Abstract
Objective:
Rebleeding of an aneurysm is a leading cause of morbidity and mortality after subarachnoid hemorrhage (SAH). Whereas numerous studies have demonstrated the risk factors associated with rebleeding, few data on complications of rebleeding, including its effect on the development of delayed cerebral ischemia (DCI), are available.
Methods:
A nested case-control study was performed on patients with rebleeding and control subjects matched for modified Fisher scale, Hunt-Hess grade, age, and sex previously entered into a prospective database. Rebleeding was defined as new hemorrhage apparent on repeat CT with or without new symptoms. Incidence and time course of DCI and hospital complications were compared. A secondary analysis of DCI and hospital complications was also performed on subjects surviving to postbleed day 7.
Results:
We identified 120 patients with rebleeding and 359 control subjects from 1996 to 2011. The rebleeding rate was 8.6%. In both the primary and secondary analyses, there was no difference in the incidence of DCI or its time course (29% vs 27%, p = 0.6; 7 ± 5 vs 7 ± 6 days, p = 0.9 for primary analysis; 39% vs 31%, p = 0.1, 7 ± 5 vs 7 ± 6 days, p = 0.6 for the secondary analysis). In a multivariate logistic regression model, rebleeding was associated with the complications of hyponatremia, respiratory failure, and hydrocephalus. Patients with rebleeding had higher rates of mortality, brain death, and poor outcomes.
Conclusions:
Rebleeding after SAH is associated with multiple medical and neurologic complications, resulting in higher morbidity and mortality, but is not associated with change of incidence or timing of DCI.
Rebleeding after aneurysmal subarachnoid hemorrhage (SAH) is a feared complication and decreases the likelihood that patients will survive with functional independence.1,2 Despite studies demonstrating the safety of early clipping,3 rebleeding remains a leading cause of morbidity and mortality,4,5 because it often occurs within 6–24 hours of the presenting bleed.6–10 A number of clinical and radiographic factors are known to increase the risk for rebleeding, including clinical severity at admission, large aneurysm size, loss of consciousness or clinical seizure at ictus, and delayed referral to neurosurgical care.1,9–12 Whereas its contribution to morbidity and mortality is well established, the mechanism by which rebleeding drives poor outcomes is not known. With respect to the incidence and time course of cerebrovascular vasospasm, experimental models show that rebleeding increases the incidence of vasospasm and hastens its onset, but little is known in humans.13,14 In addition, limited data are available as to whether rebleeding increases morbidity and mortality due to primarily neurologic complications such as delayed cerebral ischemia (DCI)7,15 or poor outcomes can be ascribed to longer intensive care unit (ICU) and hospital stays and the medical complications that ensue. To this end, we designed a retrospective case-control study on patients entered into a prospective database to determine the associated medical and neurologic complications of rebleeding, and its effect on the development and time course of DCI.
METHODS
Patient selection and data collection.
We enrolled 1,391 patients with SAH admitted to the Neurological Intensive Care Unit of Columbia University Medical Center (New York, NY) from August 1996 to January 2011 in the Columbia University SAH Outcomes Project (SHOP) and identified 120 patients with rebleeding and selected 359 control patients (1:3 case patient/control patient ratio) matched for admission modified Fisher score, Hunt-Hess grade, age (dichotomized for ≥53 years, the average age in the full database), and sex. Matching criteria were based on known risk factors for development of DCI and general demographic factors. Control patients were randomly selected from a pool of patients meeting matching criteria to fulfill a 1:3 ratio of case patients to control patients. The study was conducted in 2 parts: the primary analysis was conducted on all study patients. The secondary analysis was conducted on the subgroup of study patients who were still alive on postbleed day 7, which in the general population of SAH patients is the period of greatest risk of vasospasm.
Clinical definitions.
SAH was diagnosed by the admission CT scan or by the presence of blood and xanthochromia of the CSF if the CT scan was not diagnostic. Rebleeding was defined as either an acute deterioration in neurologic status in conjunction with new hemorrhage apparent on a CT scan or an increase in hemorrhage burden on a repeat CT scan. The primary outcome measure was DCI, defined as either the presence of symptomatic vasospasm or the presence of an infarction on a CT scan attributable to vasospasm.16 Symptomatic vasospasm was defined as clinical deterioration (i.e., a new focal deficit, a decrease in level of consciousness, or both) in the presence of confirmed vasospasm determined by CT angiography or cerebral angiography. This definition of DCI has been demonstrated to have a better predictive value for poor outcomes than (1) clinical deterioration solely due to vasospasm, (2) the presence of angiographic vasospasm irrespective of clinical status, or (3) transcranial Doppler (TCD) mean flow velocity of more than 120 cm/s.16
Decreased level of consciousness was defined as a 2-point drop in the Glasgow Coma Scale score in a 24-hour period. All patients who experienced clinical deterioration underwent CT to rule out other causes of deterioration (e.g., fever, hydrocephalus, rebleeding, or cerebral edema), followed by medical or interventional therapy as indicated. All endpoints were classified according to a priori criteria and adjudicated weekly at a SHOP database meeting. The adjudication process involves a consensus agreement of each endpoint by neurocritical care faculty (N.B., K.L., J.C., S.A.M.) after a complete review of source documentation, imaging, and laboratory tests. Low serum sodium values were not recorded in the setting of severe hyperglycemia (>250 mg/dL).
All patients received an EKG on admission. According to our management protocol, creatinine kinase-MB (between 1995 and 1998) or troponin (after 1998) was measured in all patients with an abnormal EKG (Q waves, QTc prolongation, ST-segment abnormalities, or T-wave inversion) or clinical signs or symptoms of potential cardiovascular dysfunction (pulmonary edema, hypertension or hypotension [systolic blood pressure >160 or <100 mm Hg, respectively], cardiac dysrhythmia, or chest pain). We did not distinguish between enzyme elevations due to stunned myocardium and other causes.17
Clinical management.
The medical management of patients with SAH at our institution has been described previously,1 but we highlight here the management related to rebleeding and the treatment of DCI as well as changes made to management over the study period. Aneurysm repair was performed within 24 hours whenever possible. In 2003 we began the routine use of aminocaproic acid (4-g load followed by 1 g/hour) before aneurysm clipping or coiling. A previous study from our group showed that this treatment decreased rebleeding rates in the treated group (2.7%) vs that in control patients (11.4%).18 All patients received oral nimodipine. An external ventricular drain was placed in all patients with a depressed level of consciousness and intraventricular hemorrhage or acute hydrocephalus. TCD was performed daily, and in 2006 we began the routine use of CT angiography for both initial detection of aneurysms and detection of radiographic vasospasm. All patients received 0.9% normal saline at a dose of 1 mL/kg/hour, and supplemental 5% albumin solution was administered to maintain central venous pressure at >5 mm Hg. Patients experiencing symptomatic cerebral ischemia received hypertensive hypervolemic therapy, involving a target central venous pressure of >8 mm Hg, induced hypertension with phenylephrine or norepinephrine to maintain systolic blood pressure of 180–220 mm Hg, and maintenance of cardiac index at >4.0 L/minute/m2 with milrinone or dobutamine as needed. Patients with refractory DCI underwent endovascular therapy including intra-arterial verapamil hydrochloride, angioplasty, or both.
Radiographic interpretation.
CT scans were obtained on admission, at the occurrence of major clinical events at the discretion of the attending neurointensivist, and at 14 days or discharge, whichever was sooner. All CT scans performed at other hospitals were reviewed by study staff. Admission scans were prospectively rated for acute hemorrhage using the Fisher scale, modified Fisher scale,19 and Hijdra SAH sum score.20
Outcomes.
Functional and cognitive outcomes at 3 and 12 months were assessed with the modified Rankin Scale (mRS), a global disability scale with scores that range from 0 (no symptoms) to 6 (death).
Statistical analysis.
Baseline characteristics, in-hospital complications, and outcomes were compared between the 2 groups using 1- and 2-tailed Student t tests and the Mann-Whitney U test for continuous variables and Fisher exact and χ2 tests for categorical variables. To compare the time course of the development of vasospasm, we used Kaplan-Meier survival curves. In addition, variables found to be statistically significant on univariate analysis were entered into a backward Wald multiple logistic regression model to measure complications associated with rebleeding. Complications that occurred at a frequency of <5% were excluded from the final model. Pearson product-moment correlation and χ2 analysis were used to assess the degree of intercorrelation between the significant medical complications identified in the final model. A p value of 0.05 or less was considered statistically significant. All statistical calculations were made with widely available commercial software (SPSS, Chicago, IL). All attempts were made to minimize missing data, and patients with missing data were excluded from individual analyses.
Standard protocol approvals, registrations, and patient consents.
The SHOP database uses a tiered consent process, whereby consent is obtained from patients who were able to provide consent at the time of injury. In neurologically impaired patients, family members were approached for participation in the study. If capacity was regained, patients were directly approached for consent. This process of consent and the conduct of both studies were approved by the institutional review board and were consistent with guiding principles for research involving humans.21
RESULTS
Baseline characteristics.
The rebleeding rate for the study period was 8.6% (table 1). Patients with poor Hunt-Hess grade (3, 4, or 5) represented more than 90% of case patients and matched control patients. Patients with rebleeding had a higher mean APACHE II score and larger median aneurysm size and were more likely to have a seizure at ictus. Past medical history of diabetes mellitus and renal disease did not differ between groups, although there was a higher prevalence of premorbid hypertension within the rebleeding group. Thirty percent of patients in the rebleeding group and 12% in the control group died before day 7 (p < 0.001) and were not entered in the secondary analysis.
Table 1.
Patient characteristicsa
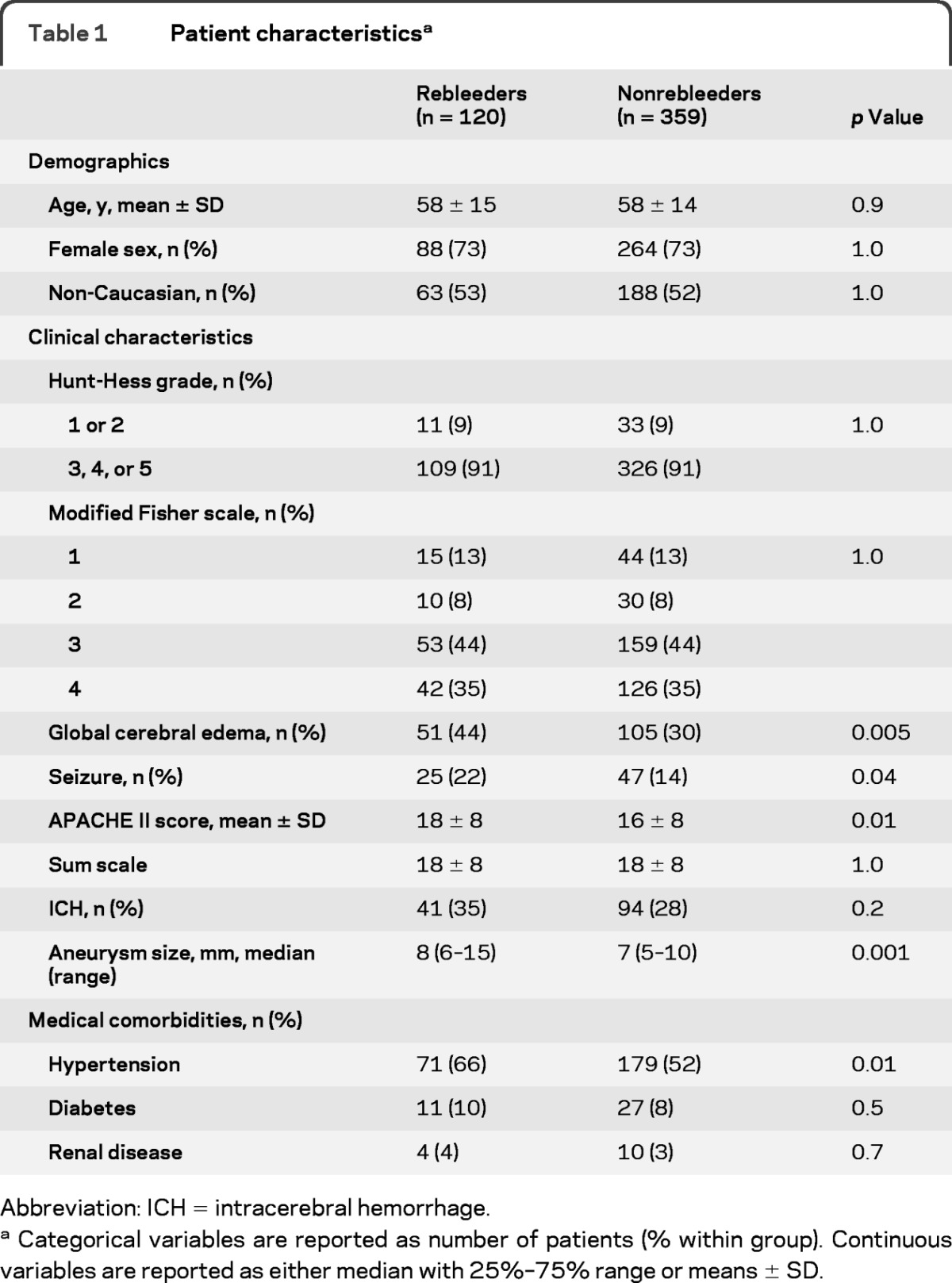
Abbreviation: ICH = intracerebral hemorrhage.
Categorical variables are reported as number of patients (% within group). Continuous variables are reported as either median with 25%–75% range or means ± SD.
Medical and neurologic complications.
The prevalence of hypotension (49% vs 33%, p = 0.002) and fever (temperature >38.5°C; 76% vs 62%, p = 0.004) was higher in patients with rebleeding, whereas the number of patients experiencing sepsis was similar (18% vs 12%, p = 0.1) (table 2). Patients with rebleeding were more likely to be intubated (90% vs 69%, p < 0.001), but the diagnosis of pneumonia was not different between the groups (36% vs 39%, p = 0.5). Rates of renal failure as well as hypernatremia and hyponatremia were higher in the rebleeding group.
Table 2.
Results
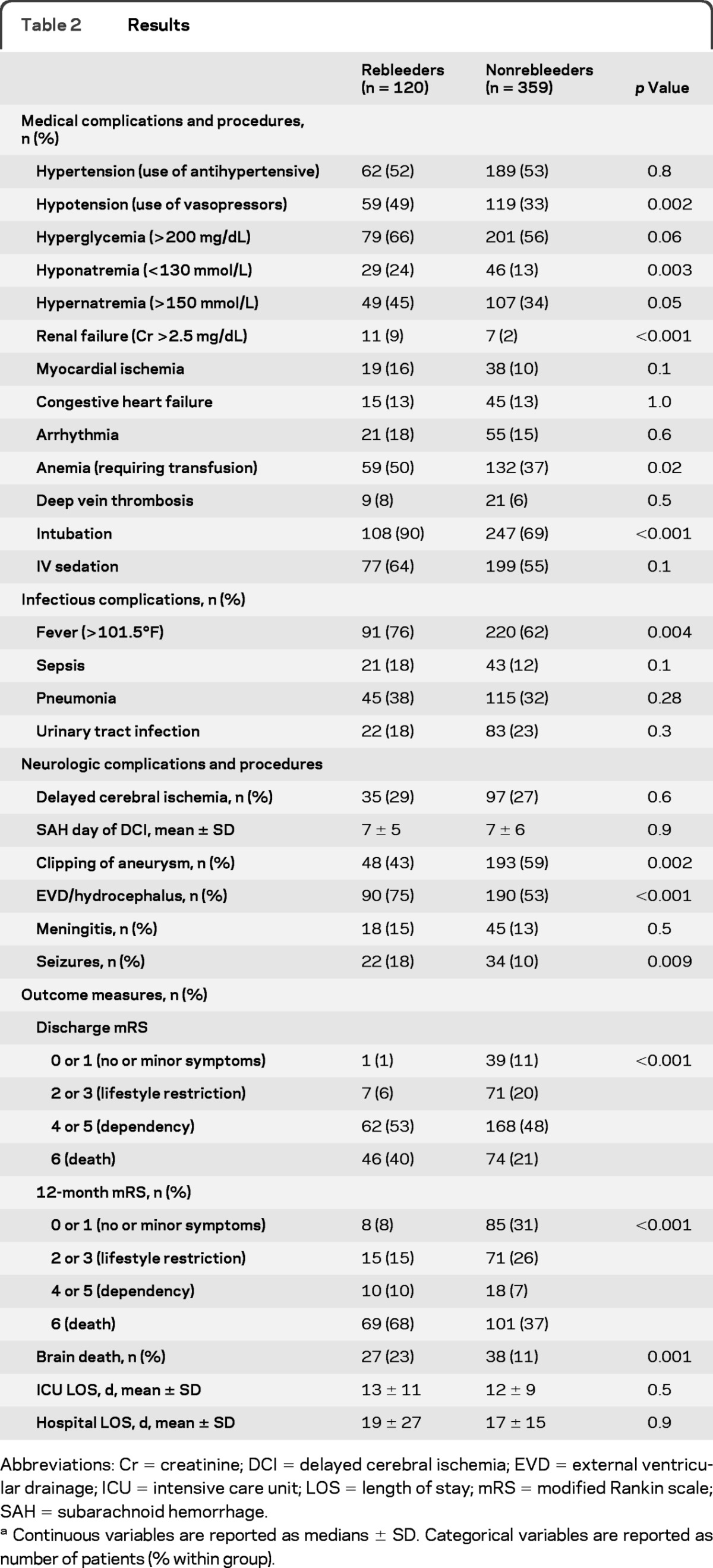
Abbreviations: Cr = creatinine; DCI = delayed cerebral ischemia; EVD = external ventricular drainage; ICU = intensive care unit; LOS = length of stay; mRS = modified Rankin scale; SAH = subarachnoid hemorrhage.
Continuous variables are reported as medians ± SD. Categorical variables are reported as number of patients (% within group).
Patients with rebleeding had higher rates of global cerebral edema, hydrocephalus, and seizures. They were also more likely to subsequently have their aneurysm clipped as opposed to coiled.
Rates of symptomatic vasospasm and DCI.
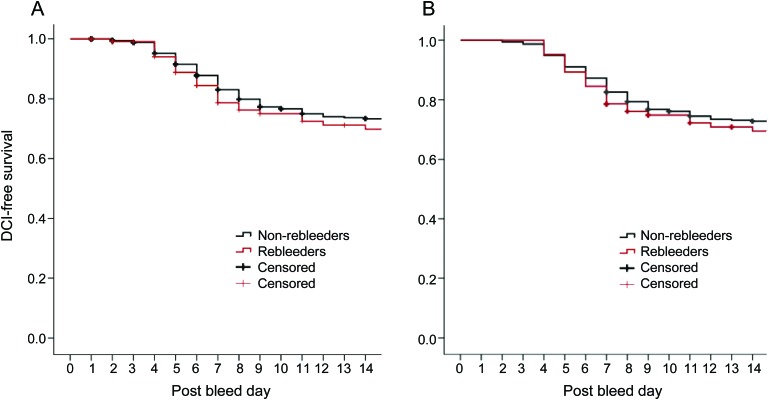
The percentage of patients developing DCI did not differ between the groups (29% vs 27%, p = 0.6) nor did the mean day of vasospasm onset (postbleed day 7 in both groups, p = 0.9). Table 3 shows the rates of the different components of DCI. There was no difference in the number of patients developing DCI due to its component criteria between the groups. The Kaplan-Meier curves demonstrate no meaningful difference in the time course of the development of vasospasm in the 2 groups (figure, A).
Table 3.
Rates of vasospasm and DCI
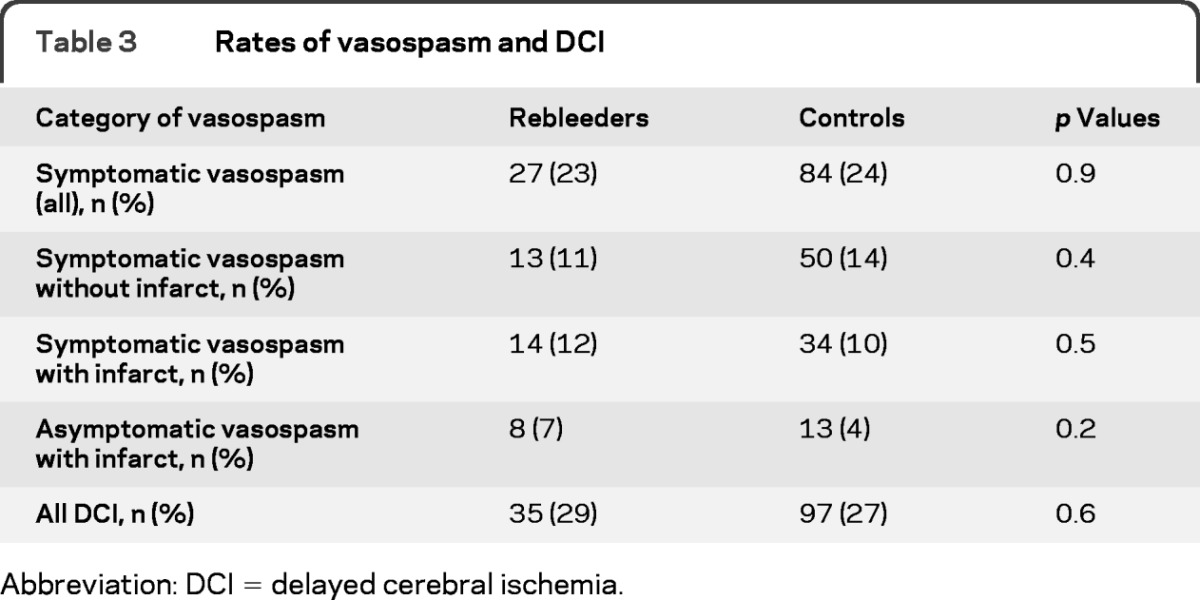
Abbreviation: DCI = delayed cerebral ischemia.
Figure. Kaplan-Meier curves for delayed cerebral ischemia (DCI)–free survival (A) in all patients and (B) in postbleed day 7 survivors.
Outcome measures.
Patients with rebleeding were more likely to progress to brain death and die during hospitalization. Hospital and ICU lengths of stay were similar between the groups. Twelve-month mRS scores were worse for patients with rebleeding, with only a small proportion recovering to functional independence.
Secondary analysis of survivors.
For those who survived to postbleed day 7, the percentage of patients developing DCI and the mean day of DCI onset (figure, B) did not differ between the groups (table 4). Patients with rebleeding who survived to day 7 were more likely to develop infectious complications, including sepsis and pneumonia, and the higher rates of medical complications seen in the rebleeding group of the primary analysis remained. Neurologic complications of global cerebral edema, hydrocephalus, and seizures also remained higher in the rebleeding group. Outcomes in those surviving to day 7 remained worse in the rebleeding group, with longer ICU and hospital lengths of stay, higher rates of brain death, and poor discharge and 12-month mRS scores.
Table 4.
Results for postbleed day 7 survivorsa
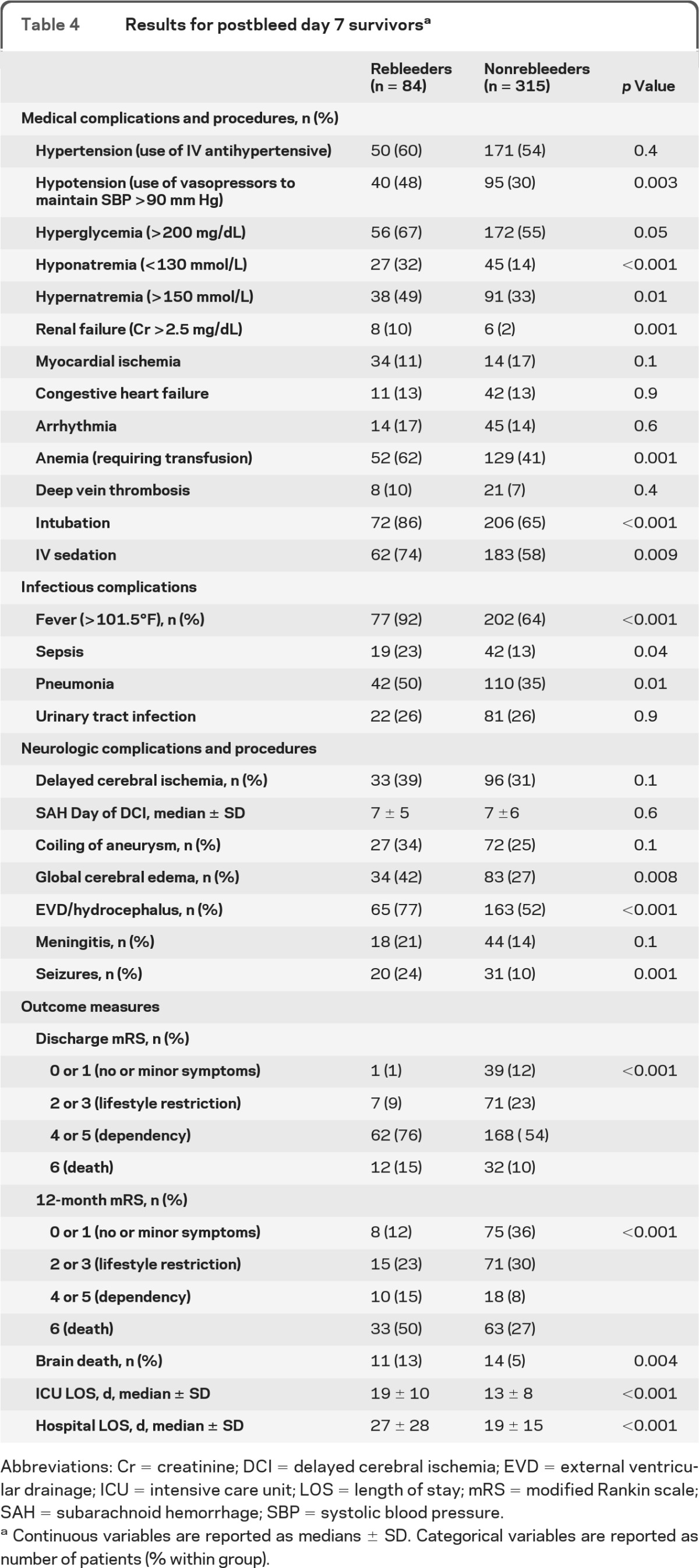
Abbreviations: Cr = creatinine; DCI = delayed cerebral ischemia; EVD = external ventricular drainage; ICU = intensive care unit; LOS = length of stay; mRS = modified Rankin scale; SAH = subarachnoid hemorrhage; SBP = systolic blood pressure.
Continuous variables are reported as medians ± SD. Categorical variables are reported as number of patients (% within group).
Multivariable logistic regression of medical complications.
In a backward Wald multivariable logistic regression of hospital complications, rebleeding was associated with hyponatremia (odds ratio [OR] 1.8, 95% confidence interval [CI] 1.04–3.2, p = 0.04), hydrocephalus (OR 1.8, 95% CI 1.1–3.1, p = 0.02), and intubation (OR 2.5, 95% CI 1.3–5.0, p = 0.008). For patients surviving to day 7, rebleeding was associated with hyponatremia (OR 2.0, 95% CI 1.1–3.6, p = 0.03), fever (OR 4.0, 95% CI 1.7–9.4, p = 0.001), and hydrocephalus (OR 1.9, 95% CI 1.1–3.7, p = 0.03).
DISCUSSION
We found that rebleeding is not associated with an increased risk of DCI and or a change in the time course of DCI development. This observation remained true even when only patients who survived to the period of greatest vasospasm risk were analyzed. As evidenced by the Kaplan-Meier curves, the 2 groups developed vasospasm at roughly the same rate from ictus.
We also identified an association between medical complications, including respiratory failure, hyponatremia, and hydrocephalus, and fever and rebleeding. Each of these measures may be a reflection of the increased injury severity incurred as a result of rebleeding. The high rates of medical complications in patients with rebleeding, a group with high mortality, is in line with previous data from our group showing the increasing contribution of medical complications to mortality in patients with SAH.22 In the subset of patients surviving to postbleed day 7, rebleeding was independently associated with more fever (temperature >38.6°C), with more than 90% of patients developing fever. We and others have previously reported the association between fever and poor outcomes, highlighting the need for aggressive fever control in patients with SAH.23–25
There are limitations to our study. The case-control design may have led to a selection bias. We attempted to minimize this by randomly selecting control patients from preset criteria and found few major differences between the 2 groups except for higher rates of premorbid hypertension, seizure at ictus, and worse APACHE II scores in the patients with rebleeding. This study also represents the experience at a single center and may not be generalizable to other centers. Repeating this study in a larger, multicenter database would be necessary to validate our main findings. We also did not prospectively track when complications occurred, which would have aided in our understanding of their importance to rebleeding, and the reason for intubation (procedural vs respiratory failure) was not recorded.
With increasingly strong evidence that early administration of an antifibrinolytic agent prevents rebleeding with minimal life-threatening complications,19 this report lends strength to the need for further study and promotion of this potentially life-saving therapy. This report also highlights the need to carefully study and manage medical complications in prospective trials of therapies designed to prevent rebleeding.
Glossary
GLOSSARY
- CI
confidence interval
- DCI
delayed cerebral ischemia
- ICU
intensive care unit
- mRS
modified Rankin scale
- OR
odds ratio
- SAH
subarachnoid hemorrhage
- SHOP
Columbia University SAH Outcomes Project
- TCD
transcranial Doppler.
AUTHOR CONTRIBUTIONS
Dr. Lord: drafting/revising the manuscript, study concept and design, analysis and interpretation of the data, statistical analysis. Dr. Fernandez: study concept and design, analysis and interpretation of the data. Dr. Schmidt: study concept and design, analysis and interpretation of the data. Dr. Mayer: drafting/revising the manuscript, study concept and design, analysis and interpretation of the data. Dr. Claassen: drafting/revising the manuscript, study concept and design, analysis and interpretation of the data. Dr. Lee: study concept and design. Dr. Connolly: study concept and design. Dr. Badjatia: drafting/revising the manuscript, study concept and design, analysis and interpretation of the data, statistical analysis.
DISCLOSURE
Dr. Lord and Dr. Fernandez report no disclosures. Dr. Schmidt has received research support from the NIH/NCRR. Dr. Mayer serves on scientific advisory boards for Edge Therapeutics, Inc., Orsan Medical Technologies Ltd., and Actelion Pharmaceuticals Ltd; has received speaker honoraria from Medivance and Astellas Pharma Inc.; receives publishing royalties for On Call: Neurology (WB Saunders, 2011), Neurological and Neurosurgical Intensive Care (Lippincott Williams & Wilkins, 2011), and Therapeutic Hypothermia (Marcel Dekker, 2010); serves as a consultant for Actelion Pharmaceuticals Ltd. and sanofi-aventis; and receives research support from the NIH and the Dana Foundation. Dr. Claassen reports no disclosures. Dr. Lee has served on scientific advisory boards for The Medicines Company and Baxter International Inc.; serves on speakers' bureaus for Baxter International Inc., UCB, Boehringer Ingelheim, The Medicines Company, and EKR Therapeutics Inc.; and has received research support from The Medicines Company. Dr. Connolly serves as a Section Editor for Neurosurgery and on the editorial boards of the Journal of Neurosurgery and World Neurosurgery; holds patent(s) re: Role of P-selectin and Complement in Stroke; receives publishing royalties for Handbook of Neurosurgery (Thieme, 2010); and receives research support from the FDA/NIH. Dr. Badjatia has received research support from the NIH/NCRR.
REFERENCES
- 1. Naidech AM, Janjua N, Kreiter KT, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol 2005; 62: 410–416 [DOI] [PubMed] [Google Scholar]
- 2. Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 1994; 25: 1342–1347 [DOI] [PubMed] [Google Scholar]
- 3. Kassell NF, Torner JC, Jane JA, Haley EC, Jr, Adams HP. The International Cooperative Study on the Timing of Aneurysm Surgery, part 2: surgical results. J Neurosurg 1990; 73: 37–47 [DOI] [PubMed] [Google Scholar]
- 4. Kassell NF, Torner JC, Haley EC, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery: part 1: overall management results. J Neurosurg 1990; 73: 18–36 [DOI] [PubMed] [Google Scholar]
- 5. Roos YB, Beenen LF, Groen RJ, Albrecht KW, Vermeulen M. Timing of surgery in patients with aneurysmal subarachnoid haemorrhage: rebleeding is still the major cause of poor outcome in neurosurgical units that aim at early surgery. J Neurol Neurosurg Psychiatry 1997; 63: 490–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laidlaw JD, Siu KH. Ultra-early surgery for aneurysmal subarachnoid hemorrhage: outcomes for a consecutive series of 391 patients not selected by grade or age. J Neurosurg 2002; 97: 250–258 [DOI] [PubMed] [Google Scholar]
- 7. Brilstra EH, Rinkel GJ, Algra A, van Gijn J. Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology 2000; 55: 1656–1660 [DOI] [PubMed] [Google Scholar]
- 8. Inagawa T, Kamiya K, Ogasawara H, Yano T. Rebleeding of ruptured intracranial aneurysm in the acute stage. Surg Neurol 1987; 28: 93–99 [DOI] [PubMed] [Google Scholar]
- 9. Laidlaw JD, Siu KH. Poor-grade aneurysmal subarachnoid hemorrhage: outcome after treatment with urgent surgery. Neurosurgery 2003; 53: 1275–1280 [DOI] [PubMed] [Google Scholar]
- 10. Steiger HJ, Fritschi J, Seiler RW. Current pattern of in-hospital aneurysmal rebleeds: analysis of a series treated with individually timed surgery and intravenous nimodipine. Acta Neurochir 1994; 127: 21–26 [DOI] [PubMed] [Google Scholar]
- 11. Rosenorn J, Eskesen V, Schmidt K, Ronde F. The risk of rebleeding from ruptured intracranial aneurysms. J Neurosurg 1987; 67: 329–332 [DOI] [PubMed] [Google Scholar]
- 12. Edner G, Ronne-Engstrom E. Can early admission reduce aneurysmal rebleeds? A prospective study on aneurysmal incidence, aneurysmal rebleeds, admission and treatment delays in a defined region. Br J Neurosurg 1991; 5: 601–608 [DOI] [PubMed] [Google Scholar]
- 13. Zabramski JM, Spetzler RF, Bonstelle C. Chronic cerebral vasospasm: effect of volume and timing of hemorrhage in a canine model. Neurosurgery 1986; 18: 1–6 [DOI] [PubMed] [Google Scholar]
- 14. Megyesi JF, Vollrath B, Cook DA, Findlay JM. In vivo animal models of cerebral vasospasm: a review. Neurosurgery 2000; 46: 448–460 [PubMed] [Google Scholar]
- 15. Gruber A, Dietrich W, Czech T, Richling B. Recurrent aneurysmal subarachnoid haemorrhage: bleeding pattern and incidence of posthaemorrhagic ischaemic infarction. Br J Neurosurg 1997; 11: 121–126 [DOI] [PubMed] [Google Scholar]
- 16. Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 2009; 40: 1963–1968 [DOI] [PubMed] [Google Scholar]
- 17. Naidech AM, Kreiter KT, Janjua N, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation 2005; 112: 2851–2856 [DOI] [PubMed] [Google Scholar]
- 18. Starke RM, Kim GH, Fernandez A, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke 2008; 39: 2617–2621 [DOI] [PubMed] [Google Scholar]
- 19. Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 2001; 32: 2012–2020 [DOI] [PubMed] [Google Scholar]
- 20. Hijdra A, van Gijn J, Nagelkerke NJ, et al. Prediction of delayed cerebral ischemia, rebleeding, and outcome after aneurysmal subarachnoid hemorrhage. Stroke 1988; 19: 1250–1256 [DOI] [PubMed] [Google Scholar]
- 21. American Physiological Society, World Medical Association General Assembly Guiding principles for research involving animals and human beings. Am J Physiol Cell Physiol 2002; 282: 3. [DOI] [PubMed] [Google Scholar]
- 22. Komotar RJ, Schmidt JM, Starke RM, et al. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery 2009; 64: 397–411 [DOI] [PubMed] [Google Scholar]
- 23. Badjatia N, Fernandez L, Schmidt JM, et al. Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case-control study. Neurosurgery 2010; 66: 696–700 [DOI] [PubMed] [Google Scholar]
- 24. Naidech AM, Bendok BR, Bernstein RA, et al. Fever burden and functional recovery after subarachnoid hemorrhage. Neurosurgery 2008; 63: 212–217 [DOI] [PubMed] [Google Scholar]
- 25. Fernandez A, Schmidt JM, Claassen J, et al. Fever after subarachnoid hemorrhage: risks factors and impact on outcome. Neurology 2007; 68: 1013–1019 [DOI] [PubMed] [Google Scholar]