Abstract
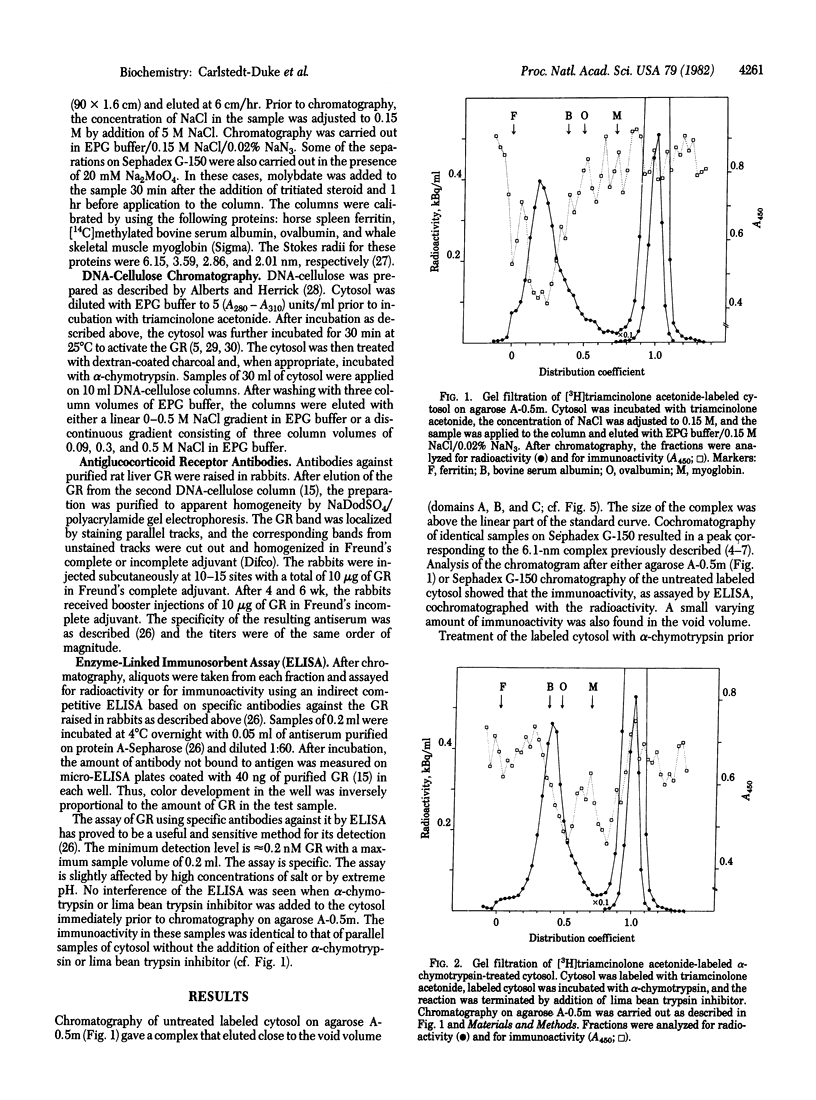
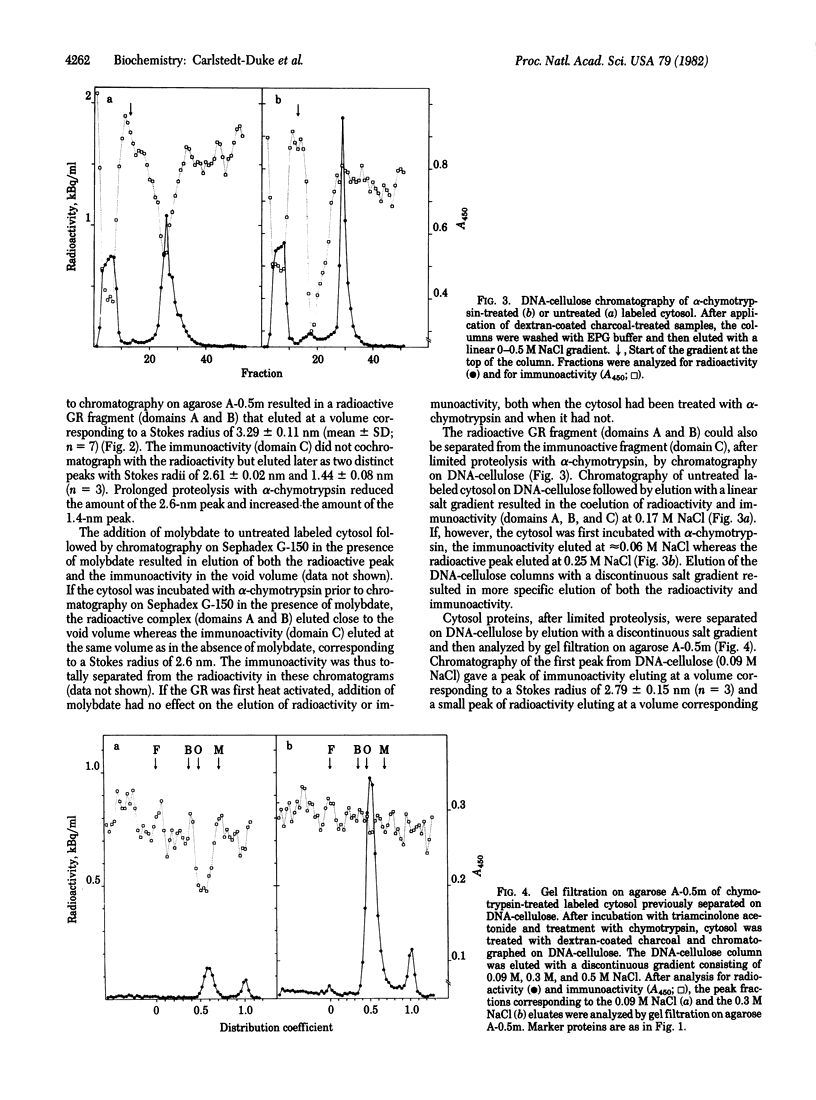
The glucocorticoid-receptor complex can be subdivided into three separate domains by limited proteolysis with trypsin or alpha-chymotrypsin. The following characteristics can be separated: steroid-binding activity (domain A), DNA-binding activity (domain B), and immunoactivity (domain C). We have previously reported the separation of the steroid-binding domain from the DNA-binding domain by limited proteolysis of the receptor with trypsin. In this paper, we report the detection by immunochemical analysis of a third domain of the glucocorticoid receptor, which does not bind hormone. Immunoactivity was detected by using specific antiglucocorticoid receptor antibodies raised in rabbits against purified rat liver glucocorticoid receptor and the assay used was an enzyme-linked immunosorbent assay. After digestion with alpha-chymotrypsin, the immunoactive region of the receptor (domain C) was separated from the other two domains (A and B). The immunoactive fragment was found to have a Stokes radius of 2.6 nm. Further digestion with alpha-chymotrypsin resulted in separation of the immunoactive fragment to give a fragment having a Stokes radius of 1.4 nm. The immunoactive domain could be separated from the half of the glucocorticoid receptor containing the steroid-binding and the DNA-binding domains (Stokes radius, 3.3 nm), by limited proteolysis of the receptor by alpha-chymotrypsin followed by gel filtration or chromatography on DNA-cellulose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atger M., Milgrom E. Mechanism and kinetics of the thermal activation of glucocorticoid hormone receptor complex. J Biol Chem. 1976 Aug 10;251(15):4758–4762. [PubMed] [Google Scholar]
- Carlstedt-Duke J., Gustaffson J. A., Wrange O. Formation and characteristics of hepatic dexamethasone-receptor complexes of different molecular weight. Biochim Biophys Acta. 1977 Apr 27;497(2):507–524. doi: 10.1016/0304-4165(77)90208-2. [DOI] [PubMed] [Google Scholar]
- Carlstedt-Duke J., Wrange O., Dahlberg E., Gustafsson J. A., Högberg B. Transformation of the glucocorticoid receptor in rat liver cytosol by lysosomal enzymes. J Biol Chem. 1979 Mar 10;254(5):1537–1539. [PubMed] [Google Scholar]
- Cidlowski J. A. Pyridoxal phosphate induced alterations in glucocorticoid receptor metabolism by proteases. Biochemistry. 1980 Dec 23;19(26):6162–6170. doi: 10.1021/bi00567a033. [DOI] [PubMed] [Google Scholar]
- Francke U., Gehring U. Chromosome assignment of a murine glucocorticoid receptor gene (Grl-1) using intraspecies somatic cell hybrids. Cell. 1980 Dec;22(3):657–664. doi: 10.1016/0092-8674(80)90541-3. [DOI] [PubMed] [Google Scholar]
- Garroway N. W., Orth D. N., Harrison R. W. Binding of cytosol receptor-glucocorticoid complexes by isolated nuclei of glucocorticoid-responsive and nonresponsive cultured cells. Endocrinology. 1976 May;98(5):1092–1100. doi: 10.1210/endo-98-5-1092. [DOI] [PubMed] [Google Scholar]
- Giannopoulos G. Glucocorticoid receptors in lung. I. Specific binding of glucocorticoids to cytoplasmic components of rabbit fetal lung. J Biol Chem. 1973 Jun 10;248(11):3876–3883. [PubMed] [Google Scholar]
- Giannopoulos G., Mulay S., Solomon S. Glucocorticoid receptors in lung. II. Specific binding of glucocorticoids to nuclear components of rabbit fetal lung. J Biol Chem. 1973 Jul 25;248(14):5016–5023. [PubMed] [Google Scholar]
- Govindan M. V. Isolation and characterization of rat liver nuclear glucocorticoid receptor. Biochim Biophys Acta. 1980 Aug 13;631(2):327–333. doi: 10.1016/0304-4165(80)90306-2. [DOI] [PubMed] [Google Scholar]
- Govindan M. V., Manz B. Three-step purification of glucocorticoid receptors from rat liver. Eur J Biochem. 1980;108(1):47–53. doi: 10.1111/j.1432-1033.1980.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Govindan M. V. Purification of glucocorticoid receptors from rat liver cytosol. Preparation of antibodies against the major receptor proteins and application of immunological techniques to study activation and translocation. J Steroid Biochem. 1979 Jul;11(1A):323–332. doi: 10.1016/0022-4731(79)90315-7. [DOI] [PubMed] [Google Scholar]
- Govindan M. V., Sekeris C. E. Purification of two dexamethasone-binding proteins from rat-liver cytosol. Eur J Biochem. 1978 Aug 15;89(1):95–104. doi: 10.1111/j.1432-1033.1978.tb20900.x. [DOI] [PubMed] [Google Scholar]
- Grove J. R., Dieckmann B. S., Schroer T. A., Ringold G. M. Isolation of glucocorticoid-unresponsive rat hepatoma cells by fluorescence-activated cell sorting. Cell. 1980 Aug;21(1):47–56. doi: 10.1016/0092-8674(80)90113-0. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Aronow L. Physicochemical properties of glucocorticoid receptors from mouse fibroblasts. Endocrinology. 1977 Feb;100(2):271–282. doi: 10.1210/endo-100-2-271. [DOI] [PubMed] [Google Scholar]
- Milgrom E., Atger M., Bailly A. Interaction of rat-liver glucocorticoid receptor with DNA. Eur J Biochem. 1976 Nov 1;70(1):1–6. doi: 10.1111/j.1432-1033.1976.tb10948.x. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K., Lan N. C., Showers M. O., Baxter J. D. Photoaffinity labeling of glucocorticoid receptors. J Biol Chem. 1981 Oct 25;256(20):10503–10508. [PubMed] [Google Scholar]
- Okret S., Carlstedt-Duke J., Wrange O., Carlström K., Gustafsson J. A. Characterization of an antiserum against the glucocorticoid receptor. Biochim Biophys Acta. 1981 Oct 12;677(2):205–219. doi: 10.1016/0304-4165(81)90087-8. [DOI] [PubMed] [Google Scholar]
- Payvar F., Wrange O., Carlstedt-Duke J., Okret S., Gustafsson J. A., Yamamoto K. R. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. R., Pickering L. A., Rollwagen F. M., Miller L. K. Mero-receptors: proteolytic fragments of receptors containing the steroid-binding site. Fed Proc. 1978 Feb;37(2):167–173. [PubMed] [Google Scholar]
- Sherman M. R., Tuazon F. B., Miller L. K. Estrogen receptor cleavage and plasminogen activation by enzymes in human breast tumor cytosol. Endocrinology. 1980 Jun;106(6):1715–1727. doi: 10.1210/endo-106-6-1715. [DOI] [PubMed] [Google Scholar]
- Stevens J., Eisen H. J., Stevens Y. W., Haubenstock H., Rosenthal R. L., Artishevsky A. Immunochemical differences between glucocorticoid receptors from corticoid-sensitive and -resistant malignant lymphocytes. Cancer Res. 1981 Jan;41(1):134–137. [PubMed] [Google Scholar]
- Stevens J., Stevens Y. W. Influence of limited proteolysis on the physicochemical and DNA-binding properties of glucocorticoid receptors from corticoid-sensitive and -resistant mouse lymphoma P1798. Cancer Res. 1981 Jan;41(1):125–133. [PubMed] [Google Scholar]
- Stevens J., Stevens Y. W. Physicochemical differences between glucocorticoid-binding components from the corticoid-sensitive and -resistant strains of mouse lymphoma P1798. Cancer Res. 1979 Oct;39(10):4011–4021. [PubMed] [Google Scholar]
- Stevens J., Stevens Y. W., Rosenthal R. L. Characterization of cytosolic and nuclear glucocorticoid-binding components in human leukemic lymphocytes. Cancer Res. 1979 Dec;39(12):4939–4948. [PubMed] [Google Scholar]
- Tsawdaroglou N. G., Govindan M. V., Schmid W., Sekeris C. E. Dexamethasone-binding proteins in cytosol and nucleus of rat thymocytes. Purification of three receptor proteins. Eur J Biochem. 1981;114(2):305–313. doi: 10.1111/j.1432-1033.1981.tb05150.x. [DOI] [PubMed] [Google Scholar]
- Westphal H. M., Beato M. The activated glucocorticoid receptor of rat liver. Purification and physical characterization. Eur J Biochem. 1980 May;106(2):395–403. doi: 10.1111/j.1432-1033.1980.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Wrange O., Carlstedt-Duke J., Gustafsson J. A. Purification of the glucocorticoid receptor from rat liver cytosol. J Biol Chem. 1979 Sep 25;254(18):9284–9290. [PubMed] [Google Scholar]
- Wrange O., Gustafsson J. A. Separation of the hormone- and DNA-binding sites of the hepatic glucocorticoid receptor by means of proteolysis. J Biol Chem. 1978 Feb 10;253(3):856–865. [PubMed] [Google Scholar]
- Yamamoto K. R., Gehring U., Stampfer M. R., Sibley C. H. Genetic approaches to steroid hormone action. Recent Prog Horm Res. 1976;32:3–32. doi: 10.1016/b978-0-12-571132-6.50008-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Stampfer M. R., Tomkins G. M. Receptors from glucocorticoid-sensitive lymphoma cells and two clases of insensitive clones: physical and DNA-binding properties. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3901–3905. doi: 10.1073/pnas.71.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]



