Abstract
The majority of colorectal cancers (CRCs) are characterized by a dysregulated canonical Wnt-signaling pathway leading to the stabilization and subsequent cellular increase and accumulation of β-catenin. After translocation into the nucleus, it acts as a transcription factor resulting in the expression of β-catenin target genes. These resemble most of the hallmarks of cancer except eternal life. The central mediator of this hallmark is hTERT (human telomerase reverse transcriptase). The hTERT gene is regulated, besides others, by the transcription factor c-Myc and, thus, indirectly via β-catenin as c-Myc is a β-catenin target gene. Interestingly, the expression patterns of hTERT and β-catenin, but not c-Myc are overlapping, probably because c-Myc is not only regulated by β-catenin, but also by many other transcription factors and pathways. Therefore, we argued that hTERT might be a direct target gene of β-catenin. In this study, we show evidence that β-catenin directly regulates the expression of the hTERT gene.
Keywords: hTERT, carcinogenesis, colorectal cancer, eternal life, hallmark of cancer, β-catenin
Introduction
The majority of human colorectal carcinomas (CRCs) are characterized by the dysregulation of the canonical Wnt-signaling pathway. This pathway controls the stability of β-catenin, which is a protein with ambivalent functions.1 On the one hand, it maintains the epithelial phenotype of cells in the context of E-Cadherin as an integral part of the zonula adherens.2 On the other, it drives together with DNA binding factors of the TCF (T-cell factor)/LEF-1 (lymphocyte enhancing factor-1) family the transcription of a variety of target genes when it has translocated into the nucleus.3 β-catenin target genes are characterized by consensus binding sites (WWCAAG) for TCFs/LEF-1 in their promoter/enhancer regions, which is known as the TCF-4 binding element (TBE).4 The expression of β-catenin target genes results in a dramatic change of the cell biology, whereby the target genes sustain the hallmarks of cancer.5 These hallmarks are epithelial-mesenchymal transition (EMT; Slug, Snail, Vimentin, Fibronectin),6 stemness,7 chemoresistance (MDR-1, multi drug resistance-gene),8 proliferation (c-Myc,9 cyclin D1,10,11 p16INK4A12), angiogenesis (VEGF, vascular endothelial growth factor),13 resistance to apoptosis (Survivin),14 as well as invasion and migration (MMP7, matrix metalloproteinase 7,8 uPA, urokinase plasminogen activator,15 Tenascin-C16), besides others. Another essential hallmark for the survival of tumor cells is eternal life, which is usually connected with the expression of the hTERT (human telomerase reverse transcriptase) gene in the majority of all tumor types or sometimes ALT (alternative telomere lengthening),17 as the lengthening of telomers is linked to the evasion from replication induced senescence and subsequently cell death.5,18 Additionally, hTERT is expressed in intestinal stem cells (SCs),19,20 which are also characterized by active Wnt-signaling what is essential for the maintenance of stemness in adult stem cells,21 as well as colorectal cancer stem cells (coCSCs).7,22 A connection between the activity of β-catenin and the regulation of the expression of the hTERT gene had already been drawn by c-Myc, which is a target gene of β-catenin9 and regulates at the same time the transcription of the hTERT gene.23 But, whereas c-Myc is found to be expressed in all tumor cells,24 hTERT was shown to be expressed only in some tumor cells mostly at the invasive front of CRC25 and thereby reflects the expression pattern of β-catenin.26 Additionally, the c-Myc-gene is not only regulated by β-catenin, but a plethora of other signaling pathways as well.27 Thus, it is reasonable that the expression of nuclear β-catenin, with all its consequences, might become decoupled from the hallmark eternal life, as other signaling traits might overdrive the expression of the c-Myc gene. Thus, we speculated that the hTERT gene might be directly regulated by β-catenin. This hypothesis was additionally supported by the finding that the hTERT gene contains at least one TCF-4 binding site.28 Here, we show evidence that hTERT is another direct target gene of β-catenin, and that therefore the hallmark eternal life is directly controlled by β-catenin in human colorectal cancer. During the preparation of this manuscript, the relationship of hTERT and β-catenin was also demonstrated in embryonic and adult stem cells as well as in colorectal cancer cell lines.29
Results
hTERT and β-catenin are coexpressed at the invasive front of human colorectal cancers
Many CRCs are characterized by an invasive front with tumor cells displaying β-catenin. These tumor cells also express β-catenin target genes.26,30 Therefore, invasive fronts of such CRCs are a useful screening tool for the identification of β-catenin target genes in human colorectal cancers. Moreover, it had already been shown that hTERT is expressed at the invasive front of CRC.25 But, no relation with the nuclear localization of β-catenin is known. Therefore, we investigated if nuclear β-catenin and hTERT displayed overlapping expression patterns employing serial histological sections of 24 human CRC with invasive fronts expressing nuclear β-catenin. It turned out that tumor cells with nuclear β-catenin (Fig. 1A) also strongly expressed hTERT (Fig. 1B). In contrast, tumor cells lacking nuclear β-catenin (Fig. 1C) did not display hTERT expression (Fig. 1D). Thus, it was reasonable to argue that β-catenin might be involved in the regulation of hTERT.
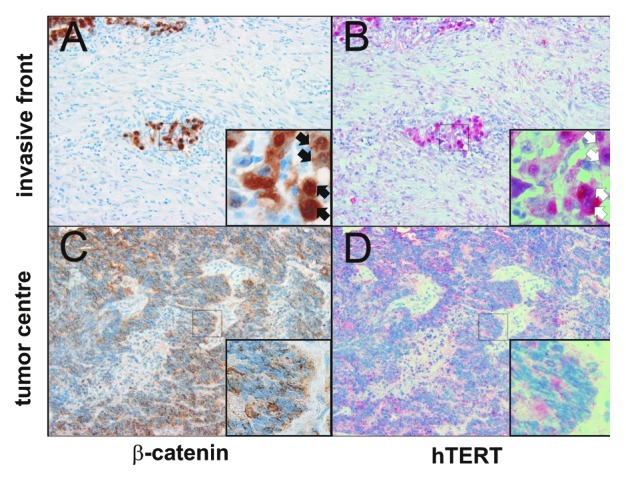
Figure 1. Coexpression of β-catenin and hTERT in tumor cells at the invasive front of colorectal tumors. (A) Tumor cells at the invasive front express nuclear β-catenin (inset, black arrows) and display a mesenchymal differentiation. (B) Corresponding tumor cells also express hTERT (inset, white arrows). (C) Tumor cells in central areas of the tumor display weak or no expression of nuclear β-catenin (inset). These cells are characterized by epithelial differentiation. (D) Corresponding tumor cells display weak or no hTERT expression (inset). At 200x magnification.
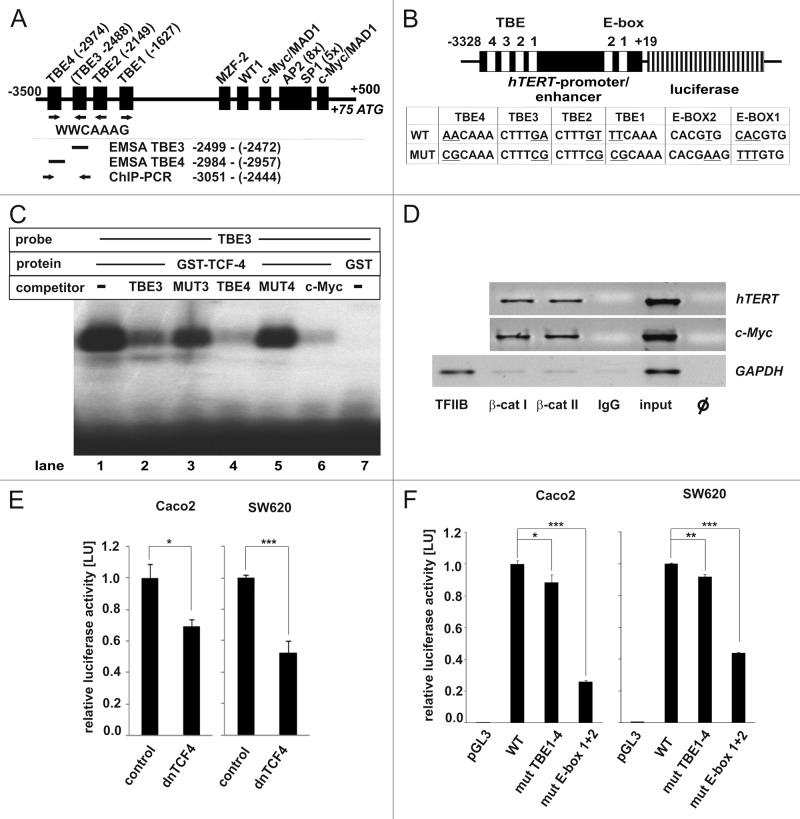
The promoter/enhancer of the hTERT gene contains four TCF-4 binding elements (TBE)
A possible link in the regulation of hTERT by β-catenin is c-myc as it is on the one hand a target gene of β-catenin9 and is known to regulate the transcription of the hTERT gene.23 But, as the hTERT gene has an outstanding role during the development of cancer5 and quite a complex transcriptional regulation,31 a more direct role of β-catenin was envisaged.
Especially, as in a ChIP-on-chip assay using TCF-4 specific antibodies chromatin containing the promoter/enhancer of the hTERT gene was detected.28 To investigate the transcriptional regulation of hTERT by β-catenin/TCF-4 in more detail, we analyzed the promoter/enhancer region of the hTERT gene (accession number AB016767) for the occurrence of consensus TBEs (WWCAAAG).28,32 Four TBEs were identified at positions -1,627 (TBE1), -2,149 (TBE2), -2,488 (TBE3) and -2,974 (TBE4), with respect to the transcription start (+1) of the hTERT gene. These four TBEs are embedded in binding elements for a variety of other transcription factors like AP2 (activation protein 2), MAD (MAX dimerization protein), c-Myc, MZF2 (myeloid zinc finger protein 2), SP1 (specificity protein 1) or WT1 (Wilms tumor 1-gene) (Fig. 2A).31 Thus, based on the composition of its promoter/enhancer region, the hTERT gene might be a putative β-catenin/TCF-4 target gene.
Figure 2. β-catenin interacts with TBEs in the hTERT promoter/enhancer and confers transcriptional activation. (A) Schematic representation of the hTERT promoter/enhancer with four located consensus motif TBEs (TCF binding elements) and other known responsive elements: MZF2 (myeloid zinc finger protein 2), WT1 (Wilms tumor 1 gene), c-Myc/MAD1 (MAX dimerization protein 1), AP2 (activation protein 2) and SP1 (specificity protein 1). The arrows below the TBEs represent the orientation of the consensus sequence WWCAAAG. The sites of the EMSA-DNA probes and competitors as well as the binding site of hTERT primer used in the ChIP-assay are indicated. Figure is not drawn to scale. (B) The hTERT promoter/enhancer luciferase reporter gene construct consists of a 3,347 bp fragment of the hTERT promoter/enhancer harboring the four TBEs (1, 2, 3, 4) and the two E-boxes driving the firefly luciferase reporter gene. Figure not drawn to scale. The table shows the mutated consensus sequences of the TBEs and E-boxes. (C) Specific binding of recombinant GST-TCF-4 (glutathion-S-transferase-T-cell-factor-4) fusion protein to a radioactively endlabeled DNA probe containing the TBE3 sequence of the hTERT- promoter/enhancer using electric mobility shift assays (EMSA). Binding was competed by adding unlabeled TBE3 or TBE4 sequence containing fragments of the hTERT promoter/enhancer or the TBE2 sequence of the c-Myc promoter/enhancer. (D) β-catenin specifically binds the genomic region of the hTERT gene containing TBE3 and TBE4 in the context of native chromatin of cultured colorectal DLD-1 cells applying ChIP (chromatin immunoprecipitation). Two different antibodies specific for β-catenin, an antibody specific for TFIIB and immunoglobulins (IgG) as controls were used. Specific binding of β-catenin was analyzed by PCRs using primer pairs spanning the genomic regions of the hTERT and c-Myc promoter/enhancer region containing the gene specific TBE3 and TBE4 or TBE2, respectively. PCRs using primer-pairs for GAPDH promoter/enhancer region served as a negative control. Antibody for TFIIB was used as systemic control for GAPDH promoter/enhancer. (E) dnTCF-4 expression plasmid suppresses the transcriptional activity of hTERT promoter/enhancer luciferase reporter gene constructs. As control pcDNA-CAT plasmid was used. (F) SW620 and Caco2 cells were transfected with constitutive active β-catenin expression plasmids to enhance Wnt-signaling. Mutation of the four TBEs or of the two E-boxes in the hTERT-promoter/enhancer results in a significant downregulation of the hTERT promoter/enhancer activity compared with the activity of the WThTERT promoter/enhancer. pGL3-basic plasmids were used as negative control. Data are represented as mean ± SD (n = 3). Error bars represent standard deviations. Student’s t test (two-sided) was used for comparisons. For the analyses, p values < 0.05 considered significant. *p < 0.05, **p < 0.01; ***p < 0.001.
β-catenin and TCF-4 bind to TBEs in the promoter/enhancer region of the hTERT gene
Next, it was investigated if TCF-4 and β-catenin bound to the TBEs in the promoter/enhancer of the hTERT gene. In a first step, it was tested if the DNA binding domain (DBD) of a recombinant TCF-4/GST (glutathione-S-transferase) fusion protein bound specifically to TBE3 containing fragments (Fig. 2A, EMSA TBE3) of the hTERT gene, which was chosen randomly from the four TBEs (Table 1) applying electric mobility shift assays (EMSAs). Therefore, a radioactively end-labeled TBE3-DNA probe was incubated with TCF-4/GST, resulting in the retardation of the migration of the probe, thus, indicating binding of TCF-4/GST to the probe (Fig. 2C, lane 1). This binding was competed by adding unlabeled TBE3- (Fig. 2C, lane 2) or TBE4-DNA probes (Fig. 2A and C, lane 3), or by adding the second TBE of the c-Myc promoter/enhancer (Fig. 2C, lane 6). No competition was seen when mutant variants of TBE3- (MUT3, Fig. 2C, lane 3) or TBE4-DNA probes (MUT4, Fig. 2C, lane 5) were taken instead. Binding was due to TCF-4, as GST alone did not bind to the radioactively end-labeled TBE3-DNA probe (Fig. 2C, lane 7). In a second step, it was investigated if β-catenin was associated with the hTERT promoter/enhancer in the context of the native chromatin employing chromatin immunoprecipitations (ChIPs). Therefore, two different β-catenin specific antibodies (β-CATI, β-CATII) were incubated together with chromatin of the cultivated colorectal cell line DLD-1. Both antibodies bound chromatin of the hTERT promoter/enhancer containing TBE3 and TBE4 (Fig. 2A, ChIP-PCR, Fig. 2D, hTERT) as well as the c-Myc promoter/enhancer (Fig. 2D, c-Myc), which was used as a positive control as their precipitates resulted in PCR products specific for the respective regions of the genes containing the TBEs. The antibody driven interaction with the chromatin was specific for TBEs as no TATA-box containing fragments of the glycerol-aldehyde-phosphate-dehydrogenase (GAPDH) gene was precipitated with β-CATI or –II (Fig. 2D, GAPDH). This absence of signals was not due to a systemic error as the DNA worked when it was used as the template (Fig. 2D, input), but most importantly, also when an antibody specific for the TATA-box binding protein B was used instead (Fig. 2D, TFIIB). Additionally, binding was not due to unspecific binding of the antibodies as isotype-specific controls also did not result in the precipitation of gene-specific template DNA fragments (Fig. 2D, IgG). Taken together, both TCF-4 and β-catenin bound specifically to TBEs of the human hTERT-gene, which is a prerequisite for a transcriptional regulation.
Table 1. Sequences of oligonucleotides.
| Electric Mobility Shift Assay (EMSA) | ||||
|---|---|---|---|---|
|
TBE3 |
ATTATTTCAAAACAAAGGTTTACAGAAA |
|
|
|
|
TBE4 |
GAGTTACCCTCCTTTGATATTTTCTGTA |
|
|
|
|
MUT3 |
ATTATTTCAACGCAGAGGTTTACAGAAA |
|
|
|
|
MUT4 |
GAGTTACCCTCCTGTGCGATTTTCTGTA |
|
|
|
| c-Myc | CTAGCGCACCTTTGATTTCTGCACCTTTGATTTCTG | |||
| Chromatin immunoprecipitation (ChIP) | ||||
|---|---|---|---|---|
|
hTERT |
ACTCGCGCTGCCCTTCTAGC ACGGTGTATCCCCAGTCTACGAAG |
617 |
400 nM 400 nM |
|
|
c-Myc |
ACAGACGCCTCCCGCACGGG CCACACCGAGAACGCACTGC |
451 |
400 nM 400 nM |
|
| GAPDH | TACTAGCGGTTTTACGGGCG TCGAACAGGAGGAGCAGAGAGCGA |
165 | 400 nM 400 nM |
|
| RNA interference (RNAi) | ||||
|---|---|---|---|---|
|
β-catenin |
CAGUUGUGGUUAAGCUCUUdTdT |
|
60 nM |
|
| GFP | AAGCUACCUGUUCCAUGGCCAdTT | 60 nM | ||
| RT-qPCR | ||||
|---|---|---|---|---|
|
β-catenin |
AGCTGACCAGCTCTCTCTTCA CCAATATCAAGTCCAAGATCAGC |
73 |
900 nM 900 nM |
21 |
|
c-Myc |
CACCAGCAGCGACTCTGA GATCCAGACTCTGACCTTTTGC |
102 |
300 nM 300 nM |
34 |
|
hTERT |
CACGCGAAACCTTCCTC ACCACTGTCTTCCGCAAGTT |
80 |
300 nM 900 nM |
46 |
| HPRT1 | TGACCTTGATTTATTTTGCATACC CGAGCAAGACGTTCAGTCCT |
102 | 900 nM 900 nM |
73 |
1 Length of amplicons resulting from gene specific PCRs.2 UPL (universal probe library) given are the numbers of the probes of the UPL (Roche). In the EMSA probes TBE sequences are underlined.
β-catenin/TCF-4 regulates the transcriptional activation of the hTERT promoter/enhancer
In a next step, the functional relevance of β-catenin/TCF-4 for the transcriptional regulation of the hTERT gene was investigated by performing transient hTERT promoter/enhancer luciferase-reporter gene assays in cultivated colorectal Caco2 and SW620 cells. For the experiment, a fragment of the hTERT promoter enhancer (–3,328/+19) driving the firefly luciferase gene23 was transiently transfected together with an expression plasmid encoding a dominant-negative form of the TCF-4 gene (dnTCF-4), which inhibits β-catenin/TCF-4 transcriptional activity.33 dnTCF-4 suppressed the activity of the hTERT promoter/enhancer luciferase reporter-gene construct to residual amounts of about 70% (Caco2) or 50% (SW620), respectively (Fig. 2E). Thus, β-catenin/TCF-4 might play a direct role in the transcriptional regulation of the hTERT gene.
As the hTERT-gene is also transcriptionally regulated by c-Myc, and both the c-Myc mRNA as well as protein are characterized by short half-lives of about 20 min,34 it might be possible that the observed effects were induced by a negative effect of dnTCF-4 on the c-Myc-gene.9 To discriminate the β-catenin/TCF-4 effects from those of c-Myc, we mutated the four TBEs and the two E-boxes (Enhancer Box, c-Myc binding site) in the hTERT promoter region (Fig. 2B). These were cotransfected together with expression plasmids encoding constitutive active β-catenin into Caco2 and SW620 cells. Here, TBE-mutated hTERT promoter/enhancer-driven luciferase reporter plasmid showed in both cell lines a significant reduction of the hTERT promoter activity compared with the WT hTERT promoter/enhancer constructs. Expectedly, the mutation of both E-boxes also resulted in a significant reduction of the hTERT promoter activity, because hTERT is a well-known target gene of c-Myc (Fig. 2F).
The transcription of the hTERT gene is directly regulated via β-catenin, but independently of c-Myc
To demonstrate the direct regulation of the hTERT gene via β-catenin, a knockdown approach applying β-catenin-specific siRNA (small interfering RNA) was chosen. To overcome the concomitant downregulation of c-Myc in this approach c-Myc-expression plasmids were cotransfected additionally, 24 h before knocking down β-catenin. Therefore, β- catenin,35 or as a control GFP-specific siRNAs, were transiently transfected into cultivated colorectal SW480 or DLD-1 cells (Fig. 3, GFP-■, β-catenin-◻) in the absence (0 ng c-Myc) or presence (600 or 2,000 ng c-Myc) of c-Myc expression plasmids. The knockdown of β-catenin resulted in a substantial reduction of both β-catenin as well as c-Myc-specific mRNA levels, thus proving the function of the experimental approach. Concentrations of the c-Myc expression plasmid were used, which resulted in comparable amounts of c-Myc that were not further influenced by the knockdown of β-catenin (Fig. 3, c-Myc). Twenty-four hours after the knockdown, cells were harvested and investigated for the expression of β-catenin, c-Myc and hTERT on the level of mRNA using RT-qPCR, normalized to the reference gene HPRT1. In SW480 cells, a trend (p < 0.08) in the loss of hTERT expression could be shown, whereas in DLD-1 cells, there is a significant downregulation of hTERT. In spite of the reconstitution of c-Myc, the β-catenin knockdown resulted in a robust loss of the expression of hTERT (Fig. 3, hTERT).
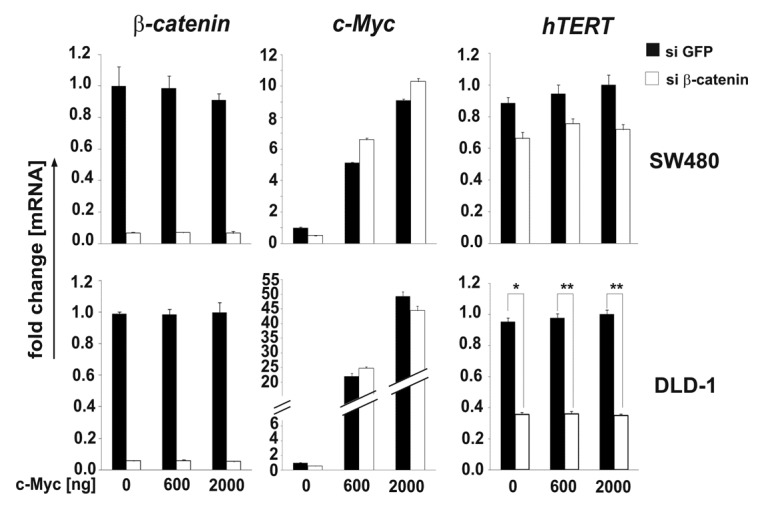
Figure 3. Knockdown of β-catenin leads to a loss of hTERT expression independently of c-Myc. Specific siRNA mediated knockdown of β-catenin leads to a substantial downregulation of hTERT- mRNA in DLD-1 and SW480 cells both in the absence or presence of c-Myc expression plasmid. Expression levels are normalized to those of the HPRT1 gene. Data are represented as mean ± SD. The experiment was done in duplicates and repeated at least twice. Representative examples are shown. Student’s t test (two-sided) was used for comparisons. For the analyses, p values < 0.05 considered significant. *p < 0.05, **p < 0.01.
Discussion
Taken together, we show evidence that β-catenin directly regulates the expression of the hTERT gene in human colorectal cancer in addition to its already known indirect effect via c-Myc.23 This finding adds the cancer hallmark eternal life5 to the many other well-known functions and hallmarks that are governed by β-catenin. Thus, β-catenin can be considered more as the master switch of colorectal carcinogenesis3 as well as colorectal cancer stemness.7
Our results described here are supported by experimental work done in embryonic and adult stem cells as well as in colorectal cancer cell lines.29 Here, we add proof that this regulation might also take place in human colorectal cancers. We showed that the expression pattern of hTERT at the invasive front of CRC25 is reflected by the expression patterns of β-catenin but not of c-Myc, as the latter is expressed in all tumor cells.24 Additionally, this tiny fraction of nuclear β-catenin-positive tumor cells generally shows a mesenchymal differentiation thus EMT26 together with the expression of mesenchymal indicator genes like fibronectin or vimentin, which are also β-catenin target genes.17,36 These points characterize these cells as colorectal cancer stem cells (coCSCs).37,38 This is additionally supported by the fact that these cells also express hTERT as coCSC express also β-catenin target genes like CD4439 or CD166.28 Therefore, it is not surprising that the number of these cells correlates with low survival,40 as does high hTERT activity.41 Because for CSC, the parallel upregulation of stemness and eternal life is relevant,5 the direct transcriptional regulation of hTERT via β-catenin leads to a coupling of stemness and eternal life.
An endogenous function of hTERT in Wnt-signaling is additionally discussed as being involved as a cofactor in a β-catenin transcriptional complex,42-44 hence there might be a positive feedback loop between hTERT and the Wnt pathway regarding the maintenance of the stemness. But this finding is challenged by other studies, which did not confirm this function of hTERT.45,46
Our results show that hTERT is a direct target gene of β-catenin and indicate, thereby, that the Wnt signaling pathway controls self-renewal and telomerase activity at the same time,47 thereby linking two essential components in the biology of colorectal cancers.
Material and Methods
Immunohistochemistry
Immunohistochemical staining of serial sections of 24 well-differentiated colorectal adenocarcinomas with an invasive front displaying nuclear β-catenin was done as described.30 Briefly, 5 μm sections were subjected to antigen retrieval using citrate buffer (DAKO), employing microwave treatment. Subsequently, the sections were incubated with antibodies specific for hTERT (Calbiochem; 1:100 - PC563T) or β-catenin (Sigma-Aldrich; 1:750 - C2206) overnight at 4°C, followed by washing. Next, binding was visualized using Envision AP (alkaline phosphatase) (DAKO) for hTERT or Envision HRP (horseradish peroxidase) (DAKO) for β-catenin staining. Finally, sections were counterstained using methylene blue according to the user’s manual. Pictures were taken employing an AnalySis photo system. The usage of the tissue blocks for research purpose was allowed by the local ethical committee of the university.
Electric mobility shift assay (EMSA)
Unlabeled competitor, or water in the case of controls, was incubated with 1 μl of crudely purified recombinant glutathione S-transferase (GST)-TCF-4 (amino acid 265–496), comprising its DNA binding domain or GST as a control in binding buffer (10 mM HEPES (pH 7.9), 60 mM KCl, 1 mM EDTA, 1 mM DTT and 4% (w/v) Ficoll, 12.5 ng/µl poly-dIdC (deoxy-inosinic-deoxy-cytidylic) acid, 62.5 μg/ml BSA) in a volume of 16 μl. After incubating for 5 min at room temperature, 0.5 ng of [32P]-ATP end-labeled double-stranded oligonucleotides (Table 1) with a specific activity of 3 × 108 dpm/μg (Hartmann Analytik) were added and the mixture incubated for a further 20 min, also at room temperature. Where mentioned, a 30-fold molar amount of unlabeled oligonucleotides (15 ng, Table 1) was used for competition. Reaction products were separated with the help of 5% (w/v) 0.25x TBE (1x TBE: 89 mM TRIS-HCl, pH 8.0, 89 mM boric acid, 2 mM EDTA) polyacrylamide slab gels.
Cell lines, cell culture
Cultivated colorectal cell lines DLD-1, SW480, SW620 and Caco2 cells (American Type Culture Collection) were maintained in DMEM with 4,500 mg/l glucose and 10% (v/v) fetal bovine serum (Invitrogen) at 5% CO2. The authenticity of the cell lines was tested by the DSMZ (German Collection of Microorganisms and Cell Lines) applying DNA typing.48 Testing for all cell lines was less than half a year ago.
Chromatin immunoprecipitation (ChIP)
ChIPs were done using ChIP-IT kits (Active Motif), following essentially the instruction manual. Briefly, chromatin of cultivated colorectal DLD-1 cells was fragmented to a size of about approximately 400 bp using ultra-sound sonification applying alternating 20 sec pulse–30 sec pause five times (G. Heinemann; HTU SONI130) on ice. Immunoprecipitation was done with the help of 2 µg of two different β-catenin specific antibodies (Sigma; β-CATI: C2,206; βCATII: clone 14; BD) or TFIIB (TATA-binding protein B of the transcription factor II)-specific antibodies (Active Motif) as well as mouse IgG (Santa Cruz Biotechnology) as isotype controls. Analysis of the reverse-crosslinked chromatin precipitates was done employing PCRs using 10% of the precipitates as template together with 400 nM of each primer spanning the region of the hTERT enhancer containing TBE3 and TBE4 (Table 1 and Fig. 2A). One percent of the chromatin (input) or water (no template) were used as the positive or negative control, respectively.
Mutation of TBE and E-box sequences
The mutations in the four TBEs and two E-boxes in the hTERT-promoter were done using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene) according to user’s instructions. The mutated consensus sequences of the TBEs and E-boxes are listed in Figure 2B.
Luciferase gene reporter assay
For the functional investigation of the relevance of β-catenin/TCF-4 for the transcriptional regulation of the hTERT gene, cultivated colorectal Caco2 and SW620 cells were transiently transfected 24 h after seeding in 24 cluster well plates. 120 ng pGL3-hTERT promoter (3328), pGL3-hTERT promoter (mut TBE1–4), pGL3-hTERT promoter (mut E-box1+2) or pGL3-basic (Promega; E1751) as negative control were transfected using Fugene 6 Transfection Reagent (Promega; E2691) in combination with 160 ng pEGFP-dnTCF-4 or pcl-neo-β-catenin-D45 encoding expression plasmid as well as 40 ng of pCMV-Renilla (Promega) for the normalization of results. After 24 h, Dual Luciferase Reporter Assay (Promega; E1960) was done according to user’s instructions. Fluorescence intensities were measured with an Orion II luminometer (Berthold) in a 96-well format and analyzed with the SIMPLICITY software package (DLR). All experiments were done in triplicates and repeated at least twice.
RNA interference, RNA isolation and quantitative reverse transcriptase PCR (RT-qPCR)
To specifically knockdown the β-catenin mRNA in cultivated DLD-1 and SW480 cells, the cells were transfected with 60 nM siRNA specific for either β-catenin or GFP (Qiagen) as the control (Table 1) using 3 μl Lipofectamine RNAiMAX (Invitrogen; 13778–150), following essentially the user’s instructions (forward transfection in the manual). To prevent a decrease of c-Myc levels in these cells, they were transiently transfected 24 h before the application of siRNA with 600 ng or 2,000 ng pUHD10–1-c-Myc - or pcDNA3-CAT-expression plasmid as the control and to fill up to a constant DNA-amount in 6 cluster well plates with the help of Fugene 6 Transfection Reagent (Roche), following the instruction manual. Another 24 h after, siRNA transfection, cells were harvested and total RNA was isolated using RNeasy kits (Qiagen; 74104). The concentrations of RNA were measured by UV-photometry. Four hundred–1,000 ng of RNA isolates were reverse transcribed in the presence of 100 μM random hexamer primers together with 200 U RevertAid Reverse Transcriptase (both Fermentas; S0142, EP0441), following the user’s recommendations. Two μl of the crude RT-reaction were used as the template in RT-qPCRs employing Light Cycler 480 Probes Master (Roche; 04902343001) together with specific primer-pairs (Table 1) and Universal ProbeLibrary Probes (Roche; Table 1), following the manufacturer`s instructions. Cp (critical point) values of RT-qPCRs specific for β-catenin, c-Myc, hTERT and the reference gene HPRT1 (hypo-xanthin phospho-ribosyl-transferase) were determined employing a LightCycler 480 device (Roche). All concentrations of β-catenin, c-Myc and hTERT-specific RNAs were normalized to those of the HPRT1 gene. All experiments were done in duplicates and repeated at least twice.
Acknowledgments
We thank Dr. Masaki Inoue (Kanazawa University, Ishikawa, Japan) for donating us the pGL3-hTERT(-3,328) luciferase reporter gene construct, Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD, USA) for kindly supplying pcl-neo-ßcatenin-D45 expression plasmid, Dr. Heiko Hermeking for generously supplying pUHD10–1-c-Myc expression plasmid and Dr. Kerstin Amann (Friedrich-Alexander-Universität, Erlangen, Germany) for providing her digital photo-equipment. We thank also Silvio K Scheel (now Qiagen cooperation) and Angela Neumann (now Institute for Pathology, Friedrich-Alexander-Universität, Erlangen, Germany) for experimental support. This work was supported by grants of the Wilhelm-Sander Stiftung (Az.: 2004.111.3) to A.J. and T.K.
Glossary
Abbreviations:
- hTERT
human telomerase reverse transcriptase
- CRC
colorectal cancer
- CSC
cancer stem cell
- EMT
epithelial-mesenchymal transition
- TCF-4
T-cell factor 4
- WT
wild-type
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21790
References
- 1.Harris TJ, Peifer M. Decisions, decisions: beta-catenin chooses between adhesion and transcription. Trends Cell Biol. 2005;15:234–7. doi: 10.1016/j.tcb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 4.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–9. doi: 10.1016/S0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes Dev. 2004;18:2225–30. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 8.Yamada T, Takaoka AS, Naishiro Y, Hayashi R, Maruyama K, Maesawa C, et al. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res. 2000;60:4761–6. [PubMed] [Google Scholar]
- 9.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 10.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 11.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wassermann S, Scheel SK, Hiendlmeyer E, Palmqvist R, Horst D, Hlubek F, et al. p16INK4a is a beta-catenin target gene and indicates low survival in human colorectal tumors. Gastroenterology. 2009;136:196–205. doi: 10.1053/j.gastro.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–4. [PubMed] [Google Scholar]
- 14.Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, et al. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61:8664–7. [PubMed] [Google Scholar]
- 15.Hiendlmeyer E, Regus S, Wassermann S, Hlubek F, Haynl A, Dimmler A, et al. Beta-catenin up-regulates the expression of the urokinase plasminogen activator in human colorectal tumors. Cancer Res. 2004;64:1209–14. doi: 10.1158/0008-5472.CAN-3627-2. [DOI] [PubMed] [Google Scholar]
- 16.Beiter K, Hiendlmeyer E, Brabletz T, Hlubek F, Haynl A, Knoll C, et al. beta-Catenin regulates the expression of tenascin-C in human colorectal tumors. Oncogene. 2005;24:8200–4. doi: 10.1038/sj.onc.1208960. [DOI] [PubMed] [Google Scholar]
- 17.Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63:2658–64. [PubMed] [Google Scholar]
- 18.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–6. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 19.Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011;30:1104–9. doi: 10.1038/emboj.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung P, Sato T, Merlos-Suárez A, Barriga FM, Iglesias M, Rossell D, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–7. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 21.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 22.Flores I, Canela A, Vera E, Tejera A, Cotsarelis G, Blasco MA. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 2008;22:654–67. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, et al. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–7. [PubMed] [Google Scholar]
- 24.Hlubek F, Brabletz T, Budczies J, Pfeiffer S, Jung A, Kirchner T. Heterogeneous expression of Wnt/beta-catenin target genes within colorectal cancer. Int J Cancer. 2007;121:1941–8. doi: 10.1002/ijc.22916. [DOI] [PubMed] [Google Scholar]
- 25.Kolquist KA, Ellisen LW, Counter CM, Meyerson M, Tan LK, Weinberg RA, et al. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet. 1998;19:182–6. doi: 10.1038/554. [DOI] [PubMed] [Google Scholar]
- 26.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–90. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 28.Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S, et al. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol. 2008;28:2732–44. doi: 10.1128/MCB.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, et al. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336:1549–54. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- 30.Jung A, Schrauder M, Oswald U, Knoll C, Sellberg P, Palmqvist R, et al. The invasion front of human colorectal adenocarcinomas shows co-localization of nuclear beta-catenin, cyclin D1, and p16INK4A and is a region of low proliferation. Am J Pathol. 2001;159:1613–7. doi: 10.1016/S0002-9440(10)63007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–38. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Wetering M, Oosterwegel M, Dooijes D, Clevers H. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 1991;10:123–32. doi: 10.1002/j.1460-2075.1991.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang R, Cheng AJ, Wang JY, Wang TC. Close correlation between telomerase expression and adenomatous polyp progression in multistep colorectal carcinogenesis. Cancer Res. 1998;58:4052–4. [PubMed] [Google Scholar]
- 34.Bernstein PL, Herrick DJ, Prokipcak RD, Ross J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev. 1992;6:642–54. doi: 10.1101/gad.6.4.642. [DOI] [PubMed] [Google Scholar]
- 35.Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9:1291–300. [PubMed] [Google Scholar]
- 36.Gradl D, Kühl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576–87. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 39.Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515–23. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127–32. doi: 10.1046/j.1365-2559.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- 41.Tatsumoto N, Hiyama E, Murakami Y, Imamura Y, Shay JW, Matsuura Y, et al. High telomerase activity is an independent prognostic indicator of poor outcome in colorectal cancer. Clin Cancer Res. 2000;6:2696–701. [PubMed] [Google Scholar]
- 42.Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman KL. Telomerase reverse transcriptase and Wnt signaling. Mol Cell Biol. 2011;31:2366–8. doi: 10.1128/MCB.05462-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strong MA, Vidal-Cardenas SL, Karim B, Yu H, Guo N, Greider CW. Phenotypes in mTERT⁺/⁻ and mTERT⁻/⁻ mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase. Mol Cell Biol. 2011;31:2369–79. doi: 10.1128/MCB.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vidal-Cardenas SL, Greider CW. Comparing effects of mTR and mTERT deletion on gene expression and DNA damage response: a critical examination of telomere length maintenance-independent roles of telomerase. Nucleic Acids Res. 2010;38:60–71. doi: 10.1093/nar/gkp855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greider CW. Molecular biology. Wnt regulates TERT--putting the horse before the cart. Science. 2012;336:1519–20. doi: 10.1126/science.1223785. [DOI] [PubMed] [Google Scholar]
- 48.Dirks WG, Faehnrich S, Estella IA, Drexler HG. Short tandem repeat DNA typing provides an international reference standard for authentication of human cell lines. ALTEX. 2005;22:103–9. [PubMed] [Google Scholar]