Abstract
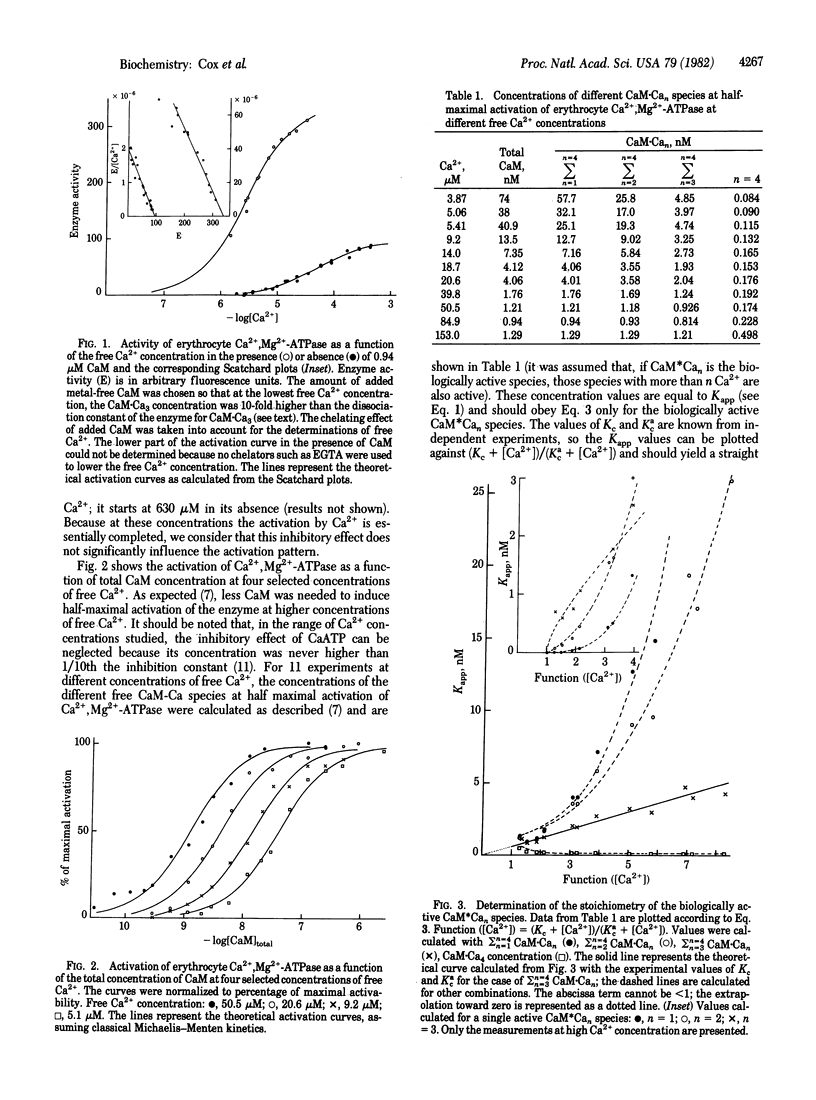
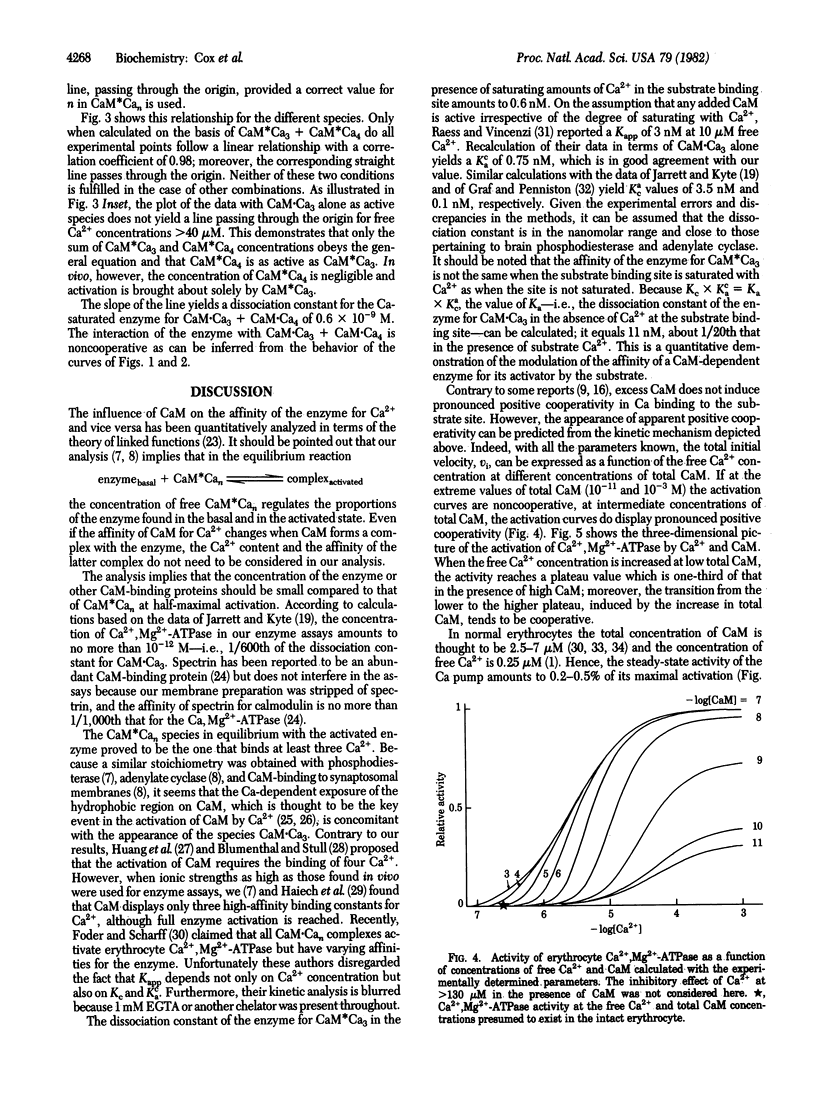
The effect of Ca2+ and calmodulin on (CaM) on the activation of Ca2+-dependent Mg2+-activated ATPase (Ca2+,Mg2+-ATPase; ATP phosphohydrolase, EC 3.6.1.3) has been carried out because of the finding that the CaM dependence of the activation varies with the concentration of free Ca2+, similarly to brain phosphodiesterase and adenylate cyclase. The study was carried out in the absence of chelating agents because they strongly interfere in the enzyme kinetics. Three main conclusions can be drawn (i) CaM-Ca3 and CaM-Ca4 together are the biochemically active species in vitro. (ii) These species bind in a non-cooperative way to the CaM-binding site of the enzyme with a dissociation constant of 6 x 10(-10) M or 1.1 x 10(-8) M, depending on whether Ca2+ saturates the substrate binding site of the enzyme or not. (iii) The binding of CaM-Ca3 to the enzyme lowers the dissociation constant of the enzyme for Ca2+ at the substrate binding site from 51.5 to 2.8 microM. Contrary to general belief, CaM does not induce pronounced positive cooperativity in the binding of Ca2+ to the enzyme. Such a cooperativity is seen only when the enzyme is incompletely saturated with the activator, but it disappears in the presence of saturating concentrations of CaM-Ca3. The rate equation proposed here accurately predicts the extent of enzyme activation over a wide range of Ca2+ and CaM concentration. In healthy erythrocytes the concentrations of Ca2+ and CaM are such that the Ca pump works with a minimal dissipation of energy, but a small increase in the intracellular Ca2+ concentration leads to a strong amplification of the pumping activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Jobore A., Roufogalis B. D. Influence of EGTA on the apparent Ca2+ affinity of Mg2+-dependent, Ca2+-stimulated ATPase in the human erythrocyte membrane. Biochim Biophys Acta. 1981 Jul 6;645(1):1–9. doi: 10.1016/0005-2736(81)90504-6. [DOI] [PubMed] [Google Scholar]
- Barnett R. E. Effect of monovalent cations on the ouabain iniibition of the sodium and potassium ion activated adenosine triphosphatase. Biochemistry. 1970 Nov 24;9(24):4644–4648. doi: 10.1021/bi00826a004. [DOI] [PubMed] [Google Scholar]
- Blumenthal D. K., Stull J. T. Activation of skeletal muscle myosin light chain kinase by calcium(2+) and calmodulin. Biochemistry. 1980 Nov 25;19(24):5608–5614. doi: 10.1021/bi00565a023. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calmodulin in the membrane transport of Ca++. Cell Calcium. 1981 Aug;2(4):353–363. doi: 10.1016/0143-4160(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Malnoë A., Stein E. A. Regulation of brain cyclic nucleotide phosphodiesterase by calmodulin. A quantitative analysis. J Biol Chem. 1981 Apr 10;256(7):3218–3222. [PubMed] [Google Scholar]
- Donaldson S. K., Kerrick W. G. Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers. J Gen Physiol. 1975 Oct;66(4):427–444. doi: 10.1085/jgp.66.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes P., Michell R. H. Human erythrocyte membranes exhibit a cooperative calmodulin-dependent Ca2+-ATPase of high calcium sensitivity. Nature. 1981 Mar 19;290(5803):270–271. doi: 10.1038/290270a0. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Foder B., Scharff O. Decrease of apparent calmodulin affinity of erythrocyte (Ca2+ + Mg2+)-ATPase at low Ca2+ concentrations. Biochim Biophys Acta. 1981 Dec 7;649(2):367–376. doi: 10.1016/0005-2736(81)90426-0. [DOI] [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- Graf E., Penniston J. T. Equimolar interaction between calmodulin and the Ca2+ ATPase from human erythrocyte membranes. Arch Biochem Biophys. 1981 Aug;210(1):257–262. doi: 10.1016/0003-9861(81)90187-9. [DOI] [PubMed] [Google Scholar]
- Haiech J., Klee C. B., Demaille J. G. Effects of cations on affinity of calmodulin for calcium: ordered binding of calcium ions allows the specific activation of calmodulin-stimulated enzymes. Biochemistry. 1981 Jun 23;20(13):3890–3897. doi: 10.1021/bi00516a035. [DOI] [PubMed] [Google Scholar]
- Hinds T. R., Andreasen T. J. Photochemical cross-linking of azidocalmodulin to the (Ca2+ + Mg2+)-ATPase of the erythrocyte membrane. J Biol Chem. 1981 Aug 10;256(15):7877–7882. [PubMed] [Google Scholar]
- Huang C. Y., Chau V., Chock P. B., Wang J. H., Sharma R. K. Mechanism of activation of cyclic nucleotide phosphodiesterase: requirement of the binding of four Ca2+ to calmodulin for activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):871–874. doi: 10.1073/pnas.78.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett H. W., Kyte J. Human erythrocyte calmodulin. Further chemical characterization and the site of its interaction with the membrane. J Biol Chem. 1979 Sep 10;254(17):8237–8244. [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1210–1216. doi: 10.1016/s0006-291x(77)80108-3. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Wierman B. M., Storm D. R. Calcium-induced exposure of a hydrophobic surface on calmodulin. Biochemistry. 1980 Aug 5;19(16):3814–3819. doi: 10.1021/bi00557a025. [DOI] [PubMed] [Google Scholar]
- Macintyre J. D., Gunn R. B. Activation and deactivation kinetics of Ca transport in inside-out erythrocyte membrane vesicles. Biochim Biophys Acta. 1981 Jun 22;644(2):351–362. doi: 10.1016/0005-2736(81)90393-x. [DOI] [PubMed] [Google Scholar]
- Malnoë A., Cox J. A., Stein E. A. Ca2+-dependent regulation of calmodulin binding and adenylate cyclase activation in bovine cerebellar membranes. Biochim Biophys Acta. 1982 Jan 12;714(1):84–92. doi: 10.1016/0304-4165(82)90129-5. [DOI] [PubMed] [Google Scholar]
- Mauldin D., Roufogalis B. D. A protein activator of Mg2+-dependent, Ca2+-stimulated ATPase in human erythrocyte membranes distinct from calmodulin. Biochem J. 1980 May 1;187(2):507–513. doi: 10.1042/bj1870507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Karlish S. J. Regulatory interaction between calmodulin and ATP on the red cell Ca2+ pump. Biochim Biophys Acta. 1980 Apr 24;597(3):631–636. doi: 10.1016/0005-2736(80)90235-7. [DOI] [PubMed] [Google Scholar]
- Muallem S., Karlish S. J. Studies on the mechanism of regulation of the red-cell Ca2+ pump by calmodulin and ATP. Biochim Biophys Acta. 1981 Sep 21;647(1):73–86. doi: 10.1016/0005-2736(81)90296-0. [DOI] [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Penniston J. T., Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981 Jan 10;256(1):395–401. [PubMed] [Google Scholar]
- Raess B. U., Vincenzi F. F. Calmodulin activation of red blood cell (Ca2+ + Mg2+)-ATPase and its antagonism by phenothiazines. Mol Pharmacol. 1980 Sep;18(2):253–258. [PubMed] [Google Scholar]
- Roufogalis B. D., Mauldin D. Regulation by calmodulin of the calcium affinity of the calcium-transport ATPase in human erythrocytes. Can J Biochem. 1980 Oct;58(10):922–927. doi: 10.1139/o80-126. [DOI] [PubMed] [Google Scholar]
- Simons T. J. Calcium-dependent potassium exchange in human red cell ghosts. J Physiol. 1976 Mar;256(1):227–244. doi: 10.1113/jphysiol.1976.sp011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Calmodulin-binding protein of erythrocyte cytoskeleton. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1063–1070. doi: 10.1016/0006-291x(81)91931-8. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Hidaka H. Hydrophobic regions function in calmodulin-enzyme(s) interactions. J Biol Chem. 1980 Dec 10;255(23):11078–11080. [PubMed] [Google Scholar]
- Waisman D. M., Gimble J. M., Goodman D. B., Rasmussen H. Studies of the Ca2+ transport mechanism of human erythrocyte inside-out plasma membrane vesicles. I. Regulation of the Ca2+ pump by calmodulin. J Biol Chem. 1981 Jan 10;256(1):409–414. [PubMed] [Google Scholar]
- Weber G. Energetics of ligand binding to proteins. Adv Protein Chem. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]



