Abstract
Nanoparticle-encapsulated thiazole antibiotic, thiostrepton, has been shown to be an effective agent for inhibiting tumor growth in solid tumor models through the inhibition of proteasomal activity by the induction of apoptosis in cancer cells. Here, we show the efficacy of thiostrepton-micelles in inhibiting tumor growth in a DEN/PB-induced liver cancer model. We also demonstrate an enhanced anticancer effect of the combination treatment of thiostrepton with bortezomib, another proteasome inhibitor in this liver cancer model.
Keywords: DEN-PB, liver cancer, bortezomib, thiostrepton, combination treatment
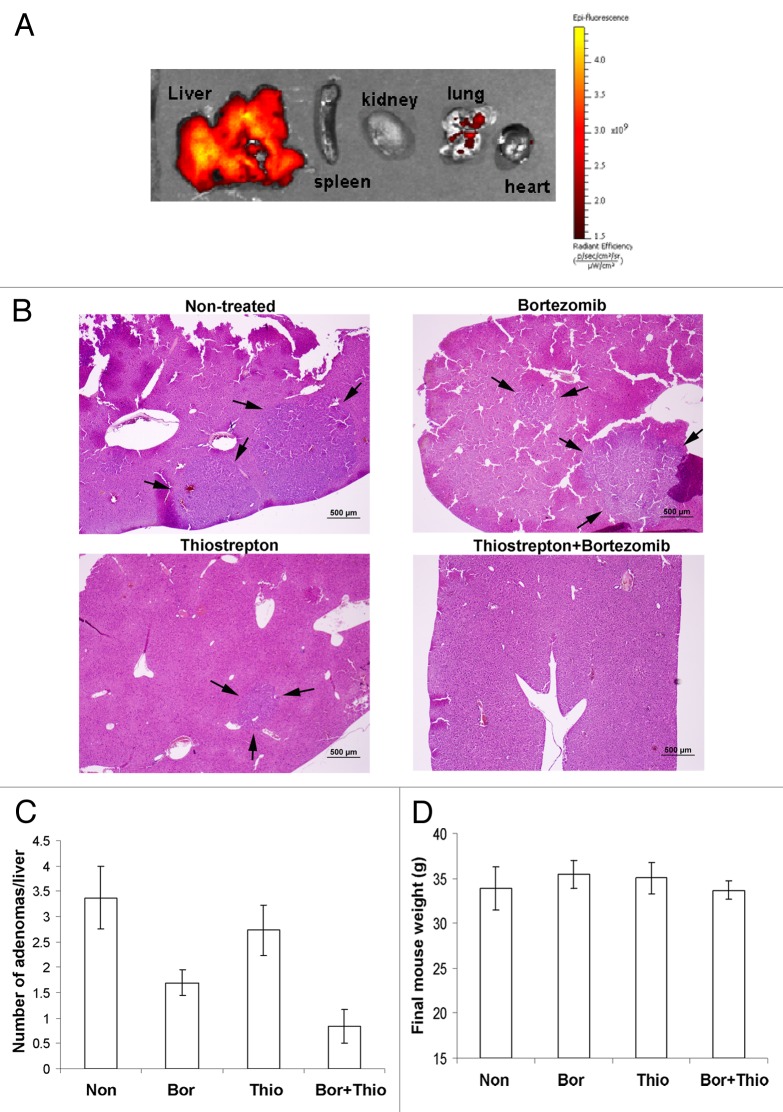
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths worldwide. Thiostrepton, a natural antibiotic derived from Streptomyces, has been shown to have anticancer effects through mechanisms of proteasome inhibition and FOXM1 suppression.1,2 The combination of thiostrepton and bortezomib induced potent apoptosis and inhibition of long-term colony formation in a wide variety of human cancer cell lines.3 The formulation of thiostrepton into micellar nanoparticles has been demonstrated to be an effective nanomedicine against tumor growth in liver cancer and breast cancer xenograft models.4 Furthermore, combination treatment with another proteasome inhibitor, bortezomib, has shown extended ability to reduce tumor growth in xenograft cancer models.5 Being nanoparticular in nature, thiostrepton-micelles have pharmacodynamic affinity for accumulation into xenograft tumors and into the liver.4 Consequently, the primary target for drug accumulation in the mouse is the liver. In this report, we demonstrate the ability of thiostrepton-micelles to accumulate in the livers of hepatocellular tumor-bearing mice and show its efficacy in reducing tumor growth. We further show the improved therapeutic effect of thiostrepton-micelles administered in combination with the proteasome inhibitor bortezomib, an FDA-approved drug.6,7 Spontaneous induction of hepatocellular neoplasia was achieved by intraperitoneal (IP) injection of the carcinogen Diethylnitrosamine (DEN) into 2-week-old neonatal male FBV mice (5 μg of DEN/g body weight in 50 μl PBS). Phenobarbitol (PB; 0.67mg/mL) was administered in the drinking water from week 4 onwards for enhanced carcinogenesis. 32 weeks following the DEN/PB protocol, animals were separated into treatment groups of, (1) non-treated control, (2) thiostrepton-micelle only 30 mg/kg, IV, (3) bortezomib only 0.5 mg/kg, IP and (4) combination of thiostrepton 30 mg/kg and bortezomib 0.5 mg/kg. Animals were treated once every other day (3 times a week) for 5 weeks before sacrifice, after which livers were removed. To study the pharmacodistribution of thiostrepton-micelle nanoparticles in mouse liver cancer models, two animals were administered with fluorescently labeled thiostrepton-micelles (30 mg/kg, IV). Four hours post-injection, it was found that over 90% of micelle-associated fluorescence was accumulated in the livers of treated animals (Fig. 1A). This occurrence is likely to be associated with the diffusion of nanoparticular species through the fenestrations within the sinusoidal capillaries of the liver vasculature.8,9 This organ-specific phenomenon renders nanoparticle drug delivery of anticancer drugs to liver cancers advantageous.
Figure 1. Analysis of hepatocellular tumor-bearing mouse livers after treatment with thiostrepton-micelles and/or bortezomib. Liver tumor models were prepared in FBV mice following the DEN/PB protocol, where neonatal pups were injected with DEN, followed by continuous exposure to PB by doping of drinking water. Thirty-two weeks post-DEN injection, treatment groups were administered with either 30 mg/kg thiostrepton-micelle, 0.5 mg/kg bortezomib or the combination of 30 mg/kg thiostrepton-micelle and 0.5 mg/kg bortezomib. Treatment continued for 5 weeks, after which animals were sacrificed and their livers prepared for histological examination. (A) Biodistributional studies of rhodamine-labeled micelle-thiostrepton (30 mg/kg) in liver tumor-bearing animals. Organ-associated fluorescence was examined 4 h post-administration using the Xenogen IVIS imaging equipment and demonstrated that the majority of micelle-thiostrepton accumulation was found in the liver of the animals. The spectral unmixing algorithm was used to differentiate between rhodamine and non-specific background autofluroescence. (B) Light microscopic evaluation of right liver lobe sections from control and treated animals. Black arrows point to the adenomas. (C) Post-treatment, the number of hepatocellular tumors in tissue sections was determined microscopically. Thiostrepton/bortezomib combination-treated animals exhibited significantly less number of adenomas compared with non-treated controls and single drug treated animals. (D) Thiostrepton-micelle, bortezomib and combination treatment did not cause significant weight loss in treated animals.
Livers were fixed in 4% paraformaldehyde, and the right lobe was paraffin-embedded, sectioned at 5 µm and stained with H&E for light microscopic evaluation. Histologically, the majority of hepatocellular tumors in all treatment groups were adenomas (Fig. 1B). Single carcinomas were detected in two individuals (one untreated control and one thiostrepton-micelle only treated). It was found that animals treated with either thiostrepton-micelle alone, bortezomib alone or with the combination of the two exhibited fewer hepatocellular neoplasms than non-treated animals (Fig. 1C). More specifically, while treatment with either thiostrepton-micelle or bortezomib alone resulted in the suppression of the number of adenomas compared with non-treated animals, the combined treatment with both drugs resulted in greater (up to 75% of control) suppression of tumor growth (Fig. 1C). Thiostrepton and bortezomib are both proteasome inhibitors, and their complementary anticancer activities have been described in xenograft cancer models.5 The potential differential binding of thiostrepton and bortezomib to the proteasome, resulting in the inhibition of both chymotrypsin and caspase-like cleavage sites likely accounts for this complementary effect.10 The body weights of treated animals did not decrease significantly compared with that of non-treated animals, indicating that the toxicity profiles of the single thiostrepton-micelle or bortezomib treatment and of the combined thiostrepton/bortezomib treatment were low (Fig. 1D).
The data presented in this communication is a further demonstration of the previously shown anticancer efficacy of thiostrepton-micelles as nanomedicine against liver cancer.4 Results also indicate that co-administration of thiostrepton-micelle with another proteasome inhibitor, bortezomib, leads to enhanced tumor suppression not only in xenograft cancer models,5 but in the current DEN/PB-induced liver cancer model as well. It is likely that the inhibition of FOXM1, an oncogenic transcription factor overexpressed in hepatocellular cancer, by proteasome inhibitors plays a role in their anticancer effect. Previous studies in this HCC model have shown similar effects on tumor inhibition by another FOXM1-inhibitor, ARF-peptide, through its suppression of FOXM1 oncogenic activity.11 In addition to FOXM1 suppression,4 the liver-targeting biodistributional properties of thiostrepton-micelles as a nanoparticular drug formulation provides for liver-specific delivery and consequent low toxicity (Fig. 1A).4 These features highlight the clinical potential of thiostrepton-micelle nanoparticles in combination with bortezomib to be adopted as a treatment option for solid tumors and particularly for liver cancers.
Grant Support
NIH grants 1RO1CA1294414 and 1R21CA134615 (A.L. Gartel).
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21290
References
- 1.Schoof S, Pradel G, Aminake MN, Ellinger B, Baumann S, Potowski M, et al. Antiplasmodial thiostrepton derivatives: proteasome inhibitors with a dual mode of action. Angew Chem Int Ed Engl. 2010;49:3317–21. doi: 10.1002/anie.200906988. [DOI] [PubMed] [Google Scholar]
- 2.Bhat UG, Halasi M, Gartel AL. Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PLoS One. 2009;4:e5592. doi: 10.1371/journal.pone.0005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandit B, Gartel AL. Thiazole antibiotic thiostrepton synergize with bortezomib to induce apoptosis in cancer cells. PLoS ONE. 2011;6:e17110. doi: 10.1371/journal.pone.0017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Gartel AL. Micelle-encapsulated thiostrepton as an effective nanomedicine for inhibiting tumor growth and for suppressing FOXM1 in human xenografts. Mol Cancer Ther. 2011;10:2287–97. doi: 10.1158/1535-7163.MCT-11-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Gartel AL. Combination with bortezomib enhances the antitumor effects of nanoparticle-encapsulated thiostrepton. Cancer Biol Ther. 2012;13:184–9. doi: 10.4161/cbt.13.3.18875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–11. [PubMed] [Google Scholar]
- 7.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2108–21. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 8.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisse E, Jacobs F, Topal B, Frederik P, De Geest B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15:1193–9. doi: 10.1038/gt.2008.60. [DOI] [PubMed] [Google Scholar]
- 10.Crawford LJ, Walker B, Ovaa H, Chauhan D, Anderson KC, Morris TC, et al. Comparative selectivity and specificity of the proteasome inhibitors BzLLLCOCHO, PS-341, and MG-132. Cancer Res. 2006;66:6379–86. doi: 10.1158/0008-5472.CAN-06-0605. [DOI] [PubMed] [Google Scholar]
- 11.Gusarova GA, Wang IC, Major ML, Kalinichenko VV, Ackerson T, Petrovic V, et al. A cell-penetrating ARF peptide inhibitor of FoxM1 in mouse hepatocellular carcinoma treatment. J Clin Invest. 2007;117:99–111. doi: 10.1172/JCI27527. [DOI] [PMC free article] [PubMed] [Google Scholar]