Abstract
Fragile X syndrome (FXS), one of the most common genetic causes of autism, results from a loss of fragile X mental retardation protein (FMRP) expression. At the molecular level, abnormal neurodevelopment is thought to result from dysregulated protein synthesis of key neural synaptic proteins, however recent evidence suggests broader roles for this protein including glutamate signaling, memory, and regulation of the critical serine/threonine regulatory kinase, glycogen synthase kinase-3 (GSK-3). In this review, genetic and molecular features of FXS are detailed in the context of FXS neuropathology. Additionally, potential mechanisms by which FMRP silencing impacts GSK-3 and GSK-3-associated signaling pathways are discussed. As GSK-3 signaling represents a central regulatory node for critical neurodevelopmental pathways, understanding how FXS results from FMRP-mediated GSK-3 dysregulation may provide novel therapeutic targets for disease-modifying interventions for FXS and related ASDs.
Keywords: Glycogen synthase kinase, fragile X, neuroinflammation, trinucucleotide repeat, microglia, lithium, flavonoids
Introduction
Fragile X syndrome (FXS), originally known as Martin-Bell syndrome [1] was first characterized in 1943 [2] is the most common cause of inherited mental retardation and is the first identified autism-related genetic disorder. The primary symptom of FXS is intellectual disability, but patients also share characteristics commonly associated with autism spectrum disorders (ASDs), such as developmental delays, communication impairments, and anxiety [3-9]. The most severely affected FXS individuals additionally display dysmorphic features, and other neurological pathology, including seizures. In this review, genetic and molecular features of FXS are detailed in the context of FXS neuropathology. Finally, potential mechanisms by which FMRP silencing impacts GSK3 and GSK3-associated signaling pathways are discussed. As GSK3 signaling represents a central regulatory node for critical neurodevelopmental pathways, understanding how FXS results from FMRP-mediated GSK3 dysregulation may provide novel therapeutic targets for disease-modifying interventions for FXS and related ASDs.
Eitiology of FXS
The first evidence regarding the molecular origin of FXS was generated in 1969, at which time a non-typical constriction, or fragile site, was observed at the end of the X chromosome in several affected individuals [10,11]. In 1991, the fragile site was mapped to a specific location in the genome [10]. The fragile X mental retardation 1 (Fmr1) gene on the X chromosome was found to yield a lack of the gene product, the fragile X mental retardation protein (FMRP), an RNA binding protein which regulates translation [3,12]. This functional loss typically occurs when there is an expansion of the CGG trinucleotide repeat in the 5′ untranslated region (5′ UTR) of the fragile X mental retardation 1 (FMR1) gene [13]. Healthy individuals usually have between 5 to 54 repeats, but fully affected individuals have greater than 200 CGG repeats on what are known as “full mutation alleles” [14]. Permutation alleles (55-200 CGG repeats) of the FMR1 gene contribute to the FXS phenotype through genetic instability and can expand into the full mutation during the process of germline transmission [15,16].
FMRP contains three RNA-binding domains and binds to a significant number of mRNAs. In in vitro studies, it has been found that dihydroxy-phenylglycine-activated protein synthesis in synaptoneurosomes is reduced in a mouse model of FXS (the Fmr1 knockout mouse), which cannot produce full-length FMRP, suggesting that FMRP is involved in this process. FMRP is generated in synaptoneurosomes in response glutamate or metabotropic glutamate receptor (mGluR) agonists. Moreover, Fmr1 knockout mice demonstrate a substantial reduction in the ability to translate mRNA in response to activation in an experimental synaptoneurosome preparation as well as a reduction in the presence of postsynaptic polyribosomal aggregates in vivo [17].
Neuronal morphology and function in FXS
FXS patients and murine models of FXS demonstrate increased long-term depression (LTD) in hippocampal synapses [18]. FMRP functions to inhibit the synthesis of proteins that stabilize LTD. With functional loss of this protein, metabotropic glutamate receptor-5 (mGluR5) remains active and increases the synthesis of proteins associated with LTD. Increased activation of mGluR5 (and consequent increase in glutamate activity) has been implicated in audiogenic seizure activity associated with FXS. A study utilizing an mGluR antagonist and lithium to treat Fmr1 knockout mice found that the treatment alleviated mGluR-induced LTD [18]. Visualization of dendrites and dendritic spines can be performed using Golgi staining, which allows for quantitative evaluation [19] of developmental pruning of neural processes [20-22]. In humans, spine density on the dendritic apical shafts of cortical pyramidal cells increases within the first few months of life [23]. Autopsy tissue of normal human subjects ranging in age from fetal to adult revealed synapse density peaks between 3 months and 3.5 years, depending on the cortical region in question [24,25]. Following this initial burst of synaptic development, synapses are selectively pruned, leaving synapse density measures at approximately 60% of their original peak numbers [26,27], although somewhat smaller losses are observed when neuron density is taken into account [25]. Regardless of the biological basis for this developmental delay, dendritic spine dysgenesis frequently characterizes neuronal morphology in disorders associated with intellectual disability [28]. Studies utilizing samples from patients with FXS have suggested that dendritic spines do not assume a normal mature size and shape and that there are more dendritic spines per unit dendrite length in the patient samples compared to unaffected individuals. Similar findings on spine size and shape have come from studies of FXS model mice in which the development of the somatosensory cortical region contains barrel-like cell arrangements that process whisker sensory information [29]. This suggests that normal dendritic pruning is impaired in the knockout mice [17] and indicates that FMRP may be required for the normal processes of maturation and elimination to occur in cerebral cortical development [17].
Structural magnetic resonance imaging (MRI) has shown a reduction in the size of the posterior cerebellar vermis which may result in the enlargement of the fourth ventricle in males with FXS [30,31]. Such gross morphological aberrations are not unique to the cerebellum, because the volume of hippocampus [32], caudate nucleus, and lateral ventricles [33,34] also have all been noted to be enlarged in FXS patients. The generalizability of these observations is controversial as several of these differences in brain morphology have not been replicated in a study using physical measurements of autopsy material from one underpowered study (2 FXS patients) [35].
At the molecular level, the consequence of the aforementioned CGG trinucleotide expansion in the 5' untranslated region of the FMR1 gene leads to a hypermethylation of the promoter region of the DNA, thus silencing transcription of the gene and resulting in the absence of FMRP. The function of FMRP has not yet been fully elucidated, although it is found to be associated with polyribosomal complexes near synapses and contains mRNA-binding domains. This suggests that it may be involved in mRNA transport or translation of proteins required for synaptic plasticity [36]. FMRP's role as an mRNA-binding protein is so critical for normal development that a point mutation in one of its RNA binding sites results in severe intellectual disability [37].
To test indirectly the role of FMRP in neuroplasticity, FMRP expression has been analyzed in rats after exposure to experimental paradigms known to induce synaptic plasticity. Regional increases in FMRP immunoreactivity were observed after training on a motor learning task or exposure to a complex environment [38]. It has also been shown that cortical levels of FMRP are elevated following sensory stimulation [39]. These observations suggest that the expression of FMRP is activity-dependent, and that the protein is involved in processes underlying synaptic plasticity [40]. Thus, it has been suggested that the loss of FMRP may lead to deficits in synaptic plasticity that could impair neuronal development [17].
Several other studies of FXS patients support that the syndrome is associated with dendritic spine dysgenesis, suggestive of abnormal neuronal development. In qualitative studies of Golgi-impregnated cortical neurons from human autopsy tissue, immature-appearing dendritic spine morphology has been described [41,42]. Specifically, long, thin, tortuous spines with prominent heads and irregular dilations on apical dendrites of pyramidal cells in layers III and V of parieto-occipital neocortex and in the pyramidal layer of allocortex have been observed. Investigators noted that this spine morphology was reminiscent of that described in children and infants with other disorders associated with intellectual disability, such as Down syndrome and Patau syndrome [43]. Decreased synaptic contact area also was found, but no other major neuropathologies were observed. The lack of altered neuronal density in FXS patients, with the absence of significant cortical atrophy in the MRI research, indicates normal neurogenesis and cell migration and no prominent atrophy of processes.
The role of GSK-3 in neurodevelopment
Glycogen synthase kinase-3 (GSK-3) regulates a variety of developmental processes, such as neurogenesis, gliogenesis, cell migration, cell morphology, and axonogenesis through interaction with a variety of signaling pathways [44-47]. GSK-3 is a partially constitutively active serine/threonine kinase that is predominantly modulated by inhibitory serine phosphorylation of its two isoforms, serine-9 on GSK-3β and serine-21 on GSK-3α [48-50].
FMRP is known to play a critical role in adult hippocampal neurogenesis and regulates adult neural stem cell (NSC) fate by modulating the translation of glycogen synthase kinase-β (GSK-3β) [51]. One study examined the effects of GSK-3β inhibition on Fmr1 knockout mice [51]. GSK-3β inhibition increased hippocampal neurogenesis and improved performance in hippocampal-dependent learning tasks. It is possible that while overall neuronal density is not significantly altered in FXS patients, a decrease in hippocampal neurogenesis through loss of FMRP and the resultant dysregulation of GSK3 contributes to the pathogenesis of the disorder.
Underlying this are changes in the inhibitory serine-phosphorylation, which has a robust impact on GSK-3 activity, as this is the cardinal mechanism by which it is regulated. The phosphoinositide-3-OH kinase (PI3K)/Akt pathway is an essential pathway for neuronal and glial survival and is also one of the main regulatory pathways for serine-phosphorylation of GSK3 [47,52]. However, GSK-3β can also be regulated by p38 mitogen-activated protein kinase (MAPK)-mediated inhibitory phosphorylation of serine-389 [53]. While GSK-3β and GSK-3β are expressed ubiquitously, GSK-3β2 is highly expressed in the central nervous system (CNS) and is found in highest concentrations during neurodevelopment [44].
GSK-3 inactivation has been associated with increased neuronal progenitor proliferation and suppressed neural differentiation. GSK-3 interacts with the canonical Wnt, sonic hedgehog (SHH), and Notch pathways to regulate proliferation [22,24,43]. The canonical Wnt/β-catenin signaling pathway involves Wnt binding to Frizzled receptors (Fzd) and Fzd binding to the protein Disheveled (Dvl). Dvl then binds and destabilizes the β-catenin destruction complex, which includes GSK-3. Therefore, GSK-3 regulates the canonical Wnt pathway by remaining bound to the Wnt complex, preventing β-catenin from translocating to the nucleus to induce gene transcription [54]. Inhibition of GSK-3 is necessary for β-catenin-mediated transcription. Wnt/β-catenin signaling is vital for adult hippocampal neurogenesis and is critical for CNS developmental processes, such as synapse and dendrite formation.
GSK-3 and fragile X syndrome
GSK-3 activity has been found to be elevated in murine models of FXS [57]. A recent study found that lithium administration increased inhibitory phosphorylation of GSK-3 isoforms, reduced audiogenic seizure activity, and improved performance on open field, elevated plus maze, and passive avoidance tests in Fmr1 knockout (KO) mice, and passive avoidance tests [52]. Fmr1 KO mice also display impaired sociability. Mines et al. (2010) found that GSK-3 inhibition with lithium improved the previously impaired social interaction of Fmr1 KO mice with a novel mouse [58].
GSK-3 activity is also associated with mGluR5 in that mGluR5 normally activates the PI3K/Akt pathway, which induces inhibitory phosphorylation of GSK-3. However, it has been shown that mGluR5 signaling and GSK-3 activities are both elevated in Fmr1 KO mice [57]. A study utilizing both lithium and the mGluR inhibitor 2-methyl-6-phenylethynyl-pyridine (MPEP) found that the inhibition of mGluR also led to the inhibition of GSK-3. Both treatments led to decreased audiogenic seizure activity and improvement on open field tests. However, treatment with both lithium and MPEP did not have an additive effect, suggesting that the pharmacologic agents may target the same signaling pathway.
Likewise, long-term depression has been found to be increased in Fmr1 KO mice [60]. One study found that Fmr1 knockout mice displayed less fear memory in contextual fear conditioning than wild-type mice and decreased long-term potentiation (LTP) in the anterior cingulated cortex and lateral amygdala (areas important for associative learning) [61].
Another way in which GSK-3 may be culpable in the cognitive deficits and altered brain pathology observed in FXS is through regulation of glycogenolysis. GSK-3 inhibits glycogen synthase, thus reducing glycogenolysis. Inhibition of glycogenolysis produces learning and memory deficits. Thus, the increased GSK-3 activity observed in FXS patients and Fmr1 KO mice may cause intellectual disability through negative regulation of glycogenolysis in the CNS.
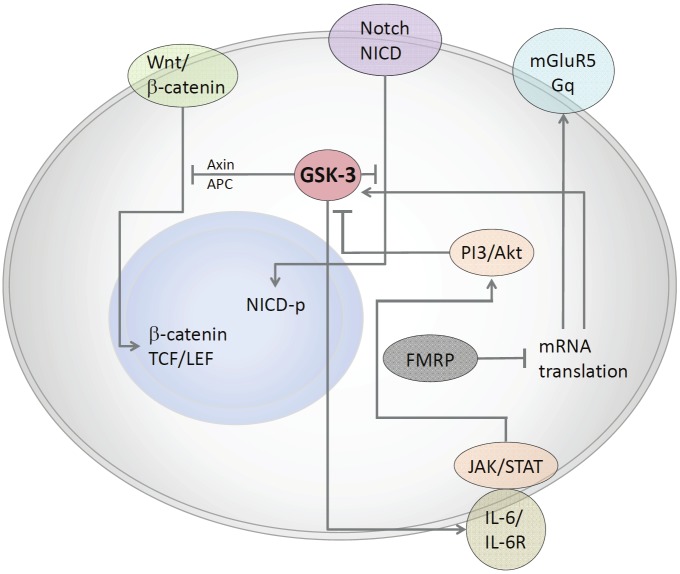
While it is evident that GSK-3 inhibition has a therapeutic effect in Fmr1 KO mice and that GSK-3 plays a role in neuronal morphology and proliferation, it is not clear whether and to what extent GSK-3 is responsible for Fragile X neuronal and brain pathogenesis. Further analysis is required to glean the role of GSK-3 in neurodevelopment in murine models of FXS (Figure 1).
Figure 1.
GSK3 regulates a variety of pathways involved in neurodevelopment. Active GSK3 regulates the canonical Wnt pathway by remaining bound to the Wnt/β-catenin destruction complex that includes APC and Axin. This complex targets β-catenin for proteasomal degradation. Inhibitory phosyphorylation of GSK3 releases β-catenin from the complex. It is then recruited to the nucleus by TCF/LEF where it induces gene transcription. GSK3β is known to phosphorylate and inhibit Notch, resulting in the inhibition of many Notch target genes. This inhibitory phosphorylation is reversed when Wnt1 is present. Inhibitory phosphorylation is induced by a number of kinases. The PI3/Akt pathway regulates GSK-3 activity by inducing inhibitory serine-phosphorylation. FMRP is deficient in Fragile X syndrome and is normally responsible for regulating mRNA transcription of metabotropic glutamate receptor-5 (mGluR5) and GSK3. It has been shown that inhibition of mGluR5 leads to the inhibition of GSK3. In addition to regulating neurodevelopmental pathways, it has been shown that GSK3 is also involved in pathways that promote inflammation. GSK3 induces IL-6 production, leading to the subsequent phosphorylation of Jak2/STAT3.
Potential therapy and future directions
FXS is generally believed to be a neuronal disorder due to the aforementioned behavioral and cognitive deficits and abnormalities in neuron morphology. Neuronal function is modulated by an array of immune cells and there is convincing evidence of neuronal dysfunction resulting from neuroinflammation [41,42]. Though a role for immune activation and associated inflammation in autism is controversial [62-64], there is evidence of activated glia in autism [65-68] and disregulated plasma cytokines associated with FXS [21]. Additionally, reactive astrocytes have been found in many brain regions of Fmr1 knockout mice. This pathology was attenuated with lithium treatment, providing further evidence of the involvement of GSK-3 in FXS [69]. Another study found that treatment of Fmr1 KO mice with minocycline (an antibiotic that exerts anti-inflammatory effects), improved dendritic spine formation and performance on behavioral tests [70].
It has been speculated that maternal immune activation (MIA) may play a role in the development of autism through activation of inflammatory pathways in utero [71]. MIA can negatively impact fetal brain development and may impair social behavior [72]. A study of MIA in normal mice revealed an increase in interleukin-6 (IL-6) [74]. IL-6 is known to induce phosphorylation of Janus kinase-2 (Jak2) and signal transducer and activator of transcription-3 (STAT3), leading to the release of proinflammatory cytokines, such as TNF-α and IL-1β. Treatment with the bioflavonoid diosmin reduced inflammation in the brain tissue of MIA offspring [72]. Another study found that GSK-3 and STAT3 enhance production of IL-6 after immune activation, GSK-3 was also found to be critical in the interferon-γ (IFN-γ)-induced activation of STAT3 [51]. Therefore, it is possible that quelling the inflammatory environment through modulation of GSK-3 is a mechanism by which a therapeutic effect may be achieved in Fmr1 KO mice.
Another way to accomplish this may be to use bioflavonoids to inhibit GSK-3 activity. It has been shown that GSK-3β activity is decreased in pancreatic cancer cells when they are treated with various citrus flavonoids [73,74]. The impetus for targeting GSK-3 in pancreatic cancer cells is that GSK-3β is over-expressed in the nucleus of these cells and causes nuclear factor-κB (NF-κB) to become active and induce an inflammatory cascade. Thus, attenuation of the inflammation leads to decreased cancer cell proliferation. Treatment with the bioflavonoid luteolin has also been shown to reduce amyloid plaques in a transgenic (Tg2576) mouse model of Alzheimer’s disease through modulation of GSK-3α [75]. To date, bioflavonoid-induced inhibition of GSK-3 has not been studied in other CNS-related disorders.
As noted earlier, lithium has been shown to be beneficial for Fmr1 KO mice in reducing the occurrence and severity of audiogenic seizures and ameliorating behavior deficits. However, cessation of lithium treatment has led to the reemergence of the FXS phenotype in Fmr1 KO mice [76]. Thus, lithium would have to be chronically administered to patients for the duration of the lifespan. Unfortunately, lithium can be highly toxic and may not be feasible as a prophylactic or therapeutic agent for pregnant mothers or young children [18,77]. Bioflavonoids and other anti-inflammatory agents that serve as GSK-3 inhibitors may prove to be more viable and safe therapeutic options in the future.
Acknowledgments
We wish to thank Dr. Michael Bengston and Dr. Tanya Murphy for the productive conversations regarding the clinical phenotypes of FXS. This work is supported by the Silver Foundation and NIH/NIMH (R21MH087849, J.T.).The authors declare no competing financial interests
References
- 1.Martin JP, Bell J. A Pedigree of Mental Defect Showing Sex-Linkage. J Neurol Psychiatry. 1943;6:154–157. doi: 10.1136/jnnp.6.3-4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- 3.Koukoui SD, Chaudhuri A. Neuroanatomical, molecular genetic, and behavioral correlates of fragile X syndrome. Brain Res Rev. 2007;53:27–38. doi: 10.1016/j.brainresrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9:1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- 5.Hagerman RJ, Ono MY, Hagerman PJ. Recent advances in fragile X: a model for autism and neurodegeneration. Curr Opin Psychiatry. 2005;18:490–496. doi: 10.1097/01.yco.0000179485.39520.b0. [DOI] [PubMed] [Google Scholar]
- 6.Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB Jr, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- 7.Kau AS, Tierney E, Bukelis I, Stump MH, Kates WR, Trescher WH, Kaufmann WE. Social behavior profile in young males with fragile X syndrome: characteristics and specificity. Am J Med Genet A. 2004;126A:9–17. doi: 10.1002/ajmg.a.20218. [DOI] [PubMed] [Google Scholar]
- 8.Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J Autism Dev Disord. 2007;37:738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 9.Loesch DZ, Bui QM, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, Tassone F, Taylor AK, Hessl D, Hagerman R, Huggins RM. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neurosci Biobehav Rev. 2007;31:315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland GR. Fragile sites on human chromosomes: demonstration of their dependence on the type of tissue culture medium. Science. 1977;197:265–266. doi: 10.1126/science.877551. [DOI] [PubMed] [Google Scholar]
- 11.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, Eussen BE, Van Ommen GJB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 12.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Warren ST, Sherman SL. The Fragile X syndrome. New York: McGraw-Hill Companies; 2001. [Google Scholar]
- 14.Sherman SL, Marsteller F, Abramowitz AJ, Scott E, Leslie M, Bregman J. Cognitive and behavioral performance among FMR1 high-repeat allele carriers surveyed from special education classes. Am J Med Genet. 2002;114:458–465. doi: 10.1002/ajmg.10303. [DOI] [PubMed] [Google Scholar]
- 15.Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proc Natl Acad Sci USA. 2010;107:9335–9339. doi: 10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagerman RJ, Hagerman PJ. The fragile X premutation: into the phenotypic fold. Curr Opin Genet Dev. 2002;12:278–283. doi: 10.1016/s0959-437x(02)00299-x. [DOI] [PubMed] [Google Scholar]
- 17.Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci USA. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messiha FS. Lithium and the neonate: developmental and metabolic aspects. Alcohol. 1986;3:107–112. doi: 10.1016/0741-8329(86)90020-0. [DOI] [PubMed] [Google Scholar]
- 19.Sholl DA. The measurable parameters of the cerebral cortex and their significance in its organization. Prog Neurobiol. 1956:324–333. [PubMed] [Google Scholar]
- 20.Brunjes PC, Schwark HD, Greenough WT. Olfactory granule cell development in normal and hyperthyroid rats. Brain Res. 1982;281:149–159. doi: 10.1016/0165-3806(82)90153-5. [DOI] [PubMed] [Google Scholar]
- 21.Falls W, Gobel S. Golgi and EM studies of the formation of dendritic and axonal arbors: the interneurons of the substantia gelatinosa of Rolando in newborn kittens. J Comp Neurol. 1979;187:1–18. doi: 10.1002/cne.901870102. [DOI] [PubMed] [Google Scholar]
- 22.Murphy EH, Magness R. Development of the rabbit visual cortex: a quantitative Golgi analysis. Exp Brain Res. 1984;53:304–314. doi: 10.1007/BF00238159. [DOI] [PubMed] [Google Scholar]
- 23.Marin-Padilla M. Number and distribution of the apical dendritic spines of the layer V pyramidal cells in man. J Comp Neurol. 1967;131:475–490. doi: 10.1002/cne.901310407. [DOI] [PubMed] [Google Scholar]
- 24.Michel AE, Garey LJ. The development of dendritic spines in the human visual cortex. Hum Neurobiol. 1984;3:223–227. [PubMed] [Google Scholar]
- 25.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 27.Huttenlocher PR, De Courten C, Garey LJ, van der Loos H. Synaptic development in human cerebral cortex. Int J Neurol. 1982;16-17:144–154. [PubMed] [Google Scholar]
- 28.Churchill JD, Grossman AW, Irwin SA, Galvez R, Klintsova AY, Weiler IJ, Greenough WT. A converging-methods approach to fragile X syndrome. Dev Psychobiol. 2002;40:323–338. doi: 10.1002/dev.10036. [DOI] [PubMed] [Google Scholar]
- 29.Greenough WT, Chang FL. Dendritic pattern formation involves both oriented regression and oriented growth in the barrels of mouse somatosensory cortex. Brain Res. 1988;471:148–152. doi: 10.1016/0165-3806(88)90160-5. [DOI] [PubMed] [Google Scholar]
- 30.Mostofsky SH, Mazzocco MM, Aakalu G, Warsofsky IS, Denckla MB, Reiss AL. Decreased cerebellar posterior vermis size in fragile X syndrome: correlation with neurocognitive performance. Neurology. 1998;50:121–130. doi: 10.1212/wnl.50.1.121. [DOI] [PubMed] [Google Scholar]
- 31.Reiss AL, Freund L, Tseng JE, Joshi PK. Neuroanatomy in fragile X females: the posterior fossa. Am J Hum Genet. 1991;49:279–288. [PMC free article] [PubMed] [Google Scholar]
- 32.Reiss AL, Lee J, Freund L. Neuroanatomy of fragile X syndrome: the temporal lobe. Neurology. 1994;44:1317–1324. doi: 10.1212/wnl.44.7.1317. [DOI] [PubMed] [Google Scholar]
- 33.Eliez S, Blasey CM, Freund LS, Hastie T, Reiss AL. Brain anatomy, gender and IQ in children and adolescents with fragile X syndrome. Brain. 2001;124:1610–1618. doi: 10.1093/brain/124.8.1610. [DOI] [PubMed] [Google Scholar]
- 34.Reiss AL, Abrams MT, Greenlaw R, Freund L, Denckla MB. Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat Med. 1995;1:159–167. doi: 10.1038/nm0295-159. [DOI] [PubMed] [Google Scholar]
- 35.Reyniers E, Martin JJ, Cras P, Van Marck E, Handig I, Jorens HZ, Oostra BA, Kooy RF, Willems PJ. Postmortem examination of two fragile X brothers with an FMR1 full mutation. Am J Med Genet. 1999;84:245–249. doi: 10.1002/(sici)1096-8628(19990528)84:3<245::aid-ajmg16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Weiler IJ, Greenough WT. Synaptic synthesis of the Fragile X protein: possible involvement in synapse maturation and elimination. Am J Med Genet. 1999;83:248–252. doi: 10.1002/(sici)1096-8628(19990402)83:4<248::aid-ajmg3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 37.De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJ. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993;3:31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- 38.Irwin SA, Swain RA, Christmon CA, Chakravarti A, Weiler IJ, Greenough WT. Evidence for altered Fragile-X mental retardation protein expression in response to behavioral stimulation. Neurobiol Learn Mem. 2000;74:87–93. [PubMed] [Google Scholar]
- 39.Todd PK, Mack KJ. Sensory stimulation increases cortical expression of the fragile X mental retardation protein in vivo. Brain Res Mol Brain Res. 2000;80:17–25. doi: 10.1016/s0169-328x(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 40.Linden DJ. The Accidental Mind: How Brain Evolution Has Given Us Love, Memory, Dreams, and God. Cambridge: Belknap Press of Harvard University Press; 2007. [Google Scholar]
- 41.Rudelli RD, Brown WT, Wisniewski K, Jenkins EC, Laure-Kamionowska M, Connell F, Wisniewski HM. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta Neuropathol. 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- 42.Wisniewski KE, Segan SM, Miezejeski CM, Sersen EA, Rudelli RD. The Fra(X) syndrome: neurological, electrophysiological, and neuropathological abnormalities. Am J Med Genet. 1991;38:476–480. doi: 10.1002/ajmg.1320380267. [DOI] [PubMed] [Google Scholar]
- 43.Marin-Padilla M. Structural abnormalities of the cerebral cortex in human chromosomal aberrations: a Golgi study. Brain Res. 1972;44:625–629. doi: 10.1016/0006-8993(72)90324-1. [DOI] [PubMed] [Google Scholar]
- 44.Castano Z, Gordon-Weeks PR, Kypta RM. The neuron-specific isoform of glycogen synthase kinase-3beta is required for axon growth. J Neurochem. 2010;113:117–130. doi: 10.1111/j.1471-4159.2010.06581.x. [DOI] [PubMed] [Google Scholar]
- 45.Ming GL, Song H. DISC1 partners with GSK3beta in neurogenesis. Cell. 2009;136:990–992. doi: 10.1016/j.cell.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu G, Huang H, Garcia Abreu J, He X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One. 2009;4:e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshimura T, Arimura N, Kawano Y, Kawabata S, Wang S, Kaibuchi K. Ras regulates neuronal polarity via the PI3-kinase/Akt/GSK-3beta/CRMP-2 pathway. Biochem Biophys Res Commun. 2006;340:62–68. doi: 10.1016/j.bbrc.2005.11.147. [DOI] [PubMed] [Google Scholar]
- 48.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. Embo J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beurel E, Jope RS. Glycogen synthase kinase-3 promotes the synergistic action of interferon-gamma on lipopolysaccharide-induced IL-6 production in RAW264.7 cells. Cell Signal. 2009;21:978–985. doi: 10.1016/j.cellsig.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem Pharmacol. 2010;79:632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- 55.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 56.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 57.Min WW, Yuskaitis CJ, Yan Q, Sikorski C, Chen S, Jope RS, Bauchwitz RP. Elevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potential. Neuropharmacology. 2009;56:463–472. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mines MA, Yuskaitis CJ, King MK, Beurel E, Jope RS. GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS One. 2010;5:e9706. doi: 10.1371/journal.pone.0009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 60.Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- 61.Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25:7385–7392. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beurel E, Jope RS. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflammation. 2009;6:9. doi: 10.1186/1742-2094-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 2009;21:264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 66.Dong WK, Greenough WT. Plasticity of non-neuronal brain tissue: roles in developmental disorders. Ment Retard Dev Disabil Res Rev. 2004;10:85–90. doi: 10.1002/mrdd.20016. [DOI] [PubMed] [Google Scholar]
- 67.Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- 68.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 69.Yuskaitis CJ, Beurel E, Jope RS. Evidence of reactive astrocytes but not peripheral immune system activation in a mouse model of Fragile X syndrome. Biochim Biophys Acta. 2010;1802:1006–1012. doi: 10.1016/j.bbadis.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 71.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parker-Athill E, Luo D, Bailey A, Giunta B, Tian J, Shytle RD, Murphy T, Legradi G, Tan J. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J Neuroimmunol. 2009;217:20–27. doi: 10.1016/j.jneuroim.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson JL, Rupasinghe SG, Stefani F, Schuler MA, Gonzalez de Mejia E. Citrus flavonoids luteolin, apigenin, and quercetin inhibit glycogen synthase kinase-3beta enzymatic activity by lowering the interaction energy within the binding cavity. J Med Food. 2011;14:325–333. doi: 10.1089/jmf.2010.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, Billadeau DD. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006;12:5074–5081. doi: 10.1158/1078-0432.CCR-06-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rezai-Zadeh K, Douglas Shytle R, Bai Y, Tian J, Hou H, Mori T, Zeng J, Obregon D, Town T, Tan J. Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer's disease beta-amyloid production. J Cell Mol Med. 2009;13:574–588. doi: 10.1111/j.1582-4934.2008.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi CH, Schoenfeld BP, Bell AJ, Hinchey P, Kollaros M, Gertner MJ, Woo NH, Tranfaglia MR, Bear MF, Zukin RS, McDonald TV, Jongens TA, McBride SM. Pharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 2011;1380:106–119. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kallen B, Tandberg A. Lithium and pregnancy. A cohort study on manic-depressive women. Acta Psychiatr Scand. 1983;68:134–139. doi: 10.1111/j.1600-0447.1983.tb06991.x. [DOI] [PubMed] [Google Scholar]