Abstract
Septin 7 is a conserved GTP-binding protein. In this study, we examined the localization and functions of Septin 7 during mouse oocyte meiotic maturation. Immunofluorescent analysis showed that intrinsic Septin 7 localized to the spindles from the pro-MI stage to the MII stage. Knockdown of Septin 7 by siRNA microinjection caused abnormal spindles and affected extrusion of the first polar body. Septin 7 mRNA tagged with myc was injected into GV stage oocytes to overexpress Septin 7. Overexpressed Myc-Septin 7 localized to the spindle and beneath the plasma membrane displaying long filaments. Fluorescence intensity of spindle α-tubulin in myc-Septin 7-injected oocytes was weaker than that of the control group, demonstrating that Septin 7 may influence recruitment of α-tubulin to spindles. MII oocytes injected with myc-Septin 7 exhibited abnormal chromosome alignment, and parthenogenetic activation failed to allow extrusion of the second polar body, suggesting that overexpression of Septin 7 may affect extrusion of the polar body by disturbing the alignment of chromosomes and regulating α-tubulin recruitment to spindles. In summary, Septin 7 may regulate meiotic cell cycle progression by affecting microtubule cytoskeletal dynamics in mouse oocytes.
Keywords: Septin 7, meiosis, mouse, oocyte, overexpression
Introduction
Septins are conserved GTPase proteins that were first found in yeasts as proteins associated with the neck filaments.1 Recent work has shown that Septins are also present in fungi, insects and vertebrates, but they do not exist in plants.2-4 The amount of Septin genes in different species vary, with seven in the budding yeast Saccharomyces cerevisiae and 14 in human.2,5 In vivo, Septins interact with the actin and microtubule cytoskeletons as well as with membranes. Current data suggest that they coordinate changes in cytoskeletal and membrane organization by acting as scaffolds that recruit factors to specific sites in a cell and/or act as barriers that segregate membrane areas into discrete domains. In mammalian cells, Septin depletion causes chromosome loss, defects in chromosome segregation and spindle elongation and a delay in cytokinesis. Septin filaments can assemble into bundles, providing templates or scaffolds localized in the discontinuous area of the plasma membrane. In mammalian systems, Septins and related proteins have also been implicated in other cellular processes, including exocytosis, apoptosis, leukemogenesis, carcinogenesis and neurodegeneration. Several human diseases including cancer and disorders including infertility are related to overexpression and mutation of Septins.6-8 Despite numerous reports in the past few years, many key questions remain about the functions of Septins. Both structure and assembly of Septin 7 have been discussed recently.8,9 Septins share a common domain organization: a conserved and distinct N-terminal GTP-binding domain and a C-terminal domain that is predicted to form a coiled-coil configuration. Many Septins possess additional extensions at their N and C termini, which have been proposed to be disordered Septins purified from Xenopus,10-13 yeast14 and Drosophila;15 mammalian cells form filaments with a diameter of 7–9 nm and of variable length.
Research on septins in yeasts provided more detailed results. At the mother-bud neck of yeasts, the Septin ring persists throughout the yeast cell cycle, acting as a spatial landmark for the proper localization of many proteins required for polarized membrane growth, spindle positioning, cytokinesis, cell wall synthesis and the selection of a new site of bud growth after cell division.16-19 In addition, the Septin cortex may allow cells to monitor local shape changes.20,21 Mammalian Septins not only localize to the plasma membrane, but also throughout the cytoplasm and are associated with the microtubule and actin cytoskeletons. Recent studies suggest that mammalian Septins function in cytoskeletal organization by acting as scaffolds for cytoskeleton-binding proteins.
Mammalian Septins have been proposed to regulate microtubules and microfilaments.22-24 Septins 2, 6 and 7 regulate cytoskeleton as a whole.12,25,26 In HeLa and MDCK cells, Septin 7 is required for stable kinetochore localization of CENP-E. Septin 7 stabilizes the kinetochore association of CENP-E by directly interacting with its C-terminal domain. Immunofluorescence result shows that Septin 7 filaments distribute along the mitotic spindle and terminate at the kinetochore marked by CENP-E. Septin 7-suppressed cells display reduced tension at kinetochores of bi-orientated chromosomes and activated the mitotic spindle checkpoint indicated by Mad2 and BubR1 labeling on these misaligned chromosomes.27 Septin 7 was suggested to be a specific subunit combining Septin 2 and Septin 6.28,29 Recent analysis reveal a heterotrimeric human SEPT 2-SEPT 6-SEPT 7 complex that was reconstituted in vitro.30 The structure of the complex exhibits a universal bipolar polymer, composed of an extended G domain, which forms oligomers and filaments by conserved interactions between adjacent nucleotide-binding sites and/or the N- and C-terminal extensions.
We recently reported the localization and roles of Septins 1 and 2 in mouse oocyte meiosis.31,32 In the present study, we explored the function of Septin 7 in oocyte meiosis through microinjection of Septin 7 siRNA to deplete Septin 7 expression and microinjection of Septin 7 mRNA to overexpress Septin 7.
Results
Subcellular localization and expression of Septin 7 during mouse oocyte meiotic maturation
To investigate the role of Septin 7 in mouse oocyte maturation, we first examined the dynamic distribution and expression of Septin 7 at different stages. Western blots showed that Septin 7 was expressed at all stages of oocyte maturation (Fig. 1A). For the subcellular localization of Septin 7, oocytes were processed for immunofluorescent staining at different stages of meiosis. Septin 7 was observed to distribute in the germinal vesicle. Shortly after GVBD (1–2 h of culture), Septin 7 began to migrate to the periphery of chromosomes until the MI spindle was formed. At MI, ATI and MII stages, Septin 7 stably localized to the spindle (Fig. 1B).
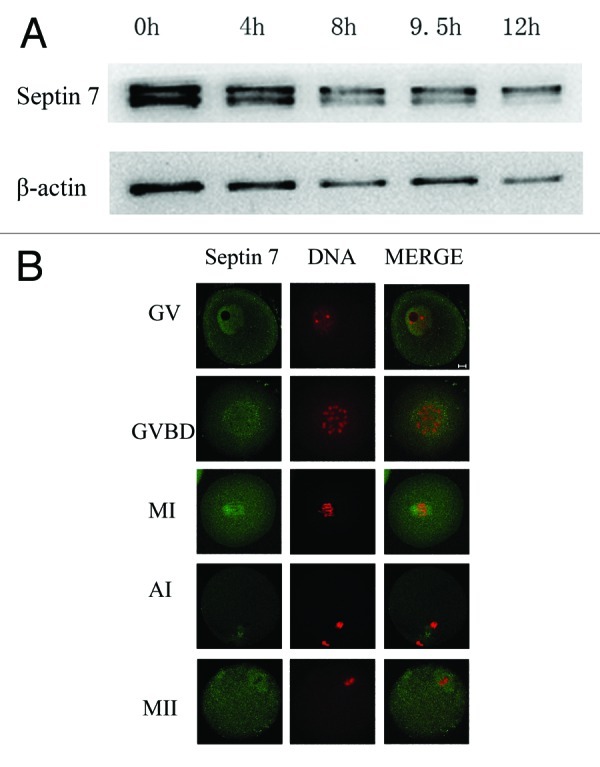
Figure 1. Subcellular localization and expression of Septin 7. (A) Samples were collected after oocytes had been cultured for 0, 2, 8, 9.5 and 12 h, corresponding to GV, GVBD, MI, AI and MII stages, respectively. The molecular mass of Septin 7 is 47 kDa and that of β-actin is 42 kDa. (B) Confocal microcopy showing immunostaining of Septin 7 (green) and DNA (red) in oocytes at GV, GVBD, pro-MI, MI, AI and MII stage. Bar = 10 μm.
Septin 7 depletion affects first polar body extrusion in mouse oocyte meiotic maturation
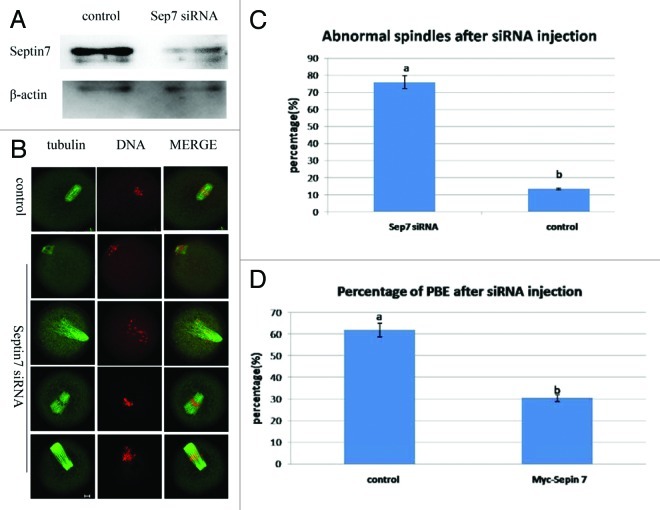
To assess its function, Septin 7 was knocked down by microinjection of Septin 7 siRNA. Compared with oocytes microinjected with control siRNA (control), western blot revealed that the expression of Septin 7 was significantly reduced in oocytes microinjected with Septin 7 siRNA (Fig. 2A). Septin 7-depleted and control oocytes were cultured for 10 h and then stained with α-tubulin and PI to assess oocyte stages. Both spindle configuration and polar body emission were disrupted after siRNA injection. The Septin 7 siRNA-injected oocytes displayed abnormal spindles, such as non-polar, mono-polar or multi-polar spindles (Fig. 2B). A total of 76% (75/99) of oocytes in the Septin 7 siRNA group displayed abnormal spindles, while only 13% (13/101) in the control group showed a similar phenotype (p < 0.05)(Fig. 2C). Only 30% (30/99) oocytes in the Septin 7 siRNA group progressed to the MII stage, while, 66% (62/101) oocytes in the control group developed to the MII stage (p < 0.05) (Fig. 2D).
Figure 2. Microinjection of Septin 7 siRNA affected spindle organization and first polar body extrusion. After microinjection of Septin 7 siRNA, the oocytes were incubated in M2 medium containing 2.5 μM milrinone for 21 h, followed by transfer to milrinone-free M2 medium for 13 h. (A) Western blot of Septin 7 in the Septin 7 siRNA group and control group. The Septin 7 molecular mass is 47 kDa and that of β-actin is 42 kDa. (B) After microinjection, oocytes microinjected with Septin 7 siRNA had abnormal spindles but the spindles of control oocytes were not affected. Double staining of α-tubulin (green) and DNA (red). Bar = 10 μm. (C) Percentage of oocytes with abnormal spindles in the Septin 7 siRNA-injected group (n = 99) and control group (n = 101). Data are presented as mean ± SE. Different superscripts indicate statistical difference (p < 0.05). (D) Percentage of PBE in the Septin 7 siRNA group (n = 99) and control group (n = 101). Data are presented as mean ± SE. Different superscripts indicate statistical difference (p < 0.05).
Localization and expression of myc-Septin 7 in mouse oocyte meiosis maturation
We overexpressed exogenous myc-Septin 7 to assess its function. Full-length Septin mRNA tagged with myc, transcribed from plasmid Septin 7 pCS2 plus in vitro, was injected into GV stage oocytes. Control mRNA was also injected into the cytoplasm of mouse oocytes. After culture for 2 h with 2.5 μM milrinone, oocytes were transferred to milrinone-free M2 medium. First, western blot was conducted to confirm the myc-Septin 7 fusion protein expression after exogenous mRNA injection (Fig. 3A). When the anti-Septin 7 antibody was used, the myc-Septin 7 group showed three bands (the above band was myc-Septin 7, other two bands were intrinsic Septin 7), while only exogenous myc-Septin 7 was detected when anti-myc antibody was used in the injection group. When stained with anti-FITC myc antibody, Septin 7 was observed to distribute as disordered filaments in the cytoplasm at the GV stage. After GVBD, myc-Septin 7 showed two patterns of location up to the MII stage. It was localized at the spindle but different from microtubule detection. It was also localized in the cytoplasm and detected as long filaments (Fig. 3B).
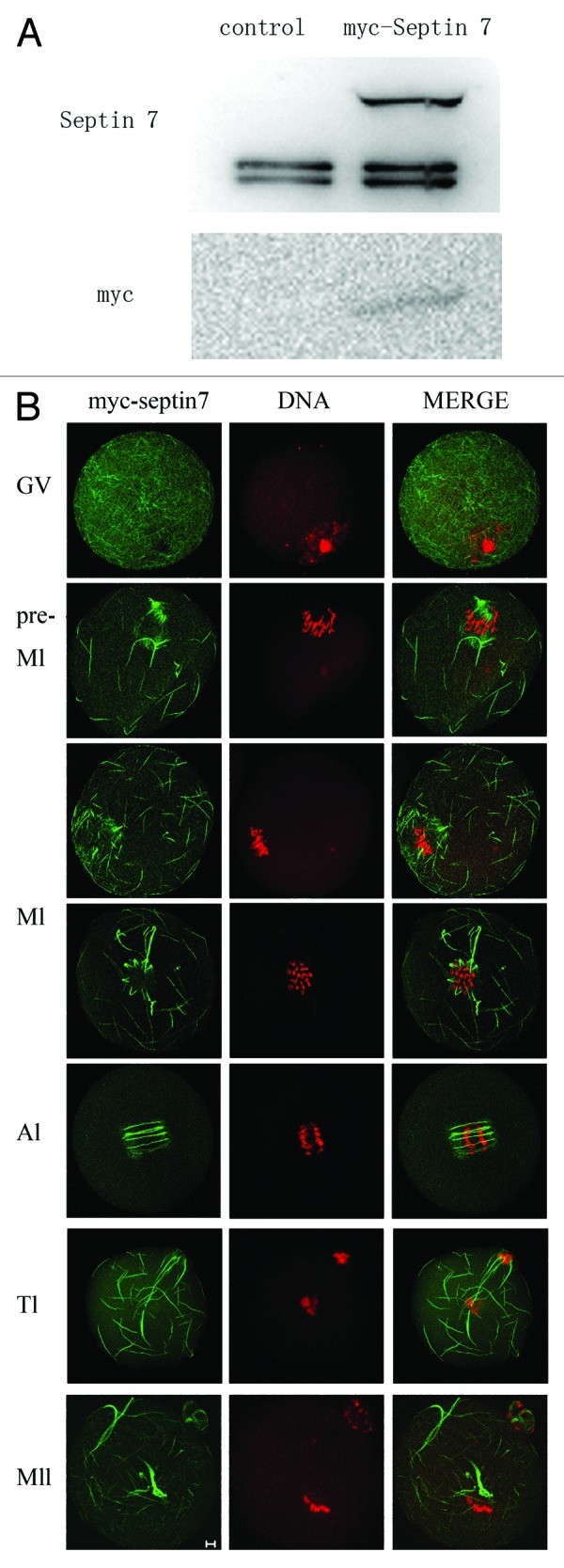
Figure 3. Western blotting and subcellular localization of exogenous myc-Septin 7. (A) Samples from control and the overexpression group were collected to test the expression of myc-Septin 7. At the top of the figure western blotting shows Septin 7 expression as detected by antibody against Septin 7. In the injection group, three bands were detected. The molecular mass of myc tag is about 10 kDa. The 57 kDa molecular mass is that of myc-Septin 7 fusion protein, and the 47 kDa molecular mass is that of endogenous Septin 7 protein. At the bottom of the figure, western blotting shows a band detected with antibody against myc. Only one band was detected in the injection group and the molecular mass of myc-Septin 7 is 56 kDa. (B) Samples were collected after oocytes had been cultured for 0, 4, 8, 9.5, 10 and 12 h, corresponding to GV, pre-MI, MI, AI, TI and MII stages, respectively. Confocal microcopy shows immunostaining of Septin 7 (green) and DNA (red) in oocytes at different stages. Bar = 10 μm.
The disordered localization of Myc-Septin 7 in the equatorial plane caused us to explore whether there is a regular pattern. Sequences of images were obtained to examine its specific localization. The images showed that myc-Septin 7 was mainly localized at the spindles and beneath the plasma membrane. Figure 4B shows the composite image of Figure 4A, and we were able to determine that myc-Septin 7 distributed in the cytoplasm appearing as a ball composed of strings.
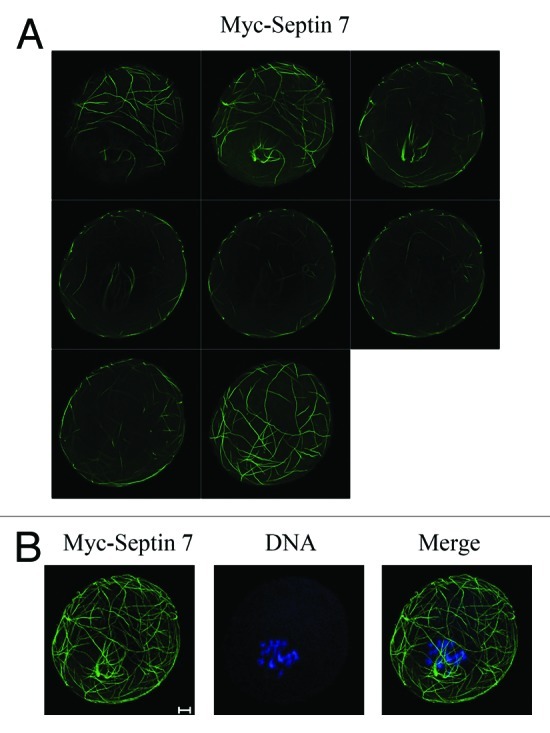
Figure 4. Sequential images showing the precise localization of myc-Septin 7. After myc-Septin 7 microinjection, oocytes injected with myc-Septin 7 were fixed and examined with a confocal laser scanning microscope. (A) Sequential images show the myc-Septin 7 subcelluar localization. (B) Composite image of Figure 4A to show the entire image of myc-Septin 7 localization. DNA was stained with Hoechst 33342. Bar = 10 μm.
Overexpression of Septin 7 affects the localization of microtubules in the spindles
Myc mRNA and myc-Septin 7 mRNA were injected separately into mouse oocytes. After microinjection, the oocytes were maintained at the GV stage for 2 h in M2 medium containing 2.5 μM milrinone and then released into M2 culture medium. To determine the relationship between Septin 7 and α-tubulin detection, the control group and the myc-Septin 7 group in MI and MII stages were collected to perform confocal microscopy. In order to compare the fluorescence intensity of α-tubulin, the parameters set was determined for consistency by scanning every oocyte. Figure 5A shows that even when the exogenous myc-Septin 7 was low, the fluorescence intensity of microtubule detection was significantly weaker than that of the control group. This result demonstrates that Septin 7 may affect the recruitment of α-tubulin to the spindle.
Figure 5. Comparison of microtubule fluorescence in the myc-Septin 7 group and control group. (A) Oocytes of the control group and the myc-Septin 7 group were cultured for 9 h and 12 h, followed by confocal observation. Parameter setup of the confocal microscope was the same in each picture. green, myc-Septin 7; purple, microtubules; red, DNA. Bar = 10 μm.
Overexpression of Septin 7 causes abnormal chromosome alignment and inhibits the extrusion of the second polar body
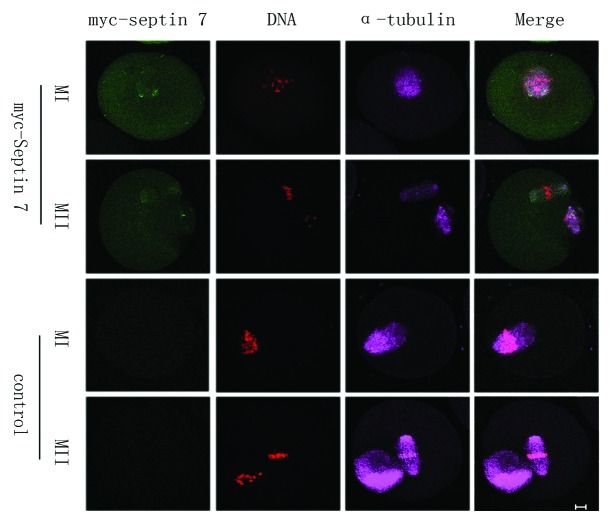
The above results revealed that overexpression of Septin 7 affected the distribution of α-tubulin at spindles, so we reasoned that Septin 7 may affect the extrusion of the second polar body. We employed parthenogenetic activation to explore this effect. Myc mRNA and myc-Septin 7 mRNA were injected separately into mouse oocytes. After microinjection, the oocytes were arrested at the GV stage for 2 h in M2-containing 2.5 μM milrinone and then released in M2 culture medium and cultured for 12 h. Parthenogenetic activation was performed by treatment of MII oocytes in the myc-Septin 7 group using SrCl2. The immunofluorescent results showed that control oocytes were able to extrude the second polar body, while the myc-Septin 7 group displayed spindles parallel to the plasma membrane beneath the first polar body. The alignment of chromosomes in the myc-Septin 7 group was extremely disordered, and some chromosomes escaped from the spindles (Fig. 6A). Data analysis showed that only 11% (6/55) oocytes in the myc-Septin 7 group extruded the second polar body, which was significantly lower than that of the control group (53%,31/58)(Fig. 6B). Besides, 100% (55/55) oocytes in myc-Septin 7 group showed abnormal spindles, but only 6% (3/58) oocytes in the control group showed a similar phenotype (p < 0.05)(Fig. 6C).
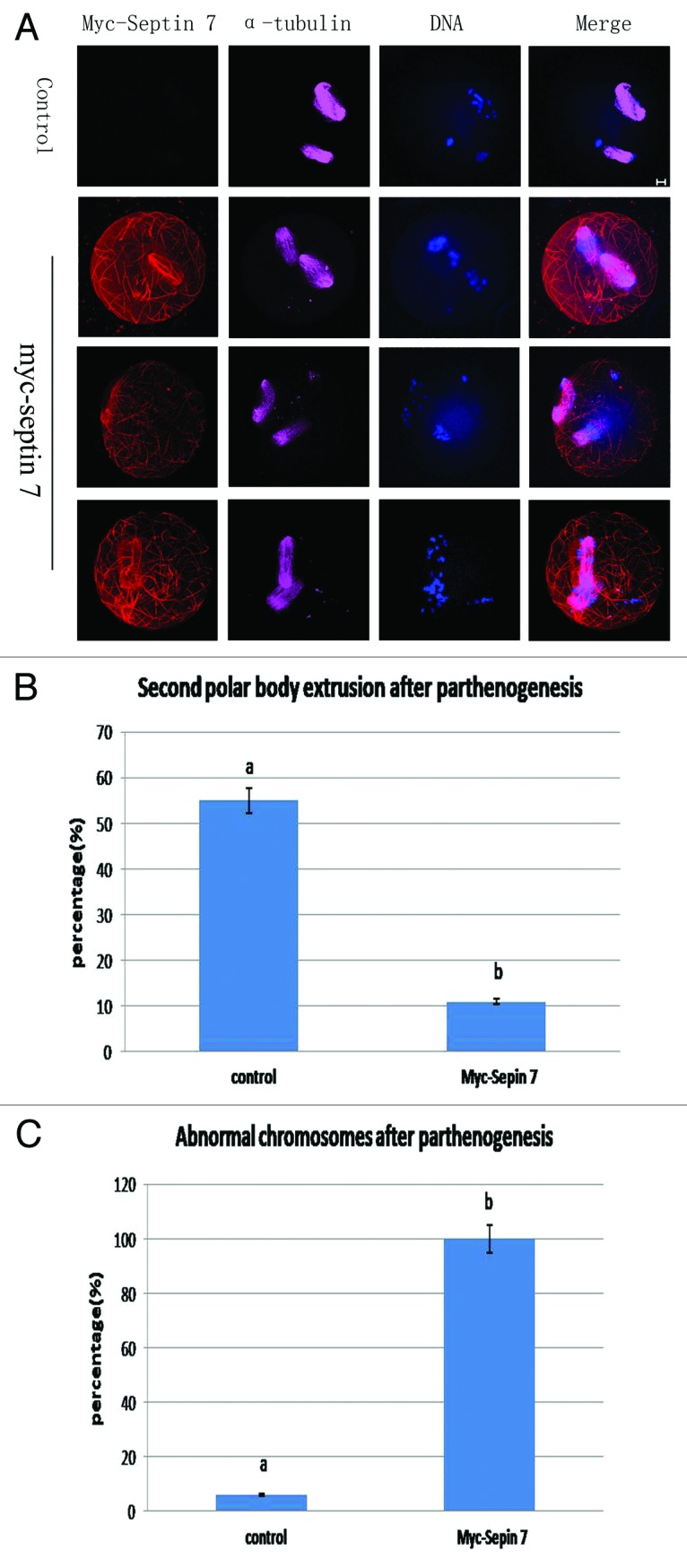
Figure 6. Septin 7 overexpression affected chromosome alignment and second polar body extrusion. (A) Oocytes of the control group and myc-Septin 7 group were cultured for 13 h, then parthenogenesis was induced by Srcl2,followed by confocal observation. Oocytes injected myc-Septin 7 had misaligned chromosomes. Green, F-actin; purple, microtubules; red, myc-Septin 7; blue, DNA. Bar = 10 μm. (B) Percentages of PBE in the Septin 7 mRNA group (55) and control group (n = 58). Data are presented as mean ± SE. Different superscripts indicate statistical difference (p < 0.05). (C) Percentage of oocytes with misaligned chromosomes in the Septin 7 mRNA-injected group (n = 55) and control group (n = 58). Data are presented as mean ± SE. Different superscripts indicate statistical difference (p < 0.05).
Discussion
Although numerous studies have been published on yeast Septins, the functions of Septins in mammalian cells are far from clear. In this study, we for the first time revealed the localization and possible functions of Septin 7 in meiotic oocytes. We demonstrated that knockdown of Septin 7 caused increase in meiotic spindle defects and decrease in the extrusion of the first polar body. Overexpression of Septin 7 disturbed the alignment of chromosomes and the recruitment of α-tubulin to the spindles, thereby affecting the extrusion of the second polar body.
Mammalian Septins are believed to have two different functions. One is chromosome segregation, and the other is cytokinesis.22,33 Cdc10 (Septin 7’s homolog) was reported to have a pivotal role in the G1/S transition in yeasts,34 but its roles in the cell cycle are unclear. It was reported that Septin 7 and 12 filaments start to appear around the acrosome at step 7 of spermiogenesis. At step 10–11 of spermiogenesis, Septin 7 and 12 form a circular structure between the edge of the acrosome and the perinuclear mantle of the manchette. With the formation of mitochondria, Septin 7 and 12 are first localized at the sperm neck and annulus. In mature spermatozoa, the Septin 7 and 12 signals are located at the sperm head, neck and midpiece with scanty signals at the tail.35,36 The functions of Septin in oocyte meiosis are largely unknown except for our two recent reports on the roles of Septins 1 and 2 in oocyte maturation.31,32
Septin 7 and Septin 9 were reported to be important in daughter cell segregation in Hela cells.37 First we used the anti-Septin 7 antibody to label the oocytes and observed the expression and localization of endogenous Septin 7 in mouse oocytes. Immunofluorescence results showed that Septin 7 was localized in the spindles. Depletion of Septin 7 expression caused abnormal spindles, suggesting that Septin 7 is important for proper meiotic spindle organization. Septin 7-depleted oocytes displayed abnormal spindles at a high rate. To test the effect on microfilaments in Septin 7-depleted oocytes with phalloidin (results not shown), we found no difference in the phenotype between the control group and the myc-Septin 7 group. The actin cap still existed during the extrusion of the second polar body. This is unlike the function reported for Septin7 in yeast, which affected cytokinesis. Our result indicates that Septin 7 serves specific roles in meiosis. Depletion of Septins caused loss of chromosomes at the equator, chromosome missegregation, abnormal spindles and incomplete cytokinesis. It was reported that SEPT 7-CENP-E interaction affected the distribution of CENP-E to the kinetochore and chromosome alignment.27 It was suggested that mammalian Septin isoforms might form a novel scaffold at the midplane of the mitotic spindle that coordinates several key steps in mammalian mitosis. Our results showed that knockdown of Septin 7 inhibited first polar body emission, while overexpression caused decreased extrusion of the second polar body. Chromosomes were not well aligned, and some became displaced from spindles and became distributed in the cytoplasm. Misaligned chromosomes may be the primary reason for the decreased extrusion of the polar body. In such oocytes, the second meiotic spindle rotation did not occur after parthenogenic activation.
To characterize the roles of Septin 7 in meiosis, we conducted overexpression experiments. When myc-Septin 7 mRNA was injected into mouse oocytes, filamentous Septin 7 distributed disorderly in the ooplasm. However, when the oocytes were scanned at different levels, we found that myc-Septin 7 mainly distributed beneath the plasma membrane and on the spindles. The localization of myc-septin 7 on the spindles did not overlap with the spindle microtubules. Microtubules provide a structural network that transports and positions membrane-bound organelles and large molecules, controlling cell division, cell movement and cell signaling. Microtubules are highly dynamic, undergoing constant assembly and disassembly. A number of cellular factors regulate this process by binding microtubules and affecting their stability.38 One family of regulators consists of the microtubule-associated proteins (MAPs), which bind and stabilize MTs.39 A novel molecular function for Septins in mammalian cells was the modulation of microtubule dynamics through interaction with MAP4.25 MAP4 was identified as a Septin binding partner. A small, proline-rich region in the C-terminal half of MAP4 bound directly to a Septin 2:6:7 heterotrimer, the trimer blocking the ability of this MAP4 fragment to bind and bundle microtubules in vitro. On the other hand, knockout of Septin 9 was reported to reduce the level of tubulin polymerization.40 In our study, myc-labeled Septin 7 mRNA was transcribed in vitro and then microinjected into the oocytes. We found that the fluorescence intensity of α-tubulin in spindles in the myc-Septin 7 group was significantly reduced (Fig. 5A), and the chromosomes were severely disordered. Most importantly, the oocytes in the myc-Septin 7 group rarely extruded the second polar body after parthenogenetic activation, and the oocytes were not able to complete meiosis. It may be possible that overexpression of Septin 7 was involved in regulating the recruitment of α-tubulin on spindles, severely affecting the alignment of chromosomes, thus affecting the progress of meiosis.
In summary, loss of Septin 7 expression affects proper meiotic spindle organization, chromosome alignment and polar body emission in mouse oocytes. Meiotic spindle assembly, polarity formation and polar body emission depend on accumulation of RanGTP in vertebrates meiotic oocytes,41 and the relationship between Ran and Septin 7 may be an interesting topic for future research.
Materials and Methods
All chemicals and culture media were purchased from Sigma Chemical Company except for those specifically mentioned.
Oocyte collection and culture
Four- to six-weeks-old KM mice were used in this study. Mouse care and use were conducted in accordance with the Animal Research Committee guidelines of the Institute of Zoology, Chinese Academy of Sciences. Mice were housed in a temperature-controlled room with proper darkness-light cycles, fed with a regular diet, and maintained under the care of the Laboratory Animal Unit, Institute of Zoology, Chinese Academy of Sciences. The mice were killed by cervical dislocation. The only procedure performed on the dead animals was the collection of oocytes from the ovary.
Oocytes were collected in M2 medium supplemented with 2.5 μM milrinone to maintain them at the germinal vesicle (GV) stage. Then oocytes were washed six times to remove the effect of milrinone and then cultured in M2 medium to the GV, GVBD, pro-MI, MI, ATI and MII stages.
Immunofluorescence and confocal microscopy
Immunofluorescence was performed as described previously.42 Oocytes were fixed with 4% paraformaldehyde/PBS (pH 7.4) for at least 30 min. After being permeabilized with 0.5% Triton X-100 at room temperature for 20 min, oocytes were blocked in 1% BSA-supplemented PBS for 1 h and then incubated with rabbit anti-Septin 7 antibody (Santa Cruz; 1:100) or anti-α-tubulin antibody (Sigma; 1:200), respectively, overnight at 4°C. After three washes with PBS containing 0.1% Tween 20 and 0.01% Triton X-100 for 5 min each, the oocytes were labeled with FITC conjugated goat-anti-rabbit IgG (Zhong Shan Jin Qiao; 1:100), TRITC conjugated goat-anti-rabbit IgG (Zhong Shan Jin Qiao; 1:100), Cy5-anti-mouse IgG (Jackson; 1:100) or FITC-anti-mouse IgG (Zhong Shan Jin Qiao; 1:100) for 1 h at room temperature and then washed three times with PBS containing 0.1% Tween-20 and 0.01% Triton X-100. The oocytes were co-stained with Hoechst 33342 or PI. Finally, the oocytes were mounted on glass slides and examined with a confocal laser scanning microscope (Zeiss LSM 510 META).
Construction of plasmids for Septin 7 and in vitro transcription of RNA
Total RNA was extracted from 150 oocytes using RN-easy micro purification kit (QIAGEN) at the GV stage and then reversely transcribed to cDNA with oligo dT primer using PrimeScript 1st Strand cDNA Synthesis Kit(TaKaRa). The full length Septin 7 coding sequence was amplified by Nested PCR with the following primers: F1,GAATCGGCGTAGGTGGTT;R1:GTTTCCTCGTTGGTTCCT;F2:TCAGGCCGGCCGATGTCGGTCAGTGCGAGAT;R2:GTTGGCGCGCCTCAAAAGATCTTGCCTTTCTTCT and then cloned at Fse Is and Asc I of pCS2 plus vector. The pCS2 plus vector, which has a myc tag, allows in vitro transcription of polyadenylated mRNA from SP6 promoter.
In vitro synthesis of capped RNAs was performed using linearized plasmids with the mMessage mMachine kit (Ambion). The mRNAs were purified on RN-easy columns (QIAGEN) and eluted in H2O to a final concentration of 2.0–3.0 mg/ml.
Microinjection of Septin 7 siRNA and myc-Septin 7
Microinjections were performed using a Nikon Diaphot ECLIPSE TE 300 (Nikon UK Ltd.) and completed within 30 min. The final concentration of the control or Septin 7 siRNA was 25 μM. 5–10 pl control siRNA or specific Septin 7 siRNA (Ambion), for which the sequence GACAAUAGUUGAUACUCCAtt was microinjected into the cytoplasm. After microinjection, the oocytes were arrested at the GV stage for 21 h in M2-containing 2.5 μM milrinone to knock down Septin 7. Similarly, the same amount of Myc mRNA and myc-Septin 7 mRNA (Ambion) was injected into mouse oocytes. After microinjection, the oocytes were arrested at the GV stage for 2 h in M2-containing 2.5 μM milrinone and then released in M2 culture medium. Each experiment consisted of three separate replicates and approximately 300 oocytes were injected in each group.
Immunoblotting analysis
Immunoblotting was performed as described previously.43 Briefly, 300 mouse oocytes were collected in SDS sample buffer and heated for 5 min at 100°C. The proteins were separated by SDS-PAGE and then electrically transferred to polyvinylidene fluoride membranes. Following transfer, the membranes were blocked in TBST containing 5% skimmed milk for 2 h, followed by incubation overnight at 4°C with rabbit polyclonal anti-Septin 7 antibody (1:500) and mouse monoclonal anti-β-actin antibody (1:1000). After washing three times in TBST, 10 min each, the membranes were incubated for 1 h at 37°C with peroxidase-conjugated rabbit anti-rabbit IgG (1:1000) and peroxidase-conjugated rabbit anti-mouse IgG, respectively. Finally, the membranes were processed using the SuperSignal West Femicrotubuleo maximum sensitivity substrate (Thermo Scientific).
Parthenogenetic activation of oocytes
After mRNA microinjection, the oocytes were arrested at the GV stage for 2 h in M2-containing 2.5 μM milrinone and then released in M2 culture medium and cultured for 13 h. Subsequently, oocytes were cultured in pre-balanced 10 mmol/L SrCl2-CZB for 6 h, then the extrusion of the second polar body was observed, and oocytes were collected for immunofluorescence analysis.
Statistical analysis
Data (mean ± SE) were from at least three replicates per experiment and analyzed by ANOVA using SPSS software (SPSS Inc.) followed by Student-Newman-Keuls test. Difference at p < 0.05 was considered to be statistically significant and different superscripts indicate the statistical difference.
Acknowledgments
We are grateful to Shi-Wen Li, Yi Hou for their technical assistance, and Drs. Bao-Zeng Xu, Jing-Shan Tong, for insightful suggestions on the manuscript. This study was supported by the National Basic Research Program of China (2012CB944404,2011CB944501) and National Natural Science Foundation of China (No. 30930065).
Glossary
Abbreviations:
- GVBD
germinal vesicle breakdown
- pro-MI
prometaphase of meiosis I
- MI
metaphase of meiosis I
- AI
anaphase of meiosis I
- TI
telophase of meiosis I
- MII
metaphase of meiosis II
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21553
References
- 1.Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976;69:717–21. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan F, Malmberg RL, Momany M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol. 2007;7:103. doi: 10.1186/1471-2148-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, et al. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–19. doi: 10.1016/S0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- 4.Spiliotis ET, Nelson WJ. Here come the septins: novel polymers that coordinate intracellular functions and organization. J Cell Sci. 2006;119:4–10. doi: 10.1242/jcs.02746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall PA, Jung K, Hillan KJ, Russell SE. Expression profiling the human septin gene family. J Pathol. 2005;206:269–78. doi: 10.1002/path.1789. [DOI] [PubMed] [Google Scholar]
- 6.Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4:681–9. doi: 10.1016/S1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- 7.Longtine MS, Bi E. Regulation of septin organization and function in yeast. Trends Cell Biol. 2003;13:403–9. doi: 10.1016/S0962-8924(03)00151-X. [DOI] [PubMed] [Google Scholar]
- 8.Weirich CS, Erzberger JP, Barral Y. The septin family of GTPases: architecture and dynamics. Nat Rev Mol Cell Biol. 2008;9:478–89. doi: 10.1038/nrm2407. [DOI] [PubMed] [Google Scholar]
- 9.McMurray MA, Thorner J. Septins: molecular partitioning and the generation of cellular asymmetry. Cell Div. 2009;4:18. doi: 10.1186/1747-1028-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versele M, Gullbrand B, Shulewitz MJ, Cid VJ, Bahmanyar S, Chen RE, et al. Protein-protein interactions governing septin heteropentamer assembly and septin filament organization in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:4568–83. doi: 10.1091/mbc.E04-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John CM, Hite RK, Weirich CS, Fitzgerald DJ, Jawhari H, Faty M, et al. The Caenorhabditis elegans septin complex is nonpolar. EMBO J. 2007;26:3296–307. doi: 10.1038/sj.emboj.7601775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheffield PJ, Oliver CJ, Kremer BE, Sheng S, Shao Z, Macara IG. Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J Biol Chem. 2003;278:3483–8. doi: 10.1074/jbc.M209701200. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of Mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/S1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- 14.Frazier JA, Wong ML, Longtine MS, Pringle JR, Mann M, Mitchison TJ, et al. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J Cell Biol. 1998;143:737–49. doi: 10.1083/jcb.143.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field CM, al-Awar O, Rosenblatt J, Wong ML, Alberts B, Mitchison TJ. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J Cell Biol. 1996;133:605–16. doi: 10.1083/jcb.133.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enserink JM, Smolka MB, Zhou H, Kolodner RD. Checkpoint proteins control morphogenetic events during DNA replication stress in Saccharomyces cerevisiae. J Cell Biol. 2006;175:729–41. doi: 10.1083/jcb.200605080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smolka MB, Chen SH, Maddox PS, Enserink JM, Albuquerque CP, Wei XX, et al. An FHA domain-mediated protein interaction network of Rad53 reveals its role in polarized cell growth. J Cell Biol. 2006;175:743–53. doi: 10.1083/jcb.200605081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadota J, Yamamoto T, Yoshiuchi S, Bi E, Tanaka K. Septin ring assembly requires concerted action of polarisome components, a PAK kinase Cla4p, and the actin cytoskeleton in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:5329–45. doi: 10.1091/mbc.E04-03-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keaton MA, Lew DJ. Eavesdropping on the cytoskeleton: progress and controversy in the yeast morphogenesis checkpoint. Curr Opin Microbiol. 2006;9:540–6. doi: 10.1016/j.mib.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Castillon GA, Adames NR, Rosello CH, Seidel HS, Longtine MS, Cooper JA, et al. Septins have a dual role in controlling mitotic exit in budding yeast. Curr Biol. 2003;13:654–8. doi: 10.1016/S0960-9822(03)00247-1. [DOI] [PubMed] [Google Scholar]
- 21.Kusch J, Meyer A, Snyder MP, Barral Y. Microtubule capture by the cleavage apparatus is required for proper spindle positioning in yeast. Genes Dev. 2002;16:1627–39. doi: 10.1101/gad.222602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiliotis ET, Kinoshita M, Nelson WJ. A mitotic septin scaffold required for Mammalian chromosome congression and segregation. Science. 2005;307:1781–5. doi: 10.1126/science.1106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13:3532–45. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vega IE, Hsu SC. The septin protein Nedd5 associates with both the exocyst complex and microtubules and disruption of its GTPase activity promotes aberrant neurite sprouting in PC12 cells. Neuroreport. 2003;14:31–7. doi: 10.1097/00001756-200301200-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kremer BE, Haystead T, Macara IG. Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol Biol Cell. 2005;16:4648–59. doi: 10.1091/mbc.E05-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low C, Macara IG. Structural analysis of septin 2, 6, and 7 complexes. J Biol Chem. 2006;281:30697–706. doi: 10.1074/jbc.M605179200. [DOI] [PubMed] [Google Scholar]
- 27.Zhu M, Wang F, Yan F, Yao PY, Du J, Gao X, et al. Septin 7 interacts with centromere-associated protein E and is required for its kinetochore localization. J Biol Chem. 2008;283:18916–25. doi: 10.1074/jbc.M710591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinoshita M. Assembly of mammalian septins. J Biochem. 2003;134:491–6. doi: 10.1093/jb/mvg182. [DOI] [PubMed] [Google Scholar]
- 29.Versele M, Thorner J. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J Cell Biol. 2004;164:701–15. doi: 10.1083/jcb.200312070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sirajuddin M, Farkasovsky M, Hauer F, Kühlmann D, Macara IG, Weyand M, et al. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–5. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Qi ST, Wang YP, Wang ZB, Ouyang YC, Hou Y, et al. Septin1 is required for spindle assembly and chromosome congression in mouse oocytes. Dev Dyn. 2011;240:2281–9. doi: 10.1002/dvdy.22725. [DOI] [PubMed] [Google Scholar]
- 32.Zhu JL, Lin SL, Li M, Ouyang YC, Hou Y, Schatten H, et al. Septin2 is modified by SUMOylation and required for chromosome congression in mouse oocytes. Cell Cycle. 2010;9:1607–16. doi: 10.4161/cc.9.8.11463. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, et al. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–47. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 34.Kinoshita M, Noda M. Roles of septins in the mammalian cytokinesis machinery. Cell Struct Funct. 2001;26:667–70. doi: 10.1247/csf.26.667. [DOI] [PubMed] [Google Scholar]
- 35.Lin YH, Lin YM, Wang YY, Yu IS, Lin YW, Wang YH, et al. The expression level of septin12 is critical for spermiogenesis. Am J Pathol. 2009;174:1857–68. doi: 10.2353/ajpath.2009.080955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao HC, Lin YH, Kuo YC, Shen CJ, Pan HA, Kuo PL. The expression pattern of SEPT7 correlates with sperm morphology. J Assist Reprod Genet. 2010;27:299–307. doi: 10.1007/s10815-010-9409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estey MP, Di Ciano-Oliveira C, Froese CD, Bejide MT, Trimble WS. Distinct roles of septins in cytokinesis: SEPT9 mediates midbody abscission. J Cell Biol. 2010;191:741–9. doi: 10.1083/jcb.201006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–42. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 39.Nogales E. Structural insights into microtubule function. Annu Rev Biochem. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- 40.Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, et al. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278:18538–43. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- 41.Dumont J, Petri S, Pellegrin F, Terret ME, Bohnsack MT, Rassinier P, et al. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol. 2007;176:295–305. doi: 10.1083/jcb.200605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang ZB, Ou XH, Tong JS, Li S, Wei L, Ouyang YC, et al. The SUMO pathway functions in mouse oocyte maturation. Cell Cycle. 2010;9:2640–6. doi: 10.4161/cc.9.13.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei L, Liang XW, Zhang QH, Li M, Yuan J, Li S, et al. BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte. Cell Cycle. 2010;9:1112–21. doi: 10.4161/cc.9.6.10957. [DOI] [PubMed] [Google Scholar]