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 29.Ding Q, He X, Xia W, Hsu JM, Chen CT, Li LY, Lee DF, Yang JY, Xie X, Liu JC, Hung MC. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3β activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007;67:4564–4571. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- 30.Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY, Lee DF, Liu JC, Zhong Q, Wang X, Hung MC. Degradation of Mcl-1 by β-TrCP Mediates Glycogen Synthase Kinase 3-Induced Tumor Suppression and Chemosensitization. Mol Cell Biol. 2007;11:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borner MM, Brousset P, Pfanner-Meyer B, Bacchi M, Vonlanthen S, Hotz MA, Altermatt HJ, Schlaifer D, Reed JC, Betticher DC. Expression of apoptosis regulatory proteins of the Bcl-2 family and p53 in primary resected non-smallcell lung cancer. Br J Cancer. 1999;79:952–958. doi: 10.1038/sj.bjc.6690152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Guttikonda S, Roberts L, Uziel T, Semizarov D, Elmore SW, Leverson JD, Lam LT. Mcl-1 is critical for survival in a subgroup of non-small-cell lung cancer cell lines. Oncogene. 2011;30:1963–1968. doi: 10.1038/onc.2010.559. [DOI] [PubMed] [Google Scholar]
- 33.Boisvert AK, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–556. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods NT, Yamaguchi H, Lee FY, Bhalla KN, Wang HG. Anoikis, initiated by Mcl-1 degradation and Bim induction, is deregulated during oncogenesis. Cancer Res. 2007;67:10744–10752. doi: 10.1158/0008-5472.CAN-07-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rungtabnapa P, Nimmannit U, Halim H, Rojanasakul Y, Chanvorachote P. Hydrogen peroxide inhibits non-small cell lung cancer cell anoikis through the inhibition of caveolin-1 degradation. Am J Physiol Cell Physiol. 2011;300:235–245. doi: 10.1152/ajpcell.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol. 2010;191:615–629. doi: 10.1083/jcb.201003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chanvorachote P, Nimmannit U, Lu Y, Talbott S, Jiang BH, Rojanasakul Y. Nitric oxide regulates lung carcinoma cell anoikis through inhibition of ubiquitin-proteasomal degradation of caveolin-1. J Biol Chem. 2009;284:28476–28484. doi: 10.1074/jbc.M109.050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mundy DI, Li PW, Luby-Phelps K, Anderson RG. Caveolin targeting to late endosome/lysosomal membranes is induced by perturbations of lysosomal pH and cholesterol content. Mol Biol Cell. 2012;23:864–880. doi: 10.1091/mbc.E11-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu CY, Wang CH, Chen TC, Lin HC, Yu CT, Kuo HP. Increased level of exhaled nitric oxide and up-regulation of inducible nitric oxide synthase in patients with primary lung cancer. Br J Cancer. 1998;78:534–541. doi: 10.1038/bjc.1998.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arias-Dìaz J, Vara E, Torres-Melero J, García C, Baki W, Ramσrez-Armengol JA, Balibrea JL. Nitrite/ nitrate and cytokine levels in bronchoalvelar lavage fluid of lung cancer patients. Cancer. 1994;74:1546–1551. doi: 10.1002/1097-0142(19940901)74:5<1546::aid-cncr2820740509>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 41.Fujimoto H, Ando Y, Yamashita T, Terazaki H, Tanaka Y, Sasaki J, Matsumoto M, Suga M, Ando M. Nitric oxide synthase activity in human lung cancer. Jpn J Cancer Res. 1997;88:1190–1198. doi: 10.1111/j.1349-7006.1997.tb00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, Nasu Y, Bangma CH, Kattan MW, Scardino PT, Thompson TC. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4:1873–1880. [PubMed] [Google Scholar]
- 43.Thompson TC. Metastasis-related genes in prostate cancer: the role of caveolin-1. Cancer Metastasis Rev. 1998-1999;17:439–442. doi: 10.1023/a:1006110326366. [DOI] [PubMed] [Google Scholar]
- 44.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 45.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 46.Chung HT, Pae HO, Choi BM, Billiar TR, Kim YM. Nitric oxide as a bioregulator of apoptosis. Biochem Biophys Res Commun. 2001;282:1075–1079. doi: 10.1006/bbrc.2001.4670. [DOI] [PubMed] [Google Scholar]
- 47.Chun SY, Eisenhauer KM, Kubo M, Hsueh AJ. Interleukin-1 beta suppresses apoptosis in rat ovarian follicles by increasing nitric oxide production. Endocrinology. 1995;136:3120–3127. doi: 10.1210/endo.136.7.7540548. [DOI] [PubMed] [Google Scholar]
- 48.Visconti R, Grieco D. New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel. 2009;12:240–5. [PubMed] [Google Scholar]
- 49.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, Richardson M, Rigaill G, Parrini MC, Lucchesi C, Bellanger D, Stern MH, Dubois T, Sastre-Garau X, Delattre O, Vincent-Salomon A, Mechta-Grigoriou F. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211–230. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luanpitpong S, Talbott SJ, Rojanasakul Y, Nimmannit U, Pongrakhananon V, Wang L, Chanvorachote P. Regulation of lung cancer cell migration and invasion by reactive oxygen species and caveolin-1. J Biol Chem. 2010;285:38832–38840. doi: 10.1074/jbc.M110.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravid D, Maor S, Werner H, Liscovitch M. Caveolin-1 inhibits cell detachment-induced p53 activation and anoikis by upregulation of insulin-like growth factor-I receptors and signaling. Oncogene. 2005;24:1338–1347. doi: 10.1038/sj.onc.1208337. [DOI] [PubMed] [Google Scholar]


