Abstract
The 40S ribosomal S6 kinase 1 (S6K1) is a conserved serine/threonine protein kinase that belongs to the AGC family of protein kinases, which also includes Akt and many others. S6K1 is the principal kinase effector downstream of the mammalian target of rapamycin complex 1 (mTORC1). S6K1 is sensitive to a wide range of signaling inputs, including growth factors, amino acids, energy levels and hypoxia. S6K1 relays these signals to regulate a growing list of substrates and interacting proteins in control of oncogenic processes, such as cell growth and proliferation, cell survival and apoptosis and cell migration and invasion. Several lines of evidence suggest an important role for S6K1 in estrogen receptor (ER)-positive breast cancer. S6K1 directly phosphorylates and activates ERα. Furthermore, S6K1 expression is estrogenically regulated. Therefore, hyperactivation of mTORC1/S6K1 signaling may be closely related to ER-positive status in breast cancer and may be utilized as a marker for prognosis and a therapeutic target.
Keywords: 17q23 amplicon, ERα, S6K1, estrogen, mTORC1, rapamycin
Introduction
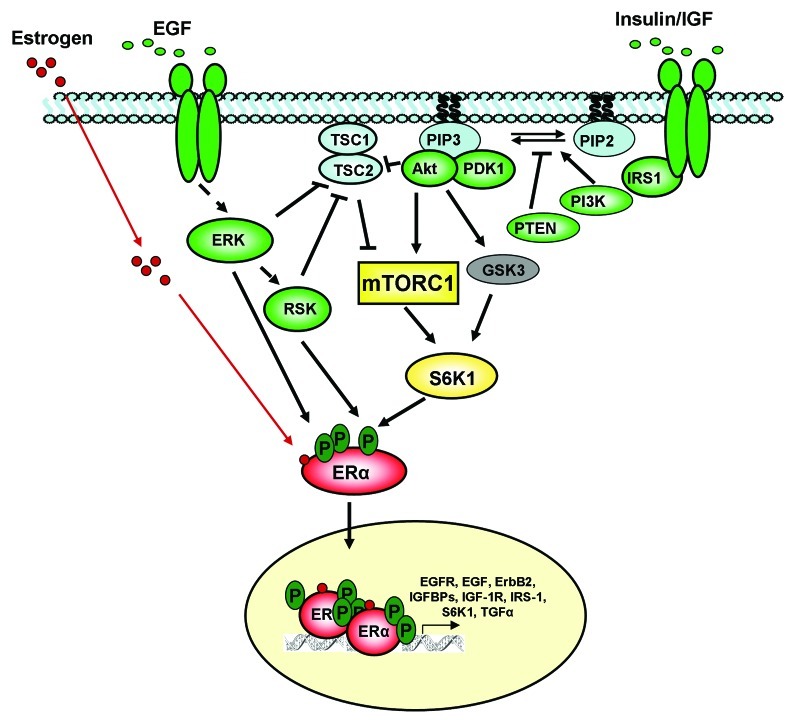
The mTORC1 signaling pathway.
Since its cloning and biochemical purification almost 20 y ago, mTOR, a conserved protein kinase, emerged as a central node in the plexus coordinating cellular growth and proliferation in response to numerous extracellular cues, including nutrient availability and growth stimuli. In eukaryotic cells, mTOR exists in two complexes. mTORC1 and mTORC2 consist of distinct sets of proteins and perform non-redundant functions.1 Rapamycin is a naturally derived inhibitor of mTORC1 and an inhibitor of cell proliferation, as manifested by its potent immunosuppressive properties and activity against solid tumors.2 Growth factor signaling to mTORC1 is primarily mediated by the phosphatidylinositol-3 kinase (PI3K) pathway and inactivation of the tuberous sclerosis complex protein TSC2 (tuberin). As illustrated in Figure 1, in response to extracellular activating stimuli, PI3K mediates cell membrane recruitment and activation of the serine/threonine protein kinases phosphatidylinositol-dependent kinase-1 (PDK1) and Akt.3,4 The lipid phosphatase PTEN opposes the action of PI3K. TSC2 functions as a heterodimer with TSC1 (hamartin) to negatively regulate mTORC1 signaling by acting as a GTPase-activating protein (GAP) for the small GTPase Rheb.5 Rheb directly binds to mTORC1 and regulates its activity in a GTP-dependent manner.6 Thus, TSC2 inhibits Rheb-dependent activation of mTORC1. Phosphorylation and inactivation of TSC2 has first been shown to occur via PI3K/Akt.7 Subsequently, several groups have shown that the Ras/ERK pathway also converges on the TSC1/2 complex. Ras-activated ERK1/2 directly phosphorylates TSC2 at sites different than Akt, resulting in TSC2 inactivation.8 The downstream effector of ERK, RSK, also phosphorylates TSC2 at a unique site, thus inactivating it.9 Therefore, TSC2 serves as a convergence point for multiple signaling inputs to mTORC1.
Figure 1. Reciprocal crosstalk between estrogen receptor (ER) α and growth factor receptor signaling pathways. Growth factor receptors activate phosphatidylinositol 3-kinase (PI3K) and MAPK signaling pathways. The effectors of these pathways, including ERK, RSK and S6K1 can then phosphorylate and activate ERα. ERα can also become activated by binding of its ligand, estrogen. When activated, ERα dimerizes, translocates to the nucleus, binds DNA and activates transcription of genes encoding components of growth factor signaling pathways, promoting a positive feed-forward pathway activation loop.
Activation of S6K1.
S6K1 is one of the best-characterized downstream targets of mTORC1, and rapamycin treatment results in rapid dephosphorylation and inactivation of S6K1.10 Regulation of S6K1 is complex, involving several mTORC1- and PI3K-mediated phosphorylation events (reviewed in ref. 11). Phosphorylation of the proline-directed sites in the C terminus (Ser411, Ser418, Ser421 and Thr424) is hypothesized to prime S6K1 for subsequent phosphorylation at critical regulatory sites. Although the kinase(s) responsible for phosphorylation of these four C-terminal sites is not known, ERK- and p38-MAPK have been implicated in their regulation. The turn motif is phosphorylated at Ser371, a site essential for S6K1 activity by a mechanism involving glycogen synthase kinase-3 (GSK-3).12 The hydrophobic motif Thr389 is also an essential and rapamycin-sensitive phosphorylation site. mTORC1 directly phosphorylates this site in vitro. Moreover, autophosphorylation has also been shown to contribute to the regulation of this site.13 Phosphorylated Thr389 creates a docking site for PDK1, which phosphorylates Thr229 at the activation loop of S6K1. Other PI3K-dependent inputs linked to the activation of S6K1 are Akt, PKCζ and λ and the small G proteins Rac1 and Cdc42.11
Signaling by S6K1.
S6K1 is an important regulator of cell size control, protein translation and cell proliferation.14 Results from S6k1 mouse knockouts uncovered a specific role for S6K1 in regulation of cell growth.15 S6K1 phosphorylates proteins that function in RNA processing and protein biogenesis to increase cellular size, which is the limiting factor for cell division.16-20 The 40S ribosomal protein S6 is the best-characterized target of S6K1.21 S6K1 has also been shown to control the proliferative aspect of cell division. Specifically, S6K1 has been reported to drive the G1/S cell cycle progression, and overexpression of S6K1 provides a significant proliferative advantage in low serum conditions,18,22,23 a hallmark of neoplastic transformation. Significantly, the upstream regulators of the mTORC1/S6K1 pathway, such as PIK3CA, PTEN, AKT, PDK1 and TSC1/2, are frequently mutated in cancer, leading to inappropriate hyperactivation of S6K1.24,25
S6K1 in ER-Positive Breast Cancer
ER-positive breast cancer and endocrine resistance.
Clinically, up to 60% of breast cancers are ER-positive, indicative of estrogen dependence for cancer cell growth.26 ER-positive breast cancers can be targeted therapeutically by antiestrogens (such as tamoxifen) or aromatase inhibitors (AIs). However, only about half of ER-positive breast cancers respond to endocrine treatments,27 and resistance frequently develops.28 Third-generation aromatase inhibitors (e.g., letrozole and anastrozole) are now considered to be the first-line treatment strategy for breast cancer.29 However, response rates range between 35% and 70% in neoadjuvant studies and tend to be less effective in advanced disease.30 Development of primary or de novo resistance frequently occurs, and even patients who have a response eventually relapse (acquired resistance). Although great strides have been made in understanding the mechanisms of resistance, for instance, implicating increased signaling via the MAPK, PI3K and mTORC1 pathways, the precise details are not fully understood.31
Regulation of ERα activity.
The binding of 17β-estradiol (estrogen), the physiological ligand of ERα, allows ERα to dissociate from the inhibitory heat shock proteins, undergo phosphorylation, dimerization and translocation to the nucleus, where ERα activates transcription of responsive genes. ERα can also become activated via phosphorylation by effectors of growth factor signaling pathways, which can either potentiate estrogen signaling or mediate estrogen-independent activation.
Several phosphorylation events occur within ERα that are essential for transcriptional activity and responsive to either estrogen, growth factors or both activating stimuli. The phosphorylation of Ser104/106 is sensitive to estrogen only and not growth factors. The kinases that have been implicated in this event are GSK3, cdk2 and MAPK.32-34 Ser118 phosphorylation is sensitive to both estrogen and growth factor signaling. While the identity of the kinase(s) that mediates estrogenic phosphorylation of Ser118 is unknown, cdk7, IKKα, MAPK and GSK3 have been shown to have direct and indirect growth factor-induced phosphorylation.32,35-37 The phosphorylation of Ser167 is mainly sensitive to growth factor stimuli and is mediated primarily by S6K1,18,38 while p90Rsk and Akt may play a secondary or indirect role.19,39 Importantly, this site is associated with tamoxifen resistance.40 PKA phosphorylates Ser236, which appears to promote the stability of ERα,41,42 as well as Ser305. Ser305 appears to be important for dimerization and transactivation activity of ERα and is phosphorylated by PKA in the presence of estrogen43 and by Akt through the IGF-I signaling pathway.44
Crosstalk between estrogen receptor and growth factor signaling pathways.
Growing evidence indicates there exists a close interaction between the mTORC1/S6K1 pathway and ER signaling (Fig. 1). Notably, endocrine resistance is often associated with ligand-independent activation of ERα signaling due to hyperactivation of the mTORC1 signaling pathway45 and can be reversed by the mTORC1 inhibitor everolimus in vitro.40,46,47 S6K1 directly phosphorylates ERα, leading to ligand-independent activation.18,19 S6K1 is also one of the kinases required for hormone-independent breast cancer cell proliferation.48 In addition, mTORC1 serves as an important signaling node for several growth factor signaling pathways that are implicated in endocrine resistance, such as MAPK and PI3K. Thus, mTORC1 hyperactivation by converging stimuli would be targeted by the addition of mTOR inhibitors to endocrine therapy.
S6K1 activates ERα and promotes proliferation of ER-positive breast cancer cells.
A large body of evidence reveals that S6K1 signaling may be particularly important for proliferation of ER-positive breast cancer cells. One molecular mechanism by which S6K1 controls proliferation is direct phosphorylation of ERα on Ser167, leading to increased ERα transcriptional activity and ER-dependent breast cancer cell proliferation. P-S6K1, a marker of S6K1 activation, has been determined to correlate with a poorer prognosis in patients with ER-positive (but not in patients with ER-negative) tumors.49 S6K1 expression levels strongly correlate with the ability of cells to proliferate in low serum conditions, a hallmark of neoplastic transformation, while suppression of S6K1 expression results in a decrease in proliferation that is very pronounced in S6K1-overexpressing cells.19 Moreover, overexpression of S6K1 increases rapamycin sensitivity of breast cancer cells.19,50 Finally, it is evident that S6K1 overexpression in breast cancer cells renders them dependent on the continuous activity of this kinase for proliferation. The apparent dependence on S6K1 is similar to oncogene addiction of cancer cells expressing Bcr-Abl, Her-2/neu receptor or a mutant c-Myc or K-ras.
17q23 amplicon in breast cancer.
Genomic amplification of genes critical for tumor initiation and progression is an important mechanism to augment the expression and activity of oncogenes. Several well-characterized examples of oncogenes amplified in breast cancer include ERRB2 (at 17q12), MYCN (at 8q24) and CCND1 (at 11q13). Owing to its biological role, S6K1 is a kinase whose activation by gene amplification would contribute to its oncogenicity. S6K1 is encoded by the RPS6KB1 gene localized to the chromosomal region 17q23, which is amplified in several types of cancer.51 Interestingly, high-level (multi-copy) amplification of RPS6KB1 is limited to breast cancer.51 This suggests that S6K1 may have a specific role in regulating the growth of breast cancer cells. The role of S6K1 in breast cancer development and progression is further supported by the observation that RPS6KB1 amplification and S6K1 overexpression and activation are associated with poor prognosis in breast cancer patients.52-55 Region 17q23 is amplified in several breast cancer cell lines and in up to 30% of primary tumors,56 while S6K1 is overexpressed and hyperactivated in the majority of cell lines and primary tumors with this amplification, more predominantly in breast cancer with BRCA1/2 mutations.19,51,52,57-59 In fact, BRCA1/2 mutations may be directly responsible for chromosomal breakage and amplifications in the 17q23 region.60
Estrogenic regulation of S6K1 expression within the 17q23 amplicon.
Our group recently determined that S6K1 expression is regulated in human breast cancer cell lines and murine mammary epithelia by estrogen/ERα in a mechanism that may involve the transcription factor GATA-3.61 S6K1 can directly phosphorylate ERα on Ser167; conversely, ERα activation leads to increased S6K1 expression, resulting in a positive co-regulatory loop.18,19,61 Thus, it appears that S6K1 expression is maintained in two modes: a basal level of expression, typical of normal cells, and an estrogen/ERα-dependent specific upregulation.61 Others have observed estrogen-mediated regulation of RPS6KB1 transcription in ZR-75–1 and MCF7 ER-positive breast cancer cell lines.62,63 The estrogenic mode of S6K1 upregulation does not seem to be limited to breast epithelia. Rps6kb1 was also observed to be estrogenically induced in uteri of ovariectomized mice.64
The existence of the feedforward regulatory loop whereby S6K1 activates ERα transcriptional activity, leading to increased expression of S6K1, is of great interest, since it not only sheds light on the mode of transcriptional regulation of S6K1 expression, but may also help in understanding the role of S6K1 overexpression in the development and progression of breast cancer. Significantly, a feedforward mechanism of transcriptional activation by ERα exists for many other genes involved in growth factor signaling pathways upstream of PI3K/mTORC1/S6K1, including IGF1-R, ErbB2, IRS-1, IGFBPs, EGFR, EGF and others,30 which underscores the importance of sustained pathway activation in breast cancer cells. Moreover, chronic activation of the PI3K/mTORC1 pathway has been associated with development of resistance to endocrine therapy.
Remarkably, the 17q23 amplicon contains the coding sequence for mir-21, a microRNA gene with oncogenic properties, located about 20kb upstream of the RPS6KB1 transcriptional unit. Mir-21 is upregulated in breast cancer and is regulated by estrogen.65,66 Mir-21 target genes include PTEN, PDCD4 and bcl‑2. RPS6KB1 expression was observed to be induced by estrogen in a study focusing on estradiol-regulated microRNAs.65 This may indicate a secondary role of miRNAs in estrogen-induced regulation of RPS6KB1. Coincidently, PTEN and PDCD4 function biochemically upstream and downstream of S6K1, respectively. The coordinate estrogenic regulation of expression of these genes together with RPS6KB1 may confer a selective proliferative advantage to breast cancer cells.
The instability of the 17q23 genomic region also results in a tandem duplication of RPS6KB1, producing a gene fusion of RPS6KB1 with VMP1 (also known as TMEM49).67 Curiously, MIR21 is included in the tandem duplication structure. The consequences of this instability include deregulated expression of cancer-associated elements such as MIR21 and RPS6KB1.
S6K as a Prognostic and a Therapeutic Target in Breast Cancer
mTOR inhibitors and markers of sensitivity.
mTOR kinase is allosterically inhibited by rapamycin and its analogs (rapalogs), such as temsirolimus (CCI‑779), everolimus (RAD-001) as well as a newer class of catalytic mTOR inhibitors. While early studies demonstrated an effect for rapalogs, especially for breast cancer,50,68 the clinical effect was variable among patients. Thus, there exists an urgent need to identify signaling proteins whose inappropriate expression or activation is predictive of the patients’ response to mTORC1 inhibition. Various upstream and downstream players in the mTORC1 pathway have been considered as markers for sensitivity. For instance, deficiency of PTEN, a gene frequently mutated in breast cancer, has been observed to correlate with rapamycin sensitivity in cell lines.50,68 Disappointingly, clinical trials proved to be inconclusive, probably owing to a relatively low incidence of PTEN mutation in sporadic breast cancer.69 A similar trial in the context of activating PIK3CA mutations didn’t produce any major objective responses.70 Akt is frequently activated by upstream PTEN or PIK3CA mutations, leading to hyperactivation of mTOR and S6K1. These mutations may make cells more sensitive to Akt or mTOR inhibitors, as the growth of the cells becomes dependent on elevated mTOR signaling. Preclinical studies of the role of Akt in sensitizing cells to mTOR inhibitors alone or in combination with endocrine agents have determined that rapamycin reverses endocrine resistance or synergizes with anti-estrogens in cells expressing activated Akt.46,71,72
Results from clinical trials with mTORC1 inhibitors in ER-positive breast cancer have been recently reported. For example, a pre-operative study of letrozole with or without everolimus reported greater tumor shrinkage for the combination.73 A very recent breast cancer trial of oral everolimus-(BOLERO-2), a phase 3 study in patients with advanced disease, showed that the addition of everolimus to endocrine therapy results in an improved clinical outcome.29 However, these studies were performed in an unselected group of patients with ER-positive disease, resulting in a variable response among patients. Work attempting to identify predictors of response following single-agent everolimus treatment noted gene expression changes in responding vs. resistant tumors, but no clear-cut pattern was observed.74
S6K1 as a marker of sensitivity to mTORC1 inhibitors.
mTOR inhibition was observed to result in the reversal of resistance in a breast cancer cell line rendered cross-resistant to tamoxifen and fulvestrant, accompanied by reduction in ERα phosphorylated on Ser167.75 Considering this is an S6K1-dependent phosphorylation site, these data imply that reversal of endocrine resistance involves S6K1 inactivation. P-S6K1 and P-S6 positively correlated with high proliferative index in letrozole-treated patients, indicative of endocrine resistance and poor patient outcome.48 Since a significant number of patients carry S6K1 amplification and exhibit S6K1 overexpression or overexpression, S6K1 could be utilized as a prognostic marker. Detection of S6K1 expression in breast cancer could be an effective way to target and treat patients most likely to respond to the combination of mTORC1 and endocrine inhibitors.
S6K1 as a therapeutic target in ER-positive breast cancer.
Targeting S6K1 may be an advantageous strategy based on the following data. First, 17q23 amplification strongly correlates with ER-positive status76 and is one of the most frequent aberrations in ER-positive invasive ductal carcinoma.77 Similarly, many other common mutations in breast cancer leading to aberrant expression and activation in IGF-1R, ErbB2, FGFR, PIK3CA, PTEN and Akt1/2 result in S6K1 hyperactivation.78 Second, RPS6KB1 was determined to be a gene whose gain in ER-positive tumors is prognostic of the metastatic capacity of human breast cancer.55 Moreover, expression of activated S6K1 in ER-positive, but not ER-negative, tumors was prognostic of a poorer prognosis and development of endocrine resistance. Third, RPS6KB1 amplification and S6K1 overexpression in ER-driven breast cancers may stem from the genomic and non-genomic co-stimulatory relationship between ERα and S6K1. Indeed, our group and others have shown that S6K1 regulates ERα transactivational activity in control of breast cancer cell proliferation by directly phosphorylating ERα on Ser167.18,19,38 Reversal of endocrine resistance by addition of everolimus resulted in decreased Ser167 phosphorylation, suggesting that this effect may be mediated through inhibition of S6K1.40 Moreover, we have shown that S6K1 expression is estrogenically regulated via ERα.61 Inhibition of the estrogenic activation of S6K1 may also be the target of action of letrozole, which has been shown to reduce phosphorylation of S6K1 and its targets, p-mTOR Ser2448 and p-S6.48,73,79 Therefore, maintaining high co-overexpression of both S6K1 and ERα may provide a selective advantage for breast cancer cells and contribute to disregulated proliferation of cells during the progression to carcinogenesis and breast cancer. Finally, S6K1 was revealed as a significant hit in a genome-wide screen for kinases important for growth of MCF7/LTED cells (ER-positive cells selected after long-term estrogen deprivation.48
Future Directions
The current challenge in the treatment of ER-positive breast cancer with regard to utilization of mTORC1 pathway inhibitors remains 2-fold. The first task is to obtain insights into the mechanistic effects of combination of endocrine and mTORC1 inhibitors. In addition, there exists a need to identify the downstream effectors of mTORC1 signaling that distinguish resistant and responsive cancers, which can subsequently be used as molecular predictors of response to mTORC1-targeted therapy. The goal is to identify and treat those patients most likely to benefit from the combination.
The identification of RPS6KB1 as an ERα-regulated gene may help further elucidate the mechanisms of breast cancer pathogenesis and may lead to the development of new targeted therapies. The investigation of the relationship between mTORC1/S6K1 and ERα would identify new mediators and determinants of endocrine escape and help develop novel cancer treatments to inhibit downstream pathways of estrogen receptor action.
Grant Support
This work was founded by grants from NIH (CA151112), Atol Charitable Trust, Wendy Will Case Cancer Fund, National Cancer Center, and Yeshiva University.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21194
References
- 1.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–68. doi: 10.1016/S1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 2.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35(Suppl):7S–14S. doi: 10.1016/S0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 3.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–50. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–8. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–13. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 7.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA. 2004;101:13489–94. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 11.Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Adv Cancer Res. 2002;86:1–39. doi: 10.1016/S0065-230X(02)86001-8. [DOI] [PubMed] [Google Scholar]
- 12.Shin S, Wolgamott L, Yu Y, Blenis J, Yoon SO. Glycogen synthase kinase (GSK)-3 promotes p70 ribosomal protein S6 kinase (p70S6K) activity and cell proliferation. Proc Natl Acad Sci USA. 2011;108:E1204–13. doi: 10.1073/pnas.1110195108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romanelli A, Dreisbach VC, Blenis J. Characterization of phosphatidylinositol 3-kinase-dependent phosphorylation of the hydrophobic motif site Thr(389) in p70 S6 kinase 1. J Biol Chem. 2002;277:40281–9. doi: 10.1074/jbc.M205168200. [DOI] [PubMed] [Google Scholar]
- 14.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–71. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 15.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–59. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–80. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–71. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 18.Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284:6361–9. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 19.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584:124–8. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma XM, Yoon SO, Richardson CJ, Jülich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008;133:303–13. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Jenö P, Ballou LM, Novak-Hofer I, Thomas G. Identification and characterization of a mitogen-activated S6 kinase. Proc Natl Acad Sci USA. 1988;85:406–10. doi: 10.1073/pnas.85.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane HA, Fernandez A, Lamb NJ, Thomas G. p70s6k function is essential for G1 progression. Nature. 1993;363:170–2. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- 23.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–16. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–64. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452–61. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1992;339:1–15. [PubMed] [Google Scholar]
- 28.Ellis M. Overcoming endocrine therapy resistance by signal transduction inhibition. Oncologist. 2004;9(Suppl 3):20–6. doi: 10.1634/theoncologist.9-suppl_3-20. [DOI] [PubMed] [Google Scholar]
- 29.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller WR, Larionov AA. Understanding the mechanisms of aromatase inhibitor resistance. Breast Cancer Res. 2012;14:201. doi: 10.1186/bcr2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452–61. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medunjanin S, Hermani A, De Servi B, Grisouard J, Rincke G, Mayer D. Glycogen synthase kinase-3 interacts with and phosphorylates estrogen receptor alpha and is involved in the regulation of receptor activity. J Biol Chem. 2005;280:33006–14. doi: 10.1074/jbc.M506758200. [DOI] [PubMed] [Google Scholar]
- 33.Rogatsky I, Trowbridge JM, Garabedian MJ. Potentiation of human estrogen receptor alpha transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J Biol Chem. 1999;274:22296–302. doi: 10.1074/jbc.274.32.22296. [DOI] [PubMed] [Google Scholar]
- 34.Thomas RS, Sarwar N, Phoenix F, Coombes RC, Ali S. Phosphorylation at serines 104 and 106 by Erk1/2 MAPK is important for estrogen receptor-alpha activity. J Mol Endocrinol. 2008;40:173–84. doi: 10.1677/JME-07-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen D, Washbrook E, Sarwar N, Bates GJ, Pace PE, Thirunuvakkarasu V, et al. Phosphorylation of human estrogen receptor alpha at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21:4921–31. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- 36.Park KJ, Krishnan V, O’Malley BW, Yamamoto Y, Gaynor RB. Formation of an IKKalpha-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol Cell. 2005;18:71–82. doi: 10.1016/j.molcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Weitsman GE, Li L, Skliris GP, Davie JR, Ung K, Niu Y, et al. Estrogen receptor-alpha phosphorylated at Ser118 is present at the promoters of estrogen-regulated genes and is not altered due to HER-2 overexpression. Cancer Res. 2006;66:10162–70. doi: 10.1158/0008-5472.CAN-05-4111. [DOI] [PubMed] [Google Scholar]
- 38.Becker MA, Ibrahim YH, Cui X, Lee AV, Yee D. The IGF pathway regulates ERα through a S6K1-dependent mechanism in breast cancer cells. Mol Endocrinol. 2011;25:516–28. doi: 10.1210/me.2010-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–24. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 40.Ghayad SE, Bieche I, Vendrell JA, Keime C, Lidereau R, Dumontet C, et al. mTOR inhibition reverses acquired endocrine therapy resistance of breast cancer cells at the cell proliferation and gene-expression levels. Cancer Sci. 2008;99:1992–2003. doi: 10.1111/j.1349-7006.2008.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen D, Pace PE, Coombes RC, Ali S. Phosphorylation of human estrogen receptor alpha by protein kinase A regulates dimerization. Mol Cell Biol. 1999;19:1002–15. doi: 10.1128/mcb.19.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai HW, Katzenellenbogen JA, Katzenellenbogen BS, Shupnik MA. Protein kinase A activation of estrogen receptor alpha transcription does not require proteasome activity and protects the receptor from ligand-mediated degradation. Endocrinology. 2004;145:2730–8. doi: 10.1210/en.2003-1470. [DOI] [PubMed] [Google Scholar]
- 43.Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, et al. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5:597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Barone I, Iacopetta D, Covington KR, Cui Y, Tsimelzon A, Beyer A, et al. Phosphorylation of the mutant K303R estrogen receptor alpha at serine 305 affects aromatase inhibitor sensitivity. Oncogene. 2010;29:2404–14. doi: 10.1038/onc.2009.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller TW, Hennessy BT, González-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–13. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beeram M, Tan QT, Tekmal RR, Russell D, Middleton A, DeGraffenried LA. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18:1323–8. doi: 10.1093/annonc/mdm170. [DOI] [PubMed] [Google Scholar]
- 47.Boulay A, Rudloff J, Ye J, Zumstein-Mecker S, O’Reilly T, Evans DB, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–28. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- 48.Fox EM, Miller TW, Balko JM, Kuba MG, Sánchez V, Smith RA, et al. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer. Cancer Res. 2011;71:6773–84. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim EK, Kim HA, Koh JS, Kim MS, Kim KI, Lee JI, et al. Phosphorylated S6K1 is a possible marker for endocrine therapy resistance in hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2011;126:93–9. doi: 10.1007/s10549-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 50.Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–23. doi: 10.1158/1078-0432.CCR-03-0043. [DOI] [PubMed] [Google Scholar]
- 51.Sinclair CS, Rowley M, Naderi A, Couch FJ. The 17q23 amplicon and breast cancer. Breast Cancer Res Treat. 2003;78:313–22. doi: 10.1023/A:1023081624133. [DOI] [PubMed] [Google Scholar]
- 52.Bärlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, et al. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J Natl Cancer Inst. 2000;92:1252–9. doi: 10.1093/jnci/92.15.1252. [DOI] [PubMed] [Google Scholar]
- 53.van der Hage JA, van den Broek LJ, Legrand C, Clahsen PC, Bosch CJ, Robanus-Maandag EC, et al. Overexpression of P70 S6 kinase protein is associated with increased risk of locoregional recurrence in node-negative premenopausal early breast cancer patients. Br J Cancer. 2004;90:1543–50. doi: 10.1038/sj.bjc.6601741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noh WC, Kim YH, Kim MS, Koh JS, Kim HA, Moon NM, et al. Activation of the mTOR signaling pathway in breast cancer and its correlation with the clinicopathologic variables. Breast Cancer Res Treat. 2008;110:477–83. doi: 10.1007/s10549-007-9746-x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Martens JW, Yu JX, Jiang J, Sieuwerts AM, Smid M, et al. Copy number alterations that predict metastatic capability of human breast cancer. Cancer Res. 2009;69:3795–801. doi: 10.1158/0008-5472.CAN-08-4596. [DOI] [PubMed] [Google Scholar]
- 56.Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–7. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Couch FJ, Wang XY, Wu GJ, Qian J, Jenkins RB, James CD. Localization of PS6K to chromosomal region 17q23 and determination of its amplification in breast cancer. Cancer Res. 1999;59:1408–11. [PubMed] [Google Scholar]
- 58.Monni O, Barlund M, Mousses S, Kononen J, Sauter G, Heiskanen M, et al. Comprehensive copy number and gene expression profiling of the 17q23 amplicon in human breast cancer. Proc Natl Acad Sci USA. 2001;98:5711–6. doi: 10.1073/pnas.091582298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu GJ, Sinclair CS, Paape J, Ingle JN, Roche PC, James CD, et al. 17q23 amplifications in breast cancer involve the PAT1, RAD51C, PS6K, and SIGma1B genes. Cancer Res. 2000;60:5371–5. [PubMed] [Google Scholar]
- 60.Savelyeva L, Claas A, Matzner I, Schlag P, Hofmann W, Scherneck S, et al. Constitutional genomic instability with inversions, duplications, and amplifications in 9p23-24 in BRCA2 mutation carriers. Cancer Res. 2001;61:5179–85. [PubMed] [Google Scholar]
- 61.Maruani DM, Spiegel TN, Harris EN, Shachter AS, Unger HA, Herrero-González S, et al. Estrogenic regulation of S6K1 expression creates a positive regulatory loop in control of breast cancer cell proliferation. Oncogene. 2012 doi: 10.1038/onc.2011.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cicatiello L, Scafoglio C, Altucci L, Cancemi M, Natoli G, Facchiano A, et al. A genomic view of estrogen actions in human breast cancer cells by expression profiling of the hormone-responsive transcriptome. J Mol Endocrinol. 2004;32:719–75. doi: 10.1677/jme.0.0320719. [DOI] [PubMed] [Google Scholar]
- 63.Hua S, Kallen CB, Dhar R, Baquero MT, Mason CE, Russell BA, et al. Genomic analysis of estrogen cascade reveals histone variant H2A.Z associated with breast cancer progression. Mol Syst Biol. 2008;4:188. doi: 10.1038/msb.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis AM, Mao J, Naz B, Kohl JA, Rosenfeld CS. Comparative effects of estradiol, methyl-piperidino-pyrazole, raloxifene, and ICI 182 780 on gene expression in the murine uterus. J Mol Endocrinol. 2008;41:205–17. doi: 10.1677/JME-08-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009;37:4850–61. doi: 10.1093/nar/gkp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, Li Y, Klinge CM. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–95. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inaki K, Hillmer AM, Ukil L, Yao F, Woo XY, Vardy LA, et al. Transcriptional consequences of genomic structural aberrations in breast cancer. Genome Res. 2011;21:676–87. doi: 10.1101/gr.113225.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu K, Toral-Barza L, Discafani C, Zhang WG, Skotnicki J, Frost P, et al. mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer. 2001;8:249–58. doi: 10.1677/erc.0.0080249. [DOI] [PubMed] [Google Scholar]
- 69.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleming GF, Ma CX, Huo D, Sattar H, Tretiakova M, Lin L, et al. Phase II trial of temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-011-1910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.deGraffenried LA, Friedrichs WE, Russell DH, Donzis EJ, Middleton AK, Silva JM, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res. 2004;10:8059–67. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 72.Sokolosky ML, Stadelman KM, Chappell WH, Abrams SL, Martelli AM, Stivala F, et al. Involvement of Akt-1 and mTOR in sensitivity of breast cancer to targeted therapy. Oncotarget. 2011;2:538–50. doi: 10.18632/oncotarget.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–7. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 74.Sabine VS, Sims AH, Macaskill EJ, Renshaw L, Thomas JS, Dixon JM, et al. Gene expression profiling of response to mTOR inhibitor everolimus in pre-operatively treated post-menopausal women with oestrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2010;122:419–28. doi: 10.1007/s10549-010-0928-6. [DOI] [PubMed] [Google Scholar]
- 75.O’Regan R, Hawk NN. mTOR inhibition in breast cancer: unraveling the complex mechanisms of mTOR signal transduction and its clinical implications in therapy. Expert Opin Ther Targets. 2011;15:859–72. doi: 10.1517/14728222.2011.575362. [DOI] [PubMed] [Google Scholar]
- 76.Hu X, Stern HM, Ge L, O’Brien C, Haydu L, Honchell CD, et al. Genetic alterations and oncogenic pathways associated with breast cancer subtypes. Mol Cancer Res. 2009;7:511–22. doi: 10.1158/1541-7786.MCR-08-0107. [DOI] [PubMed] [Google Scholar]
- 77.Han W, Han MR, Kang JJ, Bae JY, Lee JH, Bae YJ, et al. Genomic alterations identified by array comparative genomic hybridization as prognostic markers in tamoxifen-treated estrogen receptor-positive breast cancer. BMC Cancer. 2006;6:92. doi: 10.1186/1471-2407-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hynes NE, Boulay A. The mTOR pathway in breast cancer. J Mammary Gland Biol Neoplasia. 2006;11:53–61. doi: 10.1007/s10911-006-9012-6. [DOI] [PubMed] [Google Scholar]
- 79.Generali D, Fox SB, Brizzi MP, Allevi G, Bonardi S, Aguggini S, et al. Down-regulation of phosphatidylinositol 3′-kinase/AKT/molecular target of rapamycin metabolic pathway by primary letrozole-based therapy in human breast cancer. Clin Cancer Res. 2008;14:2673–80. doi: 10.1158/1078-0432.CCR-07-1046. [DOI] [PubMed] [Google Scholar]