Abstract
We have previously demonstrated that the neural stem-cell marker nestin is expressed in hair follicle stem cells. Nestin-expressing cells were initially identified in the hair follicle bulge area (BA) using a transgenic mouse model in which the nestin promoter drives the green fluorescent protein (ND-GFP). The hair-follicle ND-GFP-expressing cells are keratin 15-negative and CD34-positive and could differentiate to neurons, glia, keratinocytes, smooth muscle cells and melanocytes in vitro. Subsequently, we showed that the nestin-expressing stem cells could affect nerve and spinal cord regeneration after injection in mouse models. In the present study, we separated the mouse vibrissa hair follicle into three parts (upper, middle and lower). Each part of the follicle was cultured separately in DMEM-F12 containing B-27 and 1% methylcellulose supplemented with basic FGF. After 2 mo, the nestin-expressing cells from each of the separated parts of the hair follicle proliferated and formed spheres. Upon transfer of the spheres to RPMI 1640 medium containing 10% FBS, the nestin-expressing cells in the spheres differentiated to neurons, as well as glia, keratinocytes, smooth muscle cells and melanocytes. The differentiated cells were produced by spheres which formed from nestin-expressing cells from all segments of the hair follicle. However, the differentiation potential is greatest in the upper part of the follicle. This result is consistent with trafficking of nestin-expressing cells throughout the hair follicle from the bulge area to the dermal papilla that we previously observed. The nestin-expressing cells from the upper part of the follicle produced spheres in very large amounts, which in turn differentiated to neurons and other cell types. The results of the present study demonstrate that multipotent, nestin-expressing stem cells are present throughout the hair follicle and that the upper part of the follicle can produce the stem cells in large amounts that could be used for nerve and spinal cord repair.
Keywords: hair follicle, bulge area, nestin, outer-root sheath, stem cell, green fluorescent protein, differentiation, neuron
Introduction
Nestin-expressing cells in the hair follicle were discovered by Li, et al.1 Transgenic mice, with GFP expression driven by the nestin regulatory element [nestin-driven GFP (ND-GFP)], were used to identify the nestin-expressing cells in the hair follicle. The nestin-expressing cells were positive for the stem cell marker CD34, as well as keratin 15-negative, and could differentiate into neurons and other non-follicular cell types.2-12
When nestin-expressing hair follicle cells were implanted into the gap region of the severed sciatic nerve or the injured spine, they greatly enhanced the rate of nerve regeneration and function. The transplanted nestin-expressing cells transdifferentiated largely into Schwann cells, which are known to support neuron regrowth. The transplanted mice recovered the ability to walk normally.6,9-12
Yu, et al.13,14 isolated a population of human nestin-expressing hair follicle cells. These cells expressed neural crest and neuron stem cell markers as well as the embryonic stem cell transcription factors Nanog and Oct4. The human nestin-expressing cells proliferated as spheres, were capable of self-renewal and differentiated into multiple lineages, including myogenic, melanocytic and neuronal after in vitro clonal culture. In addition, the human hair follicle nestin-expressing cells differentiated into adipocyte, chondrocyte and osteocyte lineages and shared a similar gene expression pattern with murine skin immature neural crest cells.13,14
The nestin-expressing hair follicle cells have a small body diameter of approximately 7 mm with long extrusions, as shown by confocal imaging. We demonstrated that the bulge area (BA) is the source of the nestin-expressing stem cells of the hair follicle. The nestin-expressing stem cells migrate from the BA to the dermal papilla (DP) as well as into the surrounding skin tissues, including the epidermis.15
The present study demonstrates the relative presence of nestin-expressing multipotent cells in various regions of the hair follicle and reports a useful strategy for potential large-scale production for future clinical use of these cells for use in regenerative medicine.
Results and Discussion
ND-GFP-expressing cells are located in all regions of the hair follicle
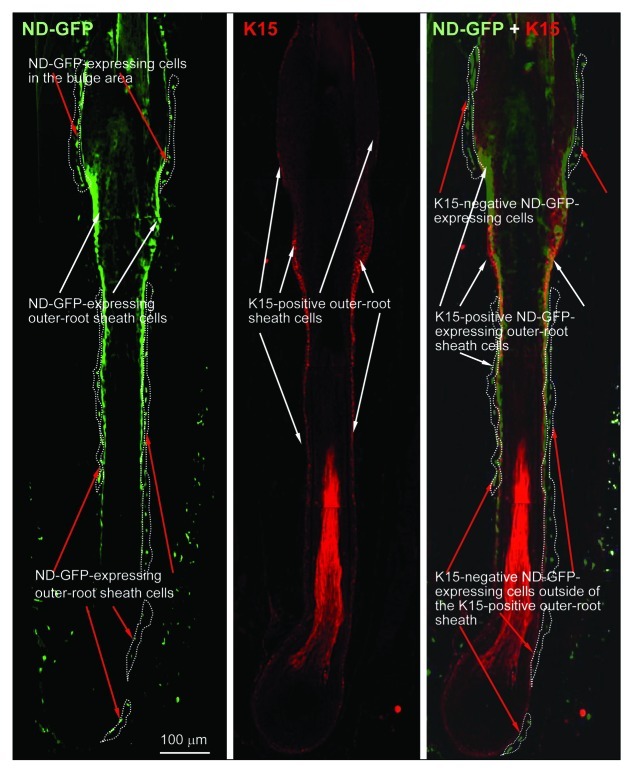
ND-GFP-expressing cells were fhound in the vibrissa follicle bulge area as well as the outside of the middle and lower the outer-root sheath. The ND-GFP cells were observed in frozen sections by immunofluorescence (Fig. 1). In contrast, immunofluorescence showed that keratin-15-expressing cells were found only in the lower two-thirds of the follicle (Fig. 1). Immunohistochemistry (IHC) staining showed that ND-GFP-expressing cells in the bulge area, as well as outside of the middle and lower outer-root sheath, were CD34-positive (data not shown), suggesting they are stem cells.
Figure 1. Frozen sections with IF staining showing presence of nestin- and keratin-15-expressing cells in various parts of the whisker hair follicle (see Materials and Methods for details).
ND-GFP cells from the upper, middle and lower parts of the whisker follicle can form spheres in culture
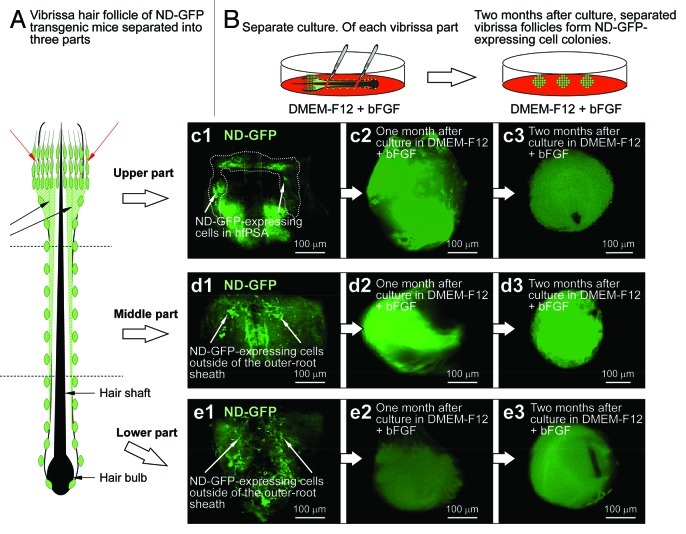
The vibrissa hair follicles excised from ND-GFP transgenic mice were physically separated into three parts from upper to lower. The whisker fragments from each region were suspended in DMEM-F12 containing B-27 and 1% methylcellulose supplemented with to basic (b) FGF every 2 d. By 2 mo in culture, ND-GFP-expressing spherical colonies had grown from each separated part of the hair follicle (Fig. 2). Differentiated cells were produced from the spherical colonies formed by each of the three separated whisker fragments were analyzed, as described below.
Figure 2. Vibrissa hair follicles from nestin-expressing green fluorescent protein (ND-GFP) transgenic mice were divided into three parts and were suspended in DMEM-F12 containing B-27 and 1% methylcellulose supplemented with bFGF every 2 d. After 2 mo, the divided ND-GFP-expressing vibrissa hair follicle fragments formed ND-GFP-expressing spherical colonies.
ND-GFP multipotent cells were present in cultures from the upper, middle and lower parts of the follicle
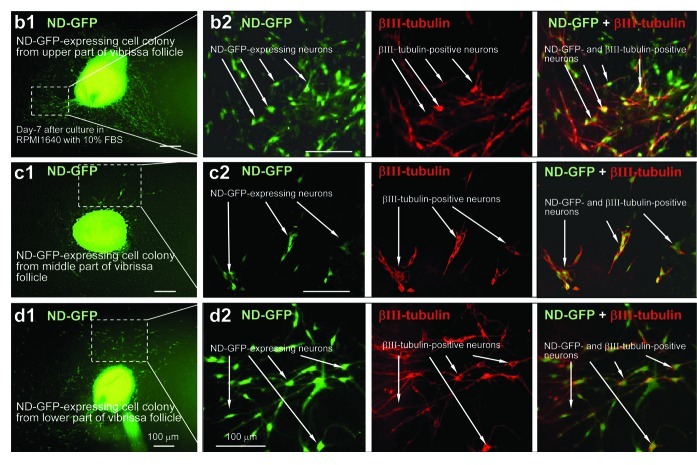
Differentiating cells migrated away from the spherical colonies, formed after 2 mo, from each of the three regions of the hair follicle within 2 d after switching the medium to RPMI 1640 with 10% FBS. Many types of differentiating cells were observed migrating from the ND-GFP-expressing spherical colonies. These differentiated cells included βIII-tubulin-positive neurons (Fig. 3) as well as K15-positive cells, K5/8-positive cells, GFAP-positive astrocytes, smooth muscle actin-positive smooth muscle cells and melanocytes containing melanin (data not shown).
Figure 3. Neurons differentiating from ND-GFP cells in spherical colonies formed from various parts of the whisker follicle. Seven days after switching to RPMI 1640 containing 10% FBS, the ND-GFP-expressing cells differentiated to βIII-tubulin-positive neurons. (b2, c2, d2) are higher magnification of (b1, c1, d1), indicated by the white-dashed box (see Materials and Methods for details).
The upper part of the vibrissa follicle is enriched in nestin-expressing, which can differentiate into neurons
The upper part of the vibrissa follicle is enriched in nestin-expressing cells. The number of neurons which differentiated from spheres grown from the upper part of the vibrissa follicle was significantly higher (p < 0.001) than from spheres formed from ND-GFP cells from the other middle or lower parts (Fig. 4). Approximately 105 cells grew out from the upper part of the hair follicle, and 2 wk after culture in DMEM-F12 medium without FBS, the growing cells formed spheres, which contained approximately 8 × 103 cells.
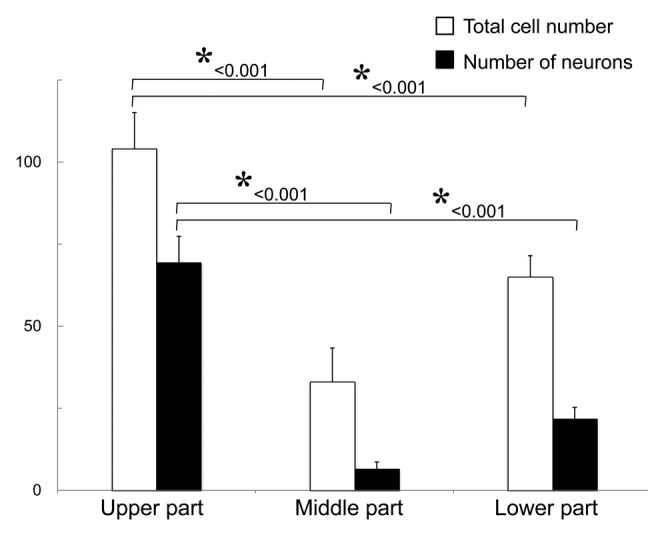
Figure 4. The number of neurons differentiating from ND-GFP-expressing spheres from the upper part of the whisker follicle was significantly higher compared with the middle and lower parts of the follicle. * p < 0.001 vs. control (see Materials and Methods for details).
Enhanced production of multipotent ND-GFP-expressing cells from the upper part of the whisker follicle
The cells from the upper part of the vibrissa follicle formed many spheres 2 wk after culture in DMEM-F12 medium without FBS (Fig. 5). The ND-GFP-expressing cells from the sphere began to differentiate to β-III-tubulin-positive neurons, K15-positive keratinocytes and smooth muscle actin-positive smooth muscle cells 2 d after transfer of spheres to RPMI medium with FBS (Fig. 6).
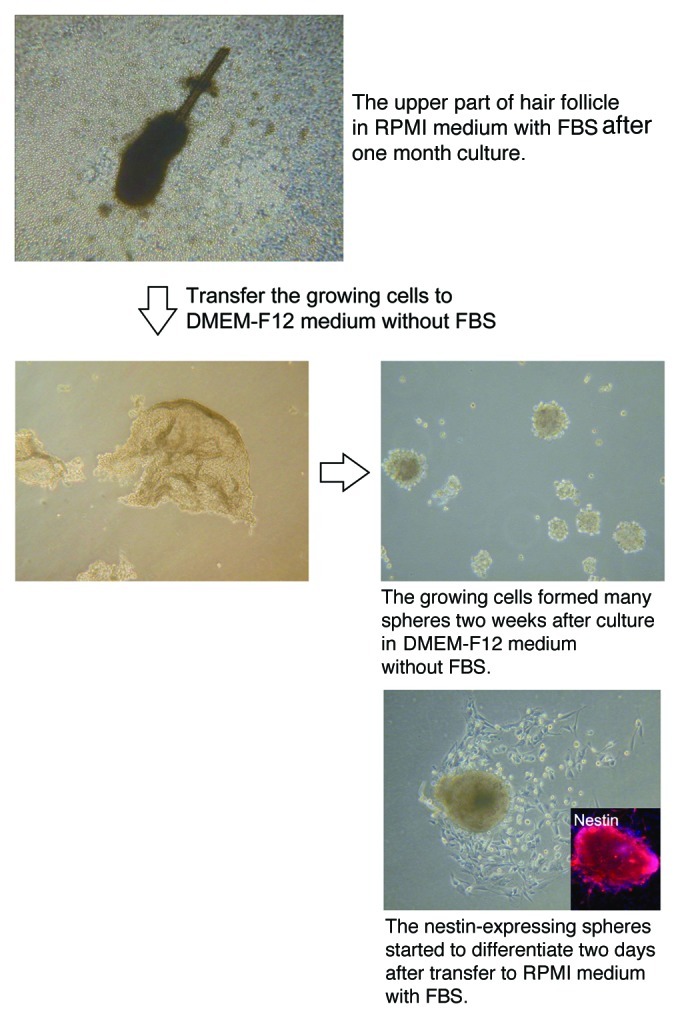
Figure 5. The upper part of the hair follicle was cultured in RPMI medium with FBS and produced numerous ND-GFP-expressing spheres (see Materials and Methods for details).
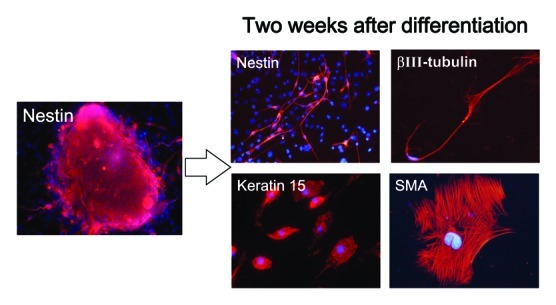
Figure 6. Cell types differentiating from spheres formed from ND-GFP cells in culture of the upper part of the whisker follicle (see Materials and Methods for details).
The fluorescent protein, GFP, whose expression is driven by the nestin promoter in transgenic mice (ND-GFP mice), enabled us to make the original observation that hair follicles contain nestin-expressing stem cells.1 These hair follicle stem cells express the stem cell marker CD34 as well as nestin, but are negative for the keratinocyte marker keratin 15, indicating their relatively undifferentiated state. The hair follicle stem cells can differentiate into neurons, glia, keratinocytes, smooth muscle cells and melanocytes in vitro and blood vessels, neurons and glial cells in vivo.7,9
The nestin-expressing hair follicle stem cells differentiated largely into Schwann cells when implanted in severed nerves9 or injured spinal cord,6,10 which may enhance neuron regrowth. The mice transplanted with nestin-expressing hair follicle stem cells in severed nerves or injured spinal cords regained the ability to walk normally. Nestin-expressing stem cells can also be readily isolated from the human scalp, thereby providing an accessible, autologous and safe source of stem cells for potential clinical use.2,11
The nestin-expressing cells, thus, have the potential as an alternative to the use of embryonal stem cells or iPS cells for regenerative medicine, which have ethical and oncogenic problems and are much more easily accessible, as well as autologous treatment. They can be readily expanded in culture after isolation from the patient’s hair follicle. Amoh, et al.,8,11 Liu, et al.,6 Mignone, et al.17 and Sieber-Blum, et al.2 have shown that nestin-expressing cells of the mouse hair follicle are multipotent. Yu, et al.13,14 and Amoh, et al.8,11 have shown that nestin-expressing cells of the human hair follicle are multipotent, suggesting their clinical potential.
The results of the present report are consistent with our previous result that the whisker nestin-expressing stem cells trafficked in between the BA and DP.15,16 The present report demonstrates that trafficking nestin-expressing cells retain their multipotency wherever they migrate in the hair follicle. The upper follicle can be used to produce large numbers of multipotent cells, which we have previously shown can effect nerve and spinal cord repair.6,10 The upper part of the follicle, therefore, can be used to produce large amounts of nestin-expressing stem cells for nerve and spinal cord repair in future clinical applications.
Materials and Methods
ND-GFP transgenic mice
Transgenic mice carrying GFP under the control of the nestin promoter ND-GFP1,17 (Research Institute for Microbial Diseases) expressing the Aequorea victoria GFP under the control of the chicken β-actin promoter and were used as a source of hair follicle stem cells. All animal experiments were conducted according to the Guidelines for Animal Experimentation at Kitasato University.
Isolation of vibrissa hair follicles
ND-GFP transgenic mice, 6–8 wk, were anesthetized with tribromoethanol (i.p. injection of 0.2 ml per 10 g of body weight of a 1.2% solution). To isolate the vibrissa follicles, the upper lip containing the vibrissa pad was cut, and the inner surface was exposed. The vibrissa follicles were dissected under a binocular microscope and plucked from the pad by pulling them gently by the neck with fine forceps, and were washed in DMEM-F12 (Gibco-BRL) containing B-27 (Gibco-BRL) and 1% penicillin/streptomycin (Gibco-BRL). All surgical procedures were done under a sterile environment.1,6,7,9,15,16
Culture of vibrissa hair follicle fragments
The vibrissa follicles were separated in three parts (upper, middle and lower) under a binocular microscope. The separate vibrissa follicle fragments were suspended in 1 ml DMEM-F12 containing B-27 with 1% methylcellulose (Sigma-Aldrich), 20 ng ml −1 basic FGF (bFGF) (Chemicon) every 2 d. Cells were cultured in 24-well dishes (Corning) in a 37°C, 5% CO2 incubator. After 2 mo, ND-GFP-expressing cells formed spherical colonies.7
Differentiation of nestin-expressing cells in spheres
For differentiation, ND-GFP-expressing cell colonies were centrifuged, the growth factor-containing supernatant medium removed, and the colonies were resuspended into fresh RPMI 1640 medium (Cellgro) containing 10% fetal bovine serum (FBS) in SonicSeal 4-well chamber slides (Nunc Inc.).11
Immunofluoresence staining (IF)
The primary antibodies that were used were: anti-βIII-tubulin monoclonal (Covance Research Products, Inc., 1:500, Tuj1 clone); anti-gamma aminobutyric acid (GABA) polyclonal (Chemicon, 1:200); anti-glial fibrillary acidic protein (GFAP) monoclonal (Molecular probes, 1:100); anti-keratin 5/8 (K5/8) monoclonal (Chemicon, 1:250); anti-keratin 15 (K15) monoclonal (Lab Vision, 1:100); anti-smooth muscle actin (SMA) monoclonal (Lab Vision, 1:200) and anti-CD34 monoclonal (BD PharMingen, 1:10). Secondary antibodies were Alexa Fluor® 568 goat anti-mouse (Molecular probes, 1:200) and Alexa Fluor® 568-conjugated goat anti-rabbit (Molecular probes, 1:200). For quantification of the percentage of cells producing a given marker protein, in any given experiment at least three fields were photographed, and the number of positive cells determined relative to the total number of cells. IF staining of cultured cells, which differentiated from ND-GFP-expressing cell colonies, were repeated in triplicate for each ND-GFP-expressing cell colony.7
Immunocytochemical staining
Immunocytochemical (IHC) staining of K15 and CD34 in the ND-GFP-expressing cell colonies was detected with the M.O.M.TM immunodetction kit (Vector laboratories, Inc.) (K15) and anti-rat (CD34) immunoglobulin horseradish peroxidase (HRP) detection kit (BD PharMingen) following manufacturer instructions. The primary antibodies were described above. Purple (VectorⓇ VIP substrate) or brown (substrate-chromogen 3, 3′-diaminobenzidine) staining was used for antigen staining. IHC stainings of ND-GFP-expressing cell colonies were repeated in triplicate for each ND-GFP-expressing cell colony.7
Fluorescence and confocal microscopy
The ND-GFP skin samples were directly observed under an Olympus IMT-2 inverted microscope equipped with a mercury lamp power supply. The microscope has a GFP filter set (Chroma Technology). An MRC-600 confocal imaging system (Bio-Rad) mounted on a Nikon Optiphot (Nikon) with a ×10 PlanApo objective was used also.11
Statistical Analysis
The experimental data are expressed as the mean ± SD. The two-tailed Student's t-test was used to compare the p value with the control.
Acknowledgments
This work was partially supported by Grant-in-Aid for Scientific Research (C) 23591653 from the Ministry of Education, Science, Sports and Culture of Japan, a grant from the Ministry of Education, Culture, Sports, Science and Technology of the Japan Government (Assistance for Strategic Creation of Research Basis, 2009–2013) and the Uehara Memorial Foundation (to Y.A.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21803
References
- 1.Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, et al. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA. 2003;100:9958–61. doi: 10.1073/pnas.1733025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231:258–69. doi: 10.1002/dvdy.20129. [DOI] [PubMed] [Google Scholar]
- 3.Sieber-Blum M, Schnell L, Grim M, Hu YF, Schneider R, Schwab ME. Characterization of epidermal neural crest stem cell (EPI-NCSC) grafts in the lesioned spinal cord. Mol Cell Neurosci. 2006;32:67–81. doi: 10.1016/j.mcn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman RM. The pluripotency of hair follicle stem cells. Cell Cycle. 2006;5:232–3. doi: 10.4161/cc.5.3.2397. [DOI] [PubMed] [Google Scholar]
- 5.Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–23. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle. 2011;10:830–9. doi: 10.4161/cc.10.5.14969. [DOI] [PubMed] [Google Scholar]
- 7.Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005;102:5530–4. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amoh Y, Kanoh M, Niiyama S, Kawahara K, Sato Y, Katsuoka K, et al. Human and mouse hair follicles contain both multipotent and monopotent stem cells. Cell Cycle. 2009;8:176–7. doi: 10.4161/cc.8.1.7342. [DOI] [PubMed] [Google Scholar]
- 9.Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, et al. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci USA. 2005;102:17734–8. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle. 2008;7:1865–9. doi: 10.4161/cc.7.12.6056. [DOI] [PubMed] [Google Scholar]
- 11.Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, et al. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: an advantageous alternative to ES and iPS cells. J Cell Biochem. 2009;107:1016–20. doi: 10.1002/jcb.22204. [DOI] [PubMed] [Google Scholar]
- 12.Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent nestin-expressing hair follicle stem cells. J Dermatol. 2009;36:1–9. doi: 10.1111/j.1346-8138.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, et al. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879–88. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol. 2010;130:1227–36. doi: 10.1038/jid.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchugonova A, Duong J, Zhang N, König K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem. 2011;112:2046–50. doi: 10.1002/jcb.23122. [DOI] [PubMed] [Google Scholar]
- 16.Duong J, Mii S, Uchugonova A, Liu F, Moossa AR, Hoffman RM. Real-time confocal imaging of trafficking of nestin-expressing multipotent stem cells in mouse whiskers in long-term 3-D histoculture. In Vitro Cell Dev Biol Anim. 2012;48:301–5. doi: 10.1007/s11626-012-9514-z. [DOI] [PubMed] [Google Scholar]
- 17.Mignone JL, Roig-Lopez JL, Fedtsova N, Schones DE, Manganas LN, Maletic-Savatic M, et al. Neural potential of a stem cell population in the hair follicle. Cell Cycle. 2007;6:2161–70. doi: 10.4161/cc.6.17.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]