Abstract
A recent comparative genomic hybridization study in our laboratory revealed considerable plasticity within the bacteriocin locus of gastrointestinal strains of Lactobacillus salivarius. Most notably, these analyses led to the identification of two novel unmodified bacteriocins, salivaricin L and salivaricin T, produced by the neonatal isolate L. salivarius DPC6488 with immunity, regulatory and export systems analogous to those of abp118, a two-component bacteriocin produced by the well characterized reference strain L. salivarius UCC118. In this addendum we discuss the intraspecific diversity of our seven bacteriocin-producing L. salivarius isolates on a genome-wide level, and more specifically, with respect to their salivaricin loci.
Keywords: Lactobacillus salivarius, bacteriocin, comparative genomic hybridization, probiotic, salivaricin
Introduction
In a recent comparative study, we investigated the diversity of the bacteriocin loci of seven Lactobacillus salivarius isolates of human and porcine intestinal origin isolated in our laboratory.1 The bacteriocin loci of the respective strains were compared with that of L. salivarius UCC118, a probiotic candidate that produces the two-component class IIb bacteriocin abp118.2 Notably, the probiotic efficacy of this bacteriocin has been reported by Corr and coworkers.3 Specifically, this study demonstrated that abp118 production was directly responsible for the inhibition of Listeria monocytogenes in a murine infection model following oral administration of L. salivarius UCC118, thereby corroborating the role of bacteriocin production in probiosis.3 Furthermore, the bacteriocin-mediated ability of L. salivarius UCC118 to influence the composition of the gut microbiota of diet induced obese (DIO) mice was recently demonstrated.4 Interestingly, abp118 did not impact total fecal bacterial numbers. Rather, an increase in the relative proportions of Bacteroidetes and Proteobacteria and a decrease in Actinobacteria were characteristic of the gut microbiota of DIO mice administered the abp118-producing probiotic in comparison to those fed a bacteriocin-deficient derivative of L. salivarius UCC118.
Possession of the genetic determinants responsible for the production of such two component class II bacteriocins is widespread among L. salivarius isolates of intestinal origin.5-8 In addition to the bacteriocin structural genes, the abp118 locus is comprised of genes involved in bacteriocin immunity (abp118IM), regulation (abp118IP, abp118K, abp118R) and transport (abp118T and abp118D), all required for efficient bacteriocin production and protection of the producing strain.2 In our study, microarray-based comparative genomic hybridization (CGH) analyses based on the genome of L. salivarius UCC118 revealed that the abp118-related genes were conserved in all test strains with the exception of one porcine isolate, L. salivarius DPC6502. The four remaining isolates of porcine origin had previously been shown to produce salivaricin P, a natural variant of abp118.5 The observation that the genes involved in bacteriocin transport were absent in the human isolate L. salivarius DPC6196, most likely explains the bacteriocin negative phenotype of this strain as the gene cluster was otherwise highly conserved. Although genes involved in abp118 regulation and transport were well conserved within the second strain of human origin, L. salivarius DPC6488, considerable diversity was evident with respect to the structural genes. Indeed, four open reading frames (ORFs) potentially encoding putative bacteriocin prepeptides were identified in the bacteriocin locus of this strain. Three of these were found to contribute to the production of two novel bacteriocins designated salivaricin T and salivaricin L, while the fourth encoded an inactive homolog of salivaricin B. Like abp118, salivaricin T is a two-component bacteriocin. However, the mature peptides of this narrow spectrum bacteriocin did not resemble those of abp118 but rather, thermophilin 13, a bacteriocin produced by Streptococcus themophilus.9 In contrast, salivaricin L is a one-peptide bacteriocin of the class IId variety that exhibited anti-Listeria activity. Overall, these analyses exposed an unprecedented level of versatility within the bacteriocin loci of the L. salivarius candidate probiotics.
Plasticity of Seven L. salivarius Genomes of Human and Porcine Origin
In this manuscript, an overview of the genome as a whole revealed that this plasticity was not exclusive to the bacteriocin locus of L. salivarius UCC118 but was reflected across 23 hyper-variable clusters within the test strains (Fig. 1, Table 1). Indeed, just 72% of the L. salivarius UCC118-specific features represented on the array were common to all seven test strains and, interestingly, 12% of features were exclusive to strain UCC118. The genome of L. salivarius UCC118 is comprised of a circular chromosome of 1.8 MB, complemented by a megaplasmid, pMP118 (242 kb; on which the genetic determinants for abp118 are located) and two smaller plasmids, pSF118–20 and pSF118–44.10 Our results indicated that the human isolate deficient for bacteriocin activity L. salivarius DPC6196 possessed the greatest percentage (88%) of UCC118-specific genes, while L. salivarius DPC6488, which produces the novel salivaricins T and L, harbored 84%. The porcine intestinal isolate L. salivarius DPC6502 displayed the greatest divergence, with 78% conservation of the UCC118 gene content. The remaining porcine isolates, L. salivarius DPC6005, DPC6027, DPC6189 and 7.3, displayed between 79% and 84% conservation. These findings were largely consistent with a previous survey of the genomic diversity of 33 L. salivarius isolates of various origins.11 We identified 96 genes that represented the regions of greatest divergence, i.e., present in strain UCC118 but absent from all seven test isolates. These were typically components of mobile DNA elements such as prophage and plasmid-associated genes, as summarized here.
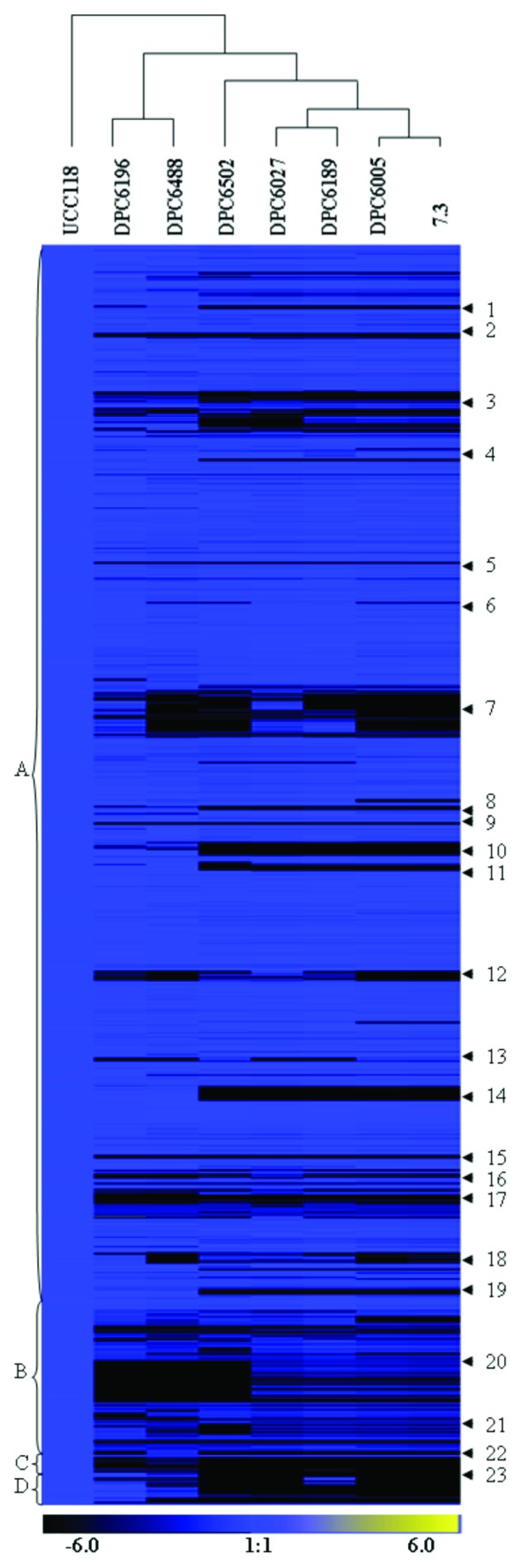
Figure 1. Analysis of genomic diversity of L. salivarius test strains with respect to L. salivarius UCC118 by CGH. Replicons are in the order of chromosome (A), pMP118 (B), pSF118–20 (C) and pSF118–44 (D). Black, blue and yellow regions represent absence, conservation or overrepresentation of CDS, respectively, corresponding to the color legend. Numbers 1 to 23 represent hyper-variable regions within the L. salivarius species, as outlined in Table 1.
Table 1. Composition of hyper-variable regions within L. salivarius species relative to L. salivarius UCC118. *The GC content of the chromosome of L. salivarius UCC118 is 32%.
| HV | Proposed function | Size (kb) | Genes | GC %* | ||
|---|---|---|---|---|---|---|
| 1 |
CRISPR genes |
|
7.786 |
|
LSL_0098-LSL_0100 |
30 |
| 2 |
Carbohydrate metabolism |
|
5.385 |
|
LSL_0142-LSL_0148 |
33 |
| 3 |
Prophage Sal2 |
|
39.622 |
|
LSL_0236-LSL_0305 |
33 |
| 4 |
Hypothetical proteins |
|
6.135 |
|
LSL_0349-LSL_0352 |
31 |
| 5 |
Hypothetical proteins |
|
1.816 |
|
LSL_0519-LSL_0521 |
26 |
| 6 |
Transposases |
|
1.583 |
|
LSL_0585-LSL_0586 |
32 |
| 7 |
Prophage Sal1 |
|
47.905 |
|
LSL_0729-LSL_0805 |
32 |
| 8 |
Type I restriction-modification system |
9.73 |
|
LSL_0915-LSL_1920 |
30 |
|
| 9 |
Hypothetical proteins |
|
2.314 |
|
LSL_0942-LSL_0945 |
30 |
| 10 |
EPS cluster 1 |
|
23.521 |
|
LSL_0975-LSL_0997 |
32 |
| 11 |
Hypothetical proteins |
|
15.795 |
|
LSL_1012-LSL_1024 |
31 |
| 12 |
Prophage Sal4 |
|
8.906 |
|
LSL_1189-LSL_1205 |
31 |
| 13 |
Mucus-binding proteins |
|
7.893 |
|
LSL_1334-LSL_1340 |
32 |
| 14 |
Hypothetical proteins |
|
23.395 |
|
LSL_1380-LSL_1401 |
35 |
| 15 |
Hypothetical proteins |
|
4.597 |
|
LSL_1492-LSL_1497 |
30 |
| 16 |
Hypothetical proteins |
|
14.441 |
|
LSL_1522-LSL_1527 |
28 |
| 17 |
EPS cluster 2 |
|
34.726 |
|
LSL_1546-LSL_1573 |
30 |
| 18 |
Prophage Sal3 |
|
10.017 |
|
LSL_1648-LSL_1666 |
31 |
| 19 |
Mannose PTS system |
|
8.253 |
|
LSL_1708-LSL_1716 |
32 |
| 20 |
Conjugation region |
|
67.138 |
|
LSL_1808-LSL_1869 |
32 |
| 21 |
Bacteriocin locus |
|
11.008 |
|
LSL_1906-LSL_1924 |
30 |
| 22 |
Mannose pts system |
|
4.609 |
|
LSL_1949-LSL_1955 |
32 |
| 23 |
Small plasmids |
pSF118–20 |
20.417 |
|
LSL_1960-LSL_1986 |
39 |
| pSF118–44 | 44.013 | LSL_1987-LSL_2037 | 39 | |||
Regions of Greatest Divergence
Neither of two complete prophage of L. salivarius UCC118, Sal1 and Sal2 (corresponding to hyper-variable regions HV 7 and HV 3 respectively), were fully conserved in any of the seven test strains. With respect to the plasmid content, the conservation of LSL_1739 (repA) indicated the presence of repA-type megaplasmids in all strains. The megaplasmid encoded choloylglycine hydrolase (LSL_1801), primarily responsible for the bile-salt hydrolase activity of L. salivarius UCC118,12 was also well conserved in all strains while hypothetical proteins, pseudogenes and transposases were largely responsible for diversity with respect to pMP118-related genes in the test strains. Notably, a remnant of a conjugal plasmid transfer locus in pMP118 (HV 20) was not conserved in either of the human test strains nor the porcine isolate L. salivarius DPC6502. Although genes associated with the smallest replicon of strain UCC118, pSF118–20, were generally absent from all test strains, L. salivarius DPC6488 DNA hybridized to probes corresponding to the replication proteins of both of the smaller replicons (LSL_1965 and LSL_2000), indicating the presence of somewhat related plasmids in this strain. The human isolate L. salivarius DPC6196 was the only strain in which the genes of pSF118–44 were almost completely conserved. LSL_2000 was also conserved in strain DPC6189 indicating that this strain may also harbor a pSF118–44-like plasmid. However, the genes associated with this replicon were absent from all other test strains of porcine origin.
Regions Distinguishing Isolates of Human and Porcine Origin
Interestingly, a hierarchical tree that was generated on the basis of the variability of the data, sub-grouped the respective test strains of human and porcine origin (Fig. 2), with the latter group displaying greatest diversity with respect to the human-associated L. salivarius UCC118. Although, it may be possible that this is a result of the small number of test strains investigated in this instance or perhaps due to an imbalance of strains from these individual hosts. Gene clusters to which this distinction was attributed were both chromosomally and megaplasmid located and often associated with fitness, niche adaptation, and potentially the probiotic functionality of the strains (Fig. 1, Table 1). It is possible, for example, that the absence of the Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) -associated genes represented by hyper-variable region 1 (HV 1) and genes associated with a type I restriction–modification system (HV 8), features which confer resistance to foreign DNA elements, in all of the porcine test strains may render these isolates susceptible to phage attack within the GIT.
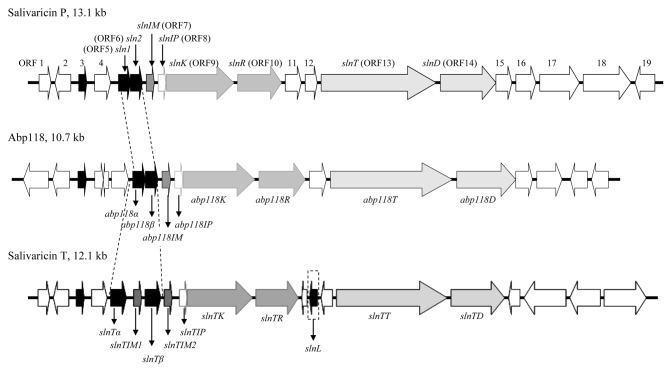
Figure 2. Comparative representation of the salivaricin P gene cluster with that of abp118 and salivaricin T/L. Black and charcoal arrows indicate bacteriocin structural and predicted immunity genes, respectively, while genes involved in regulation and transport are indicated by gray and those encoding hypothetical proteins by white arrows. The similarities of the putative protein products encoded by the respective gene clusters are outlined in Table 2.
Protection and stress tolerance as well as adhesion and in vivo persistence are also among the many benefits associated with exopolysaccharide (EPS) production which may be important factors for colonization and survival within the GIT.13 Both EPS clusters 1 (HV 10) and 2 (HV 17) of strain UCC118 were identified as strain specific traits. Although many of the genes associated with cluster 2 were not well conserved in any of the test strains, cluster 1 was clearly absent from all porcine derived isolates.
The presence of multiple mannose phosphotrasnferase systems (pts) has been associated with enhanced metabolic versatility of microorganisms, as well as horizontal gene transfer events.14 Therefore, it is notable that two of the four mannose pts systems of L. salivarius UCC118 (HV 19 and 22) were also absent in all of the porcine derived test strains.
Bacteriocin Loci of Porcine-Derived Test Strains
Despite the absence of the aforementioned features, the porcine isolates included in this study were originally recovered from intestinal origins as a consequence of their associated antimicrobial activity.15,16 The production of organic acids, hydrogen peroxide and bacteriocins may all contribute to this phenotype, however, the widespread distribution of the salivaricin P locus in L. salivarius isolates of porcine origin may be indicative of its importance for colonization of the porcine GIT. Further substantiating this hypothesis, findings by Walsh et al.17 revealed that the salivaricin P-producing component L. salivarius DPC6005 predominated within the porcine ileum over four counterparts orally administered as a probiotic formulation.17 This strain was among four porcine intestinal isolates included in our study, L. salivarius DPC6005, DPC6027, DPC6189 and 7.3, which were previously shown to produce this natural variant of abp118. The homology of the individual salivaricin P structural genes sln1 and sln2 of each of these strains was previously established.5 This conservation is also evident from our corresponding CGH data; however, diversity was evident elsewhere within the salivaricin P loci of each of the producing strains. This diversity, coupled with the revelation of novelties within the corresponding gene cluster of L. salivarius DPC6488, encouraged further analysis of the salivaricin P gene cluster, as described in detail below.
A representative salivaricin P gene cluster, consisting of a contiguous sequence of 13,256 nucleotides, was amplified and sequenced using L. salivarius DPC6005 template DNA and oligonucleotide primers designed based on the sequence of the abp118 locus. Nineteen putative ORFs were identified, which were arranged in a similar manner to the genetic determinants of the abp118 and salivaricin T/L loci of L. salivarius UCC118 and L. salivarius DPC6488, respectively (graphically represented in Fig. 2). An alignment revealed that the 10.7 kb abp118 locus (accession number AF408405)2 shared 90% similarity with the salivaricin P sequence of strain DPC6005 and functions were assigned to the products encoded by eight putative ORFs of the salivaricin P cluster based on homology with their UCC118 counterparts (Table 2). In agreement with our data, Barrett and coworkers previously revealed that the structural genes encoding the two component salivaricin P peptides, sln1 and sln2, share 98% and 97% identity with abp118α and abp118β, respectively, which corresponds to 100% and 95% identity, respectively, between the corresponding mature bacteriocin sequences.5 The deduced product of a single ORF upstream of the structural genes, ORF4, displayed similarity to the bacteriocin-like prepeptide products of both of the UCC118 associated genes LSL_1918 and LSL_1920 (95% and 70%, respectively), which may be indicative of a gene duplication event at this site. The deduced protein encoded by ORF3 exhibited 94% identity with the salivaricin B bacteriocin precursor peptide, produced by L. salivarius M6, and its inactive UCC118 (LSL_1921) and DPC6488-associated homologs.2,18 This peptide was not detected during the purification of the antimicrobial components of L. salivarius DPC6005 and thus, is also considered inactive in this strain.5 Immediately downstream of the structural genes are two putative ORFs potentially encoding immunity (ORF7) and induction (ORF8) proteins which share 80% and 60% identity with the analogous proteins encoded by UCC118, respectively. The similarity of the putative induction peptide of the salivaricin P regulatory system lies mainly within the double-glycine leader sequence [17 amino acids (aa)], as the mature peptides (22 aa) share just 40% identity. It is, thus, not surprising that the histidine kinase encoded by slnK displayed just 69% homology with its abp118 counterpart, AbpK. Indeed, these two proteins exhibited greatest diversity in the N-terminal domain responsible for sensing the cognate induction peptide. Although SlnK shares 93% similarity with AbpK of L. salivarius DSM20555 (accession number EEJ73430), DSM20555 does not possess an anti-Listeria phenotype.6 The proteins encoded by the genes adjacent to slnK shared greater than 95% homology with the response regulator and the gene products involved in transport of abp118 (Table 2). The sequence and putative ORFs downstream of the designated transport system exhibit little similarity with the abp118 locus. However, the proteins encoded by ORF15 and ORF16 display similarity to the hypothetical proteins encoded by LSL_1832 and LSL_1831, two genes located approximately 74 kb upstream of the abp118 gene cluster on pMP118, perhaps indicating the occurrence of a recombination event. Inverted repeat sequences typical of rho-independent transcription termination signals were identified at three locations. Those downstream of ORF2 and ORF18, with calculated ΔG of -20.10 kcal/mol and -19.50 kcal/mol,19 respectively, may represent the beginning and end of the salivaricin P operon, respectively. The third possible rho-independent terminator was identified downstream of sln2 (ΔG of -22.10 kcal/mol) and may serve as an attenuator to ensure a higher transcription level of the bacteriocin structural genes than the ORFs downstream, a feature frequently observed in the genetic loci of regulated bacteriocins.9,20,21 Although novel bacteriocin genes or remnants thereof were not identified, the sequence data of the salivaricin P locus of DPC6005 strongly correlated with our CGH data.
Table 2. Proteins encoded by the salivaricin P locus and similarities to their homologs. *Percentage identity was determined using BLAST. †Accession number of sequence directly submitted to EMBL Database.
| ORF (gene) | Size (aa) | Function | Homolog | Identity (%)* | Reference |
|---|---|---|---|---|---|
| ORF 1 |
65 |
Conserved hypothetical protein |
Conserved hypothetical protein of L. salivarius DSM20555 |
95 [62/65] |
EEJ73426† |
| ORF 2 |
87 |
Conserved hypothetical protein |
Conserved hypothetical protein of L. salivarius DSM20555 |
98 [86/87] |
EEJ73427† |
| ORF 3 |
57 |
Bacteriocin-like prepeptide |
Salivaricin B prepeptide |
94 [54/57] |
(18) |
| ORF 4 |
85 |
Bacteriocin-like prepeptide |
LSL_1918 of L. salivarius UCC118 |
95 [81/85] |
(10) |
| ORF 5 (sln1) |
64 |
Salivaricin P prepeptide Sln1 |
Abp118 bacteriocin α prepeptide (LSL_1917) |
100 [64/64] |
(2) |
| ORF 6 (sln2) |
68 |
Salivaricin P prepeptide Sln2 |
Abp118 bacteriocin β prepeptide (LSL_1916) |
97 [66/68] |
(2) |
| ORF 7 (slnIM) |
44 |
Putative salivaricin P immunity protein |
Abp118 IM (LSL_1915) of L. salivarius UCC118 |
80 [33/41] |
(2) |
| ORF 8 (slnIP) |
39 |
Putative salivaricin P induction peptide |
Abp118 IP (LSL_1914) of L. salivarius UCC118 |
60 [24/40] |
(2) |
| ORF 9 (slnK) |
430 |
Sensory transduction histidine kinase |
AbpK of L. salivarius DSM20555 |
93 [401/430] |
EEJ73430† |
| ORF 10 (slnR) |
266 |
Response regulator |
AbpR (LSL_1912) of L. salivarius UCC118 |
96 [255/264] |
(2) |
| ORF 11 |
79 |
Hypothetical membrane spanning protein |
LSL_1911 of L. salivarius UCC118 |
88 [70/79] |
(10) |
| ORF 12 |
65 |
Hypothetical protein |
Hypothetical protein HMPREFOS45_1706 of L. salivarius DSM20555 |
92 [60/65] |
EEJ73433† |
| ORF 13 (slnT) |
719 |
Salivaricin P ABC-transporter protein |
AbpT (LSL_1910) of L. salivarius UCC118 |
97 [698/719] |
(2) |
| ORF 14 (slnD) |
382 |
Salivaricin P export accessory protein |
AbpD (LSL_1909) of L. salivarius UCC118 |
95 [365/381] |
(2) |
| ORF 15 |
73 |
Hypothetical protein |
LSL_1832 of L. salivarius UCC118 |
87 [64/73] |
(10) |
| ORF 16 |
134 |
Hypothetical protein |
LSL_1831 of L. salivarius UCC118 |
82 [110/133] |
(10) |
| ORF 17 |
209 |
Hypothetical protein |
no homologs |
|
|
| ORF 18 |
315 |
Hypothetical protein |
no homologs |
|
|
| ORF 19 | 106 | Conserved hypothertical protein | Conserved hypothertical protein L. salivarius DSM20555 | 95 [84/88] | EEJ73436† |
Considering the bacteriocin-mediated ability of L. salivarius to modulate the gut microbiota, in particular with respect to providing protection against Listeria infection, this hitherto unknown level of intra-species diversity with respect to bacteriocin production by intestinal L. salivarius isolates is of considerable significance. In addition, the consequence of this diversity is probably that strains can adapt to very different gastrointestinal environments as evidenced by the delineation between human and porcine strains in this study.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/21417
References
- 1.O’Shea EF, O’Connor PM, Raftis EJ, O’Toole PW, Stanton C, Cotter PD, et al. Production of multiple bacteriocins from a single locus by gastrointestinal strains of Lactobacillus salivarius. J Bacteriol. 2011;193:6973–82. doi: 10.1128/JB.06221-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn S, van Sinderen D, Thornton GM, Holo H, Nes IF, Collins JK. Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118. Microbiology. 2002;148:973–84. doi: 10.1099/00221287-148-4-973. [DOI] [PubMed] [Google Scholar]
- 3.Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CG. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A. 2007;104:7617–21. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy EF, Cotter PD, Hogan A, O’Sullivan O, Joyce A, Fouhy F, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 2012 doi: 10.1136/gutjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- 5.Barrett E, Hayes M, O’Connor P, Gardiner G, Fitzgerald GF, Stanton C, et al. Salivaricin P, one of a family of two-component antilisterial bacteriocins produced by intestinal isolates of Lactobacillus salivarius. Appl Environ Microbiol. 2007;73:3719–23. doi: 10.1128/AEM.00666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Canchaya C, Fang F, Raftis E, Ryan KA, van Pijkeren JP, et al. Distribution of megaplasmids in Lactobacillus salivarius and other lactobacilli. J Bacteriol. 2007;189:6128–39. doi: 10.1128/JB.00447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilasombut K, Sakpuaram T, Wajjwalku W, Nitisinprasert S, Swetwiwathana A, Zendo T, et al. Purification and amino acid sequence of a bacteriocin produced by Lactobacillus salivarius K7 isolated from chicken intestine. Songklanakarin J Sci Technol. 2006;28:121–31. [Google Scholar]
- 8.Vera Pingitore E, Hébert EM, Nader-Macías ME, Sesma F. Characterization of salivaricin CRL 1328, a two-peptide bacteriocin produced by Lactobacillus salivarius CRL 1328 isolated from the human vagina. Res Microbiol. 2009;160:401–8. doi: 10.1016/j.resmic.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Marciset O, Jeronimus-Stratingh MC, Mollet B, Poolman B. Thermophilin 13, a nontypical antilisterial poration complex bacteriocin, that functions without a receptor. J Biol Chem. 1997;272:14277–84. doi: 10.1074/jbc.272.22.14277. [DOI] [PubMed] [Google Scholar]
- 10.Claesson MJ, Li Y, Leahy S, Canchaya C, van Pijkeren JP, Cerdeño-Tárraga AM, et al. Multireplicon genome architecture of Lactobacillus salivarius. Proc Natl Acad Sci U S A. 2006;103:6718–23. doi: 10.1073/pnas.0511060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raftis EJ, Salvetti E, Torriani S, Felis GE, O’Toole PW. Genomic diversity of Lactobacillus salivarius. Appl Environ Microbiol. 2011;77:954–65. doi: 10.1128/AEM.01687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang F, Li Y, Bumann M, Raftis EJ, Casey PG, Cooney JC, et al. Allelic variation of bile salt hydrolase genes in Lactobacillus salivarius does not determine bile resistance levels. J Bacteriol. 2009;191:5743–57. doi: 10.1128/JB.00506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebeer S, Vanderleyden J, De Keersmaecker SC. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008;72:728–64. doi: 10.1128/MMBR.00017-08. [Table of Contents.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zúñiga M, Comas I, Linaje R, Monedero V, Yebra MJ, Esteban CD, et al. Horizontal gene transfer in the molecular evolution of mannose PTS transporters. Mol Biol Evol. 2005;22:1673–85. doi: 10.1093/molbev/msi163. [DOI] [PubMed] [Google Scholar]
- 15.Casey PG, Casey GD, Gardiner GE, Tangney M, Stanton C, Ross RP, et al. Isolation and characterization of anti-Salmonella lactic acid bacteria from the porcine gastrointestinal tract. Lett Appl Microbiol. 2004;39:431–8. doi: 10.1111/j.1472-765X.2004.01603.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Shea EF, Gardiner GE, O’Connor PM, Mills S, Ross RP, Hill C. Characterization of enterocin- and salivaricin-producing lactic acid bacteria from the mammalian gastrointestinal tract. FEMS Microbiol Lett. 2009;291:24–34. doi: 10.1111/j.1574-6968.2008.01427.x. [DOI] [PubMed] [Google Scholar]
- 17.Walsh MC, Gardiner GE, Hart OM, Lawlor PG, Daly M, Lynch B, et al. Predominance of a bacteriocin-producing Lactobacillus salivarius component of a five-strain probiotic in the porcine ileum and effects on host immune phenotype. FEMS Microbiol Ecol. 2008;64:317–27. doi: 10.1111/j.1574-6941.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 18.Cataloluk O. Molecular characterization of the gene encoding for the salivaricin B activity and its flanking sequences. Turk J Biol. 2001;25:379–86. [Google Scholar]
- 19.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–15. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAuliffe O, O’Keeffe T, Hill C, Ross RP. Regulation of immunity to the two-component lantibiotic, lacticin 3147, by the transcriptional repressor LtnR. Mol Microbiol. 2001;39:982–93. doi: 10.1046/j.1365-2958.2001.02290.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuipers OP, Beerthuyzen MM, de Ruyter PG, Luesink EJ, de Vos WM. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem. 1995;270:27299–304. doi: 10.1074/jbc.270.45.27299. [DOI] [PubMed] [Google Scholar]