Life-long hematopoietic demands are filled by a pool of hematopoietic stem cells (HSC) with self-renewal and multipotential differentiation ability. Generation of reactive oxygen species (ROS) at low and moderate levels is required for HSC activity.1 However, a sustained, abnormal increase in ROS production occurs under aging and genotoxic stress, which inhibits HSC self-renewal and induces HSC senescence and hematopoietic dysfunction.2 How ROS levels are restored in HSC, and ROS-induced DNA damage prevented is not well understood, but both HSC autonomous and non-cell autonomous are likely at play.
In the bone marrow (BM), HSC activity requires a nurturing hematopoietic microenvironment (HM). While multiple mechanisms are arguably responsible for the control of the HM on HSC activity, all of them can be sorted into three categories: cytokine receptor-ligand interaction, the interaction of adhesion molecules on hematopoietic cells with BM stromal cells or the extracellular matrix, or direct cell-to-cell communication between stromal cells and HSC.
In patients undergoing cancer therapy, the functional reserve of normal BM HSC largely determines the hematological toxicity associated with the use of cytostatic drugs and significantly influences their morbi-mortality. The functional reserve of HSC depends on the number of cells and the intrinsic ability of HSC to self-renew and differentiate in an uncompromised microenvironment and is controlled by a number of modifier genes. These factors often result in a heterogenous, unpredictable level of therapy-related toxicity. Cell cycle-specific chemotherapy agents are widely used in cancer therapy. Fluorouracil (5-FU), an antimetabolite with thymidlate synthase inhibitory activity, is used in the treatment of high-proliferative, tissue-derived cancers, such as gastrointestinal carcinomas and aggressive breast cancer. Administration of 5-FU spares quiescent HSC survival3 but induces significant DNA damage through ROS production.4
Gap junctions (GJs) represent a system of cell-to-cell communication in the BM microenvironment. Although the existence of GJs in the BM has been known for over 30 y,5 their function remains unclear. GJ channels are formed by dodecamers of protein subunits called connexins (Cxs). Cx43 is the predominant Cx expressed in the BM5 and has been shown to be part of the signature of HSC.6 It is downregulated during differentiation to progenitors7 and upregulated in the endosteal space of the BM in 5-FU-treated mice.8 The function of HSC Cx43 was unknown.
Using hematopoietic-specific constitutive knockout murine models (Vav1-Cre/Cx43flox/flox), we recently demonstrated the mechanism of impaired hematopoietic recovery after in vivo 5-FU challenge.9 While basal Cx43 deficiency does not impair HSC self-renewal after 5-FU administration, HSC from hematopoietic-Cx43-deficient mice cannot enter the cell cycle and succumb to apoptosis or senesce. Senescence was associated with high levels of ROS and impaired cell cycle entry through activation of p38MAPK/p16inka and Foxo1, probably through a regulatory feedback loop. The loss-of-function in Cx43-deficient HSC activity could be reverted by antioxidant therapy with N-acetyl-l-cysteine in vivo, or by the reintroduction of Cx43 using a lentivirus vector system, indicating that Cx43 is a crucial molecule in the maintenance of HSC fitness after 5-FU treatment.
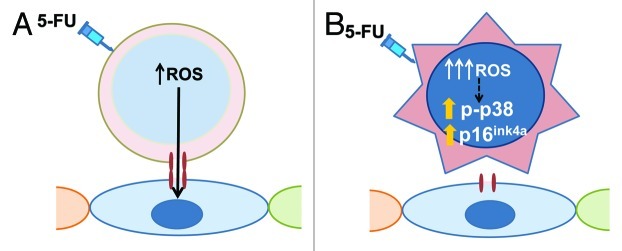
Cx43 mediates BM stromal cell adhesion to HSC.10 When wild-type HSC and progenitors (HSC/P) with high levels of ROS were deposited on BM stromal cells, the intracellular ROS was transferred to the stromal cells. A deficiency of Cx43 in the HSC resulted in impaired ROS transfer. Reintroduction of Cx43 rescued ROS transfer from the HSC/P to the stromal cells, indicating that HSC Cx43 expression plays a cell-autonomous effect. Interestingly, the deficiency of Cx43 in the HM phenocopies the deficiency of Cx43 in the HSC compartment with respect to its inability to regenerate hematopoiesis after stress. This indicates that Cx43 expression in the HM is similarly required for hematopoietic regeneration, and suggests that Cx43-to-Cx43 heterocellular interactions between HSC/P cells and the HM are required for an adequate regenerative response after chemotherapy. Altogether, these data indicate that Cx43 plays a novel role of the HM-mediated scavenging of HSC ROS and prevents HSC damage (Fig. 1). Whether prevention of loss-of-function of Cx43 may result in prevention of chemotherapy-induced HSC damage remains to be seen, but these results provide preliminary evidence that intervention on GJ intercellular communication may become a valid strategy.
Figure 1. The role of HSC Cx43 in ROS scavenging by the hematopoietic microenvironment. (A) Following 5-FU administration, normal HSC are able to eliminate a significant part of the excess of ROS through Cx43-mediated channels between HSC and BM stromal cells. (B) ROS levels accumulate in Cx43-deficient HSC and activate the p38 MAPK-p16ink4a pathway, resulting in HSC apoptosis and senescence.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21589
References
- 1.Owusu-Ansah E, et al. Nature. 2009;461:537–41. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito K, et al. Nat Med. 2006;12:446–51. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 3.Berardi AC, et al. Science. 1995;267:104–8. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- 4.Hwang PM, et al. Nat Med. 2001;7:1111–7. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell FR. Anat Rec. 1980;196:101–7. doi: 10.1002/ar.1091960110. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, et al. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsberg EC, et al. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosendaal M, et al. J Cell Sci. 1994;107:29–37. doi: 10.1242/jcs.107.1.29. [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi Ishikawa E, et al. Proc Natl Acad Sci USA. 2012;109:9071–6. doi: 10.1073/pnas.1120358109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schajnovitz A, et al. Nat Immunol. 2011;12:391–8. doi: 10.1038/ni.2017. [DOI] [PubMed] [Google Scholar]