Abstract
Recent studies from our laboratory revealed that enterocyte brush border microvilli release small vesicles laden with host defense machinery into the intestinal lumen. In this addendum, we introduce a multi-faceted model for the function of these lumenal vesicles in the gut; we also consider some of the important unanswered questions that must be addressed in order to develop our understanding of this novel aspect of innate intestinal immunity.
Keywords: alkaline phosphatase, brush border, lipopolysaccharide, myosin-1a, vesicle
The intestinal lumen is home to an astoundingly large population of microbes, many of which contribute to normal physiological function.1 To prevent these and other, potentially pathogenic bacteria from stimulating inflammation in host tissues, the lumen is enclosed by a tightly regulated barrier formed from a monolayer of intestinal epithelial cells, also referred to as ‘enterocytes’. Enterocytes carry out both absorption and barrier functions in part via an apical array of actin-based microvilli collectively known as the brush border. While a significant percentage of brush border proteins are involved in nutrient processing, nutrient absorption, and the maintenance of microvillar structure, others play roles in host defense.2 Among the most abundant of these is intestinal alkaline phosphatase (IAP), a GPI-anchored enzyme responsible for cleaving phosphate groups from bacterial compounds, including the potent pro-inflammatory toxin, lipopolysaccharide (LPS).3 Dephosphorylation of these molecules renders them less effective at activating host cell toll-like receptors (TLRs)4,5. Studies in the last decade have demonstrated that IAP expression is stimulated by intestinal microbes6 and that the resulting higher levels of IAP protect against microbe-induced inflammation.7,8 Moreover, treatment with exogenous IAP can limit inflammation upon toxigenic insult in vivo.9,10 Thus, IAP is a critical component of innate intestinal immunity.
Work in our laboratory previously demonstrated that specialized membrane vesicles (lumenal vesicles, LVs) are released from the distal tips of enterocyte microvilli.11,12 Biochemical analysis of LVs revealed that IAP11 is concentrated on the lumenal surface of these membranes11; such topology would allow the enzyme access to lumenal toxins and further suggested a physiological function for LVs in host defense. Our more recent studies showed that LVs are in fact able to interact with and limit the pro-inflammatory potential of both bacteria and bacterial products.13 Based on these recently published findings, we propose a multi-faceted model for LV function in gut host defense (Fig. 1). First, IAP-enriched LVs are released into the intestinal lumen where they likely function in detoxifying pro-inflammatory bacterial toxins such as LPS, which accumulate during the bacterial life cycle. Second, LVs also bind directly to bacteria, which may serve to aggregate microbes and facilitate their clearance from the intestine, or prevent the adherence of virulent species to the enterocyte apical surface, thereby reducing the probability of infection. Finally, because LVs also exhibit bactericidal properties, they might regulate the gut microbiota by preventing harmful overgrowth.
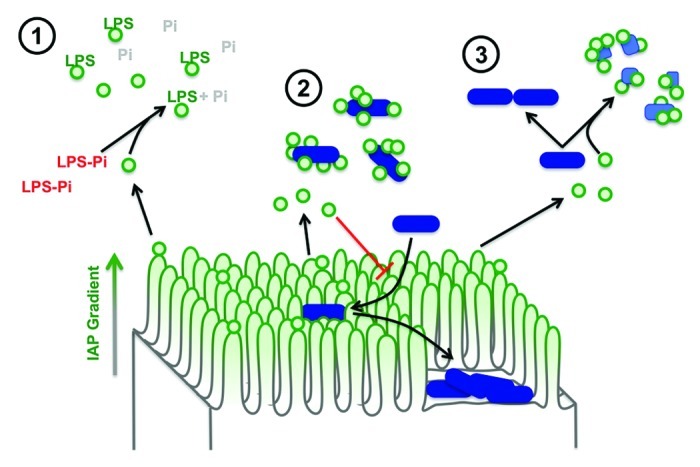
Figure 1. A multi-faceted role for LVs in gut homeostasis. The enterocyte brush border produces IAP-enriched LVs, which are found both in the gut lumen and trapped in the mucus layer. As a result, LVs are well positioned to: 1) detoxify soluble bacterial LPS using IAP catalytic activity, 2) bind lumenal bacteria to facilitate clearance and to prevent potential pathogens from adhering to the epithelial surface, and 3) help regulate microbial populations by exerting a bactericidal effect.
One important question is whether LVs are produced in large enough numbers to exert a significant effect on gut homeostasis. From a purely theoretical standpoint, the vast numbers of microvilli that extend into the lumen suggest an enormous potential for LV production to impact mucosal physiology. For example, the rodent small bowel contains approximately ~1012 microvilli and an estimated ~108 microbes. Thus, LV producing organelles outnumber bacterial organisms by at least four orders of magnitude. It is also worth noting that our recently described studies of LV function13 were performed using at most a concentration of 200 μg/ml (total LV protein). This level is equivalent to the physiological concentration of LVs captured from lumen wash preparations and was sufficient to produce robust effects in assays of bacterial attachment and growth.13 Finally, although LVs can be collected from lumen wash samples,11,13 our preliminary ultrastructural and immuno-fluorescence analyses suggest that a large fraction of LVs are trapped in the mucus layer that protects enterocytes. Such trapping is likely to further increase the effective lifetime and thus, the steady-state level of LVs present in the gut lumen.
If LV production does play a role in limiting host-microbe interactions in the gut, defective vesicle production would be expected to produce significant perturbations in gut homeostasis such as inflammation and a dysregulated microbiota. Mice that lack the shedding motor, myosin-1a (Myo1a), produce fewer LVs and those that are formed lack the characteristic enrichment of IAP.11 These animal do shows signs of chronic low level inflammation, including a greater number of goblet cells per villus, increases in the cytokines IL-1α and IL-6, and shifts in the small intestine microbiota at the family level (unpublished observations). Despite these differences, however, Myo1a KO mice show no overt phenotype,14 most likely as a result of compensatory mechanisms such as the expression of the closely related motor, myosin-1d.15 In this light, additional animal models that demonstrate more complete loss of LV production must be developed before we can fully appreciate the full range of LV functions in vivo.
Several important questions regarding the mechanism of LV production remain unanswered. How is the GPI-anchored protein IAP targeted to microvillar tips, and why is this enrichment lost in the Myo1a KO brush border11? Our unpublished observations show that microvillar tips are enriched in cholesterol, which provides the backbone for lipid raft formation.16 Moreover, microvilli and LVs both contain raft-associated annexin A2 as well as A13b,2,17 proteins that have been implicated in membrane organization and deformation.18 Thus, one possibility is that a distinct lipid raft-like environment, which favors high membrane curvature, exists at the distal tips of microvilli and helps to attract machinery that might drive LV budding. High levels of cholesterol would also favor the enrichment of IAP, which is anchored to the external leaflet via a GPI-anchor.19 An alternative possibility is that ESCRT complex components mediate vesicle formation. ESCRT complexes are involved in membrane abscission, and as such play a role in viral budding, cytokinesis and multi-vesicular body formation.20 Given that these events are topologically similar to LV release from microvillar tips, ESCRT proteins are also good candidates for driving this process. Beyond these speculative points, a detailed investigation of protein and lipid composition at microvillar tips has yet to be described, but will be needed before we fully understand the molecular mechanism of this novel facet of gut host defense.
Acknowledgments
This work was supported by National Institutes of Health DK075555 (MJT), American Heart Association Predoctoral Fellowship (DAS), and a Vanderbilt University Innovation and Discovery in Engineering And Science award (MJT).
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/21247
References
- 1.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.McConnell RE, Benesh AE, Mao S, Tabb DL, Tyska MJ. Proteomic analysis of the enterocyte brush border. Am J Physiol Gastrointest Liver Physiol. 2011;300:G914–26. doi: 10.1152/ajpgi.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poelstra K, Bakker WW, Klok PA, Kamps JA, Hardonk MJ, Meijer DK. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am J Pathol. 1997;151:1163–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–25. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 5.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–5. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 6.Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol. 2006;297:374–86. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–82. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramasamy S, Nguyen DD, Eston MA, Alam SN, Moss AK, Ebrahimi F, et al. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm Bowel Dis. 2011;17:532–42. doi: 10.1002/ibd.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beumer C, Wulferink M, Raaben W, Fiechter D, Brands R, Seinen W. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J Pharmacol Exp Ther. 2003;307:737–44. doi: 10.1124/jpet.103.056606. [DOI] [PubMed] [Google Scholar]
- 10.van Veen SQ, van Vliet AK, Wulferink M, Brands R, Boermeester MA, van Gulik TM. Bovine intestinal alkaline phosphatase attenuates the inflammatory response in secondary peritonitis in mice. Infect Immun. 2005;73:4309–14. doi: 10.1128/IAI.73.7.4309-4314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McConnell RE, Higginbotham JN, Shifrin DA, Jr., Tabb DL, Coffey RJ, Tyska MJ. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol. 2009;185:1285–98. doi: 10.1083/jcb.200902147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConnell RE, Tyska MJ. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J Cell Biol. 2007;177:671–81. doi: 10.1083/jcb.200701144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shifrin DA, Jr., McConnell RE, Nambiar R, Higginbotham JN, Coffey RJ, Tyska MJ. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr Biol. 2012;22:627–31. doi: 10.1016/j.cub.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell. 2005;16:2443–57. doi: 10.1091/mbc.E04-12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benesh AE, Nambiar R, McConnell RE, Mao S, Tabb DL, Tyska MJ. Differential localization and dynamics of class I myosins in the enterocyte microvillus. Mol Biol Cell. 2010;21:970–8. doi: 10.1091/mbc.E09-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karnovsky MJ, Kleinfeld AM, Hoover RL, Klausner RD. The concept of lipid domains in membranes. J Cell Biol. 1982;94:1–6. doi: 10.1083/jcb.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massey-Harroche D, Mayran N, Maroux S. Polarized localizations of annexins I, II, VI and XIII in epithelial cells of intestinal, hepatic and pancreatic tissues. J Cell Sci. 1998;111:3007–15. doi: 10.1242/jcs.111.20.3007. [DOI] [PubMed] [Google Scholar]
- 18.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–61. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 19.Paulick MG, Bertozzi CR. The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins. Biochemistry. 2008;47:6991–7000. doi: 10.1021/bi8006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]


