Abstract
Introduction
Chemotherapy prolongs survival without substantially impairing quality of life for medically fit patients with advanced non-small cell lung cancer (NSCLC), but population-based studies have shown that only 20 to 30% of these patients receive chemotherapy. These earlier studies have relied on Medicare-linked Surveillance, Epidemiology, and End Results (SEER) data, thus excluding the 30 to 35% of lung cancer patients younger than 65 years. Therefore, we determined the use of chemotherapy in a contemporary, diverse NSCLC population encompassing all patient ages.
Methods
We performed a retrospective analysis of patients diagnosed with stage IV NSCLC from 2000 to 2007 at the University of Texas Southwestern Medical Center. Demographic, treatment, and outcome data were obtained from hospital tumor registries. The association between these variables was assessed using univariate analysis and multivariate logistic regression.
Results
In all, 718 patients met criteria for analysis. Mean age was 60 years, 58% were men, and 45% were white. Three hundred fifty-three patients (49%) received chemotherapy. In univariate analysis, receipt of chemotherapy was associated with age (53% of patients younger than 65 years versus 41% of patients aged 65 years and older; p = 0.003) and insurance type (p < 0.001). In a multivariate model, age and insurance type remained associated with receipt of chemotherapy. For individuals receiving chemotherapy, median survival was 9.2 months, compared with 2.3 months for untreated patients (p < 0.001).
Conclusions
In a contemporary population representing the full age range of patients with advanced NSCLC, chemotherapy was administered to approximately half of all patients—more than twice the rate reported in some earlier studies. Patient age and insurance type are associated with receipt of chemotherapy.
Keywords: Non small-cell lung cancer, Chemotherapy, Metastatic, Practice patterns, Insurance
Chemotherapy has a well-defined role in the treatment of medically fit patients with advanced non-small cell lung cancer (NSCLC). Compared with best supportive care, chemotherapy prolongs overall survival without substantially impairing quality of life.1–5 Nevertheless, population-based studies from the 1990s have shown that only 22 to 31% of patients with advanced NSCLC ever receive chemotherapy during the course of their disease.6–8 Potential reasons for this seemingly low treatment rate include advanced patient age, poor performance status, and comorbidities; referring and treating physician practice patterns; and patient preference.9
Even considering these factors, these earlier studies may have underestimated current rates of treatment for advanced NSCLC. First, these studies used Medicare-linked Surveillance, Epidemiology, and End Results (SEER) data and therefore included only patients aged 65 years and older.6–8 In the United States, more than 30% of individuals with lung cancer are younger than 65 years.10 Second, most of these studies reported data from the 1990s, a time frame that may not reflect contemporary practice patterns. Over the past 10 years, advances in diagnosis, treatment, and supportive care may have expanded the patient population considered for cancer-directed therapy. For example, increased use of positron emission tomography scans has resulted in earlier detection of extrathoracic disease, conceivably leading to diagnosis of advanced-stage NSCLC earlier in the clinical course, when patients may be more medically fit to receive chemotherapy.11 In addition, the number of therapies for NSCLC has grown in recent years, including relatively well-tolerated agents such as erlotinib and pemetrexed. Administration of these and other therapies has been enhanced by improved antiemetics and more convenient formulations of hematopoietic growth factors.
Given these considerable changes to the clinical care of patients with NSCLC, as well as the absence of data on the substantial proportion of individuals younger than 65 years, we performed a single-institution study encompassing a diverse population diagnosed with stage IV NSCLC from 2000 to 2007. Based on the considerations described above, we hypothesized that this cohort would have higher rates of chemotherapy administration than those previously reported.
MATERIALS AND METHODS
Study Setting
This study was approved by the University of Texas Southwestern Medical Center at Dallas (UT Southwestern) Institutional Review Board. The study sample was drawn from UT Southwestern-associated clinical facilities, including Parkland Health and Hospital System (PHHS), University Hospital, and the Harold C. Simmons Cancer Center. PHHS consists of a 968-bed public hospital and outpatient clinics that provide care to primarily indigent and uninsured residents of Dallas County. University Hospital (415 beds) serves as the primary medical and surgical referral hospital for UT Southwestern. The Simmons Cancer Center is a freestanding outpatient diagnostic and treatment facility. All three sites are located in Dallas, Texas.
Dallas County is the eighth most populous county in the United States, with an estimated 2.4 million residents in 2008 of whom 39% were Hispanic, 35% were white, and 21% were African American.12
Data Extraction
Patients diagnosed with stage IV NSCLC between January 1, 2000, and December 31, 2007, were identified through UT Southwestern-associated tumor registries. The 2000–2007 time period was selected because (1) sufficient data were first recorded by the tumor registries in 2000 and (2) the 2007 cutoff provided sufficient follow-up time for survival outcomes. Patient data were obtained from the PHHS and UT Southwestern tumor registries. The tumor registries identify cases through review of pathology records, clinic schedules, and hospital admission and discharge records. Certified tumor registrars abstract data directly from the patients’ medical records, according to standards established by the American College of Surgeons Commission on Cancer, SEER/National Cancer Institute, and the National Program of Cancer Registries. More than 150 data fields are collected per patient, including demographics, cancer diagnosis and stage, treatment, and follow-up. After initial cancer diagnosis and treatment, the tumor registries contact patients and their medical providers every 6 months to obtain follow-up data. These data are then reported to the Texas State Cancer Registry and to the Commission on Cancer’s National Cancer Database.
Recording and Definition of Variables
For each subject, the following data were recorded: age, gender, race, insurance type, date of diagnosis, whether or not chemotherapy was administered, and date of last known follow-up or death. Race was categorized as white, Hispanic, African American, or other. Insurance type was recorded as one of the following: no insurance, Medicaid (a federal/state health care program for low-income families), Medicare (a federal health care program for individuals aged 65 years and older), and private insurance. The designation “no insurance” predominantly includes individuals ultimately treated under a county health plan that provides patients access to all diagnostic and treatment modalities within PHHS. Overall survival was defined as the interval between date of diagnosis and date of death.
Statistical Analysis
Descriptive statistics (medians/means for continuous variables and percentages for discrete variables) were generated for baseline demographic and clinical characteristics. Both univariate and multivariate logistic regression models were used to explore the association between baseline characteristics, year of diagnosis, and receipt of chemotherapy. In these analyses, age was dichotomized as younger than 65 years and 65 years and older; year of diagnosis was dichotomized as 2000–2003 and 2004–2007. In the multivariate model, we included subject age, gender, race, insurance type, and year of diagnosis. These predictive variables were identified without using any model selection methods. We analyzed the association between baseline patient characteristics, year of diagnosis, receipt of chemotherapy, and overall survival using univariate and multivariate Cox regression. Again, no model selection methods were specified in the multivariate model. All reported p values are two sided.
All statistical analyses were performed using SAS 9.1 Service Pack 4 for Windows (SAS Institute Inc., Cary, NC).
RESULTS
Study Population
A total of 718 patients met criteria for analysis. Gender, race, and year of diagnosis were available for all patients. Insurance type was available for 697 patients (97%). Median follow-up was 5.0 months, and 679 patients (95%) were followed until death. The mean patient age was 60 years; 58% were men and 45% were white. Additional baseline patient characteristics are listed in Table 1.
TABLE 1.
Baseline Patient Characteristics
| Characteristics | |
|---|---|
| Total number | 718 |
| Age (yr) | 60 ± 11 |
| Gender | |
| Male | 419 (58) |
| Female | 299 (42) |
| Race/ethnicity | |
| Non-Hispanic white | 322 (45) |
| African-American | 292 (41) |
| Hispanic | 80 (11) |
| Asian/other | 24 (3) |
| Insurance type | |
| No insurance | 271 (38) |
| Medicaid | 46 (6) |
| Medicare | 179 (25) |
| Private insurance | 201 (28) |
| Unknown | 21 (3) |
| Year of diagnosis | |
| 2000–2003 | 331 (46) |
| 2004–2007 | 386 (54) |
| Chemotherapy administration | |
| Yes | 353 (49) |
| No | 365 (51) |
Values are given as n (%) or mean ± SD.
Chemotherapy Administration
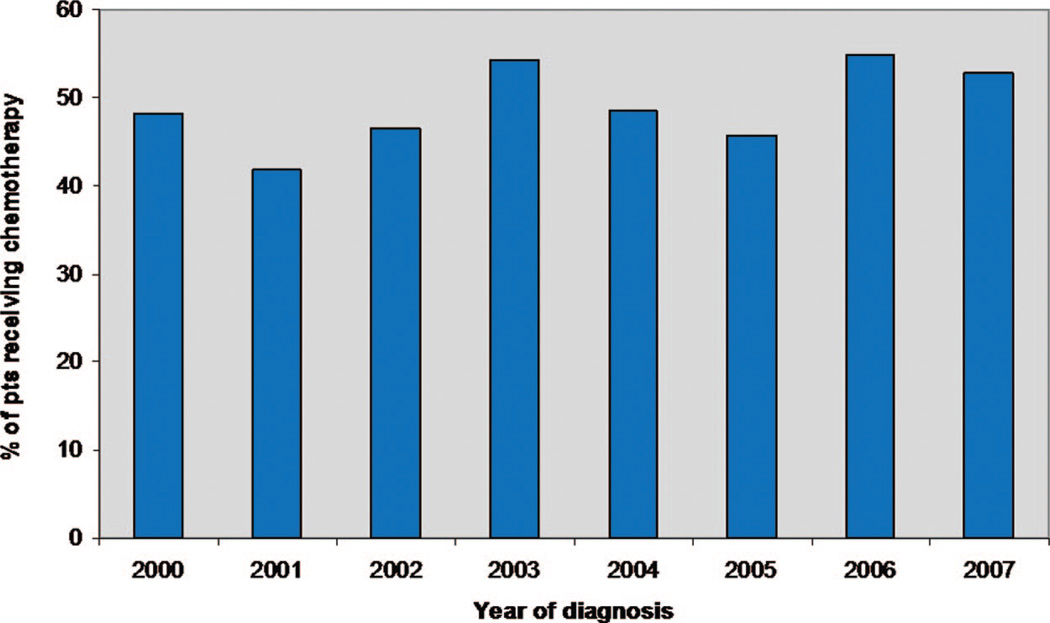
Overall, 353 patients (49%) received chemotherapy. In univariate analysis (Table 2), age was associated with receipt of chemotherapy (p = 0.003). Among patients younger than 65 years, 53% received chemotherapy, compared with 41% of those aged 65 years and older. Insurance type was also associated with chemotherapy administration (p < 0.001). Among individuals with private health insurance, 60% received chemotherapy, compared with 57% of those with Medicaid, 45% of those with Medicare, and 40% of those with no insurance. Subject gender, race, and year of diagnosis were not significantly associated with receipt of chemotherapy. Forty-seven percent of men received chemotherapy and 52% of women received chemotherapy (p = 0.23). There was a nonsignificant trend toward increased use of chemotherapy for white patients (53%) compared with non-white patients (46%) (p = 0.09). The rate of chemotherapy administration by year of diagnosis ranged from 42% in 2001 to 55% in 2006, but no clear trend was apparent (Figure 1).
TABLE 2.
Association Between Subject Characteristics and Administration of Chemotherapy
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient Characteristics |
Odds Ratio for Receiving Chemotherapy |
95% CI | p | Overall p Value |
Odds Ratio for Receiving Chemotherapy |
95% CI | p | Overall p Value |
| Age | 0.003 | 0.003 | ||||||
| <65 yr | 1.62 | 1.18–2.22 | 1.96 | 1.26–3.06 | ||||
| ≥65 yr | Reference | Reference | ||||||
| Gender | 0.23 | 0.43 | ||||||
| Male | Reference | Reference | ||||||
| Female | 1.20 | 0.89–1.62 | 1.13 | 0.83–1.54 | ||||
| Race | 0.09 | 0.37 | ||||||
| White | Reference | Reference | ||||||
| Non-white | 0.77 | 0.58–1.04 | 0.87 | 0.64–1.18 | ||||
| Insurance type | <0.001 | <0.001 | ||||||
| No insurance | 0.46 | 0.31–0.65 | <0.001 | 0.44 | 0.30–0.64 | <0.001 | ||
| Medicaid | 0.86 | 0.45–1.64 | 0.65 | 0.80 | 0.41–1.54 | 0.50 | ||
| Medicare | 0.55 | 0.36–0.82 | 0.004 | 0.85 | 0.51–1.41 | 0.53 | ||
| Private insurance | Reference | Reference | ||||||
| Year of diagnosis | 0.37 | 0.51 | ||||||
| 2000–2003 | Reference | Reference | ||||||
| 2004–2007 | 1.14 | 0.85–1.53 | 1.11 | 0.82–1.51 | ||||
CI, confidence interval.
FIGURE 1.
Chemotherapy administration for stage IV non-small cell lung cancer (NSCLC) by year (2000–2007).
In multivariate analysis (Table 2), patient age and insurance type remained significantly associated with chemotherapy administration. Compared with older individuals, patients younger than 65 years were more likely to receive chemotherapy (odds ratio 1.96; 95% confidence interval, 1.26–3.06; p = 0.003). Compared with individuals with private insurance, those with no insurance were less likely to receive chemotherapy (odds ratio 0.44; 95% confidence interval 0.30–0.64; p < 0.001). Subject gender, race, and year of diagnosis were not associated with receipt of chemotherapy.
Survival Analysis
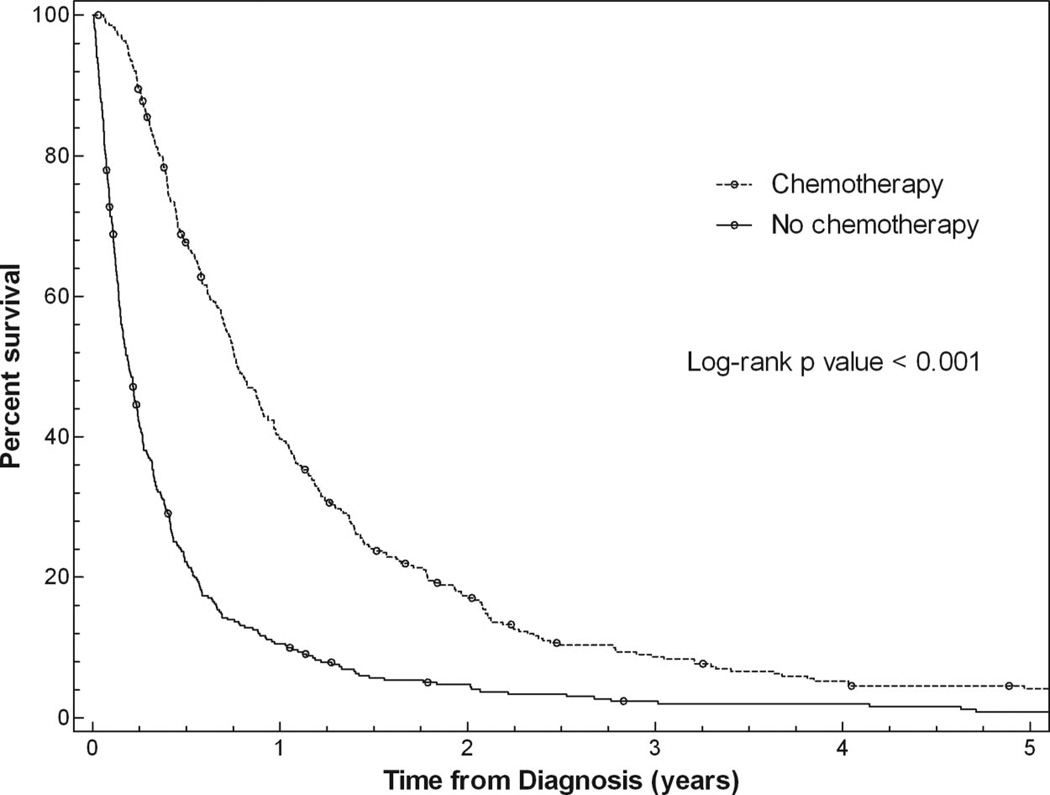
Median survival for all patients was 5.0 months. In univariate analysis (Table 3), overall survival was associated with subject gender (p = 0.02), insurance type (p = 0.01), and receipt of chemotherapy (p < 0.001) but was not associated with age, race, or year of diagnosis. Median overall survival for men was 4.6 months, compared with 5.9 months for women. Patients with private health insurance had a median survival of 6.1 months versus a median survival of 4.2 months for patients with no insurance. Patients who received chemotherapy had a median survival of 9.2 months versus 2.3 months for untreated patients (see Figure 2). In multivariate analysis (Table 3), survival remained significantly associated with gender (p = 0.04) and receipt of chemotherapy (p < 0.001) but was no longer associated with insurance type (p = 0.23).
TABLE 3.
Association Between Subject Characteristics and Overall Survival
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient Characteristics | Hazard Ratio for Death |
95% CI | p | Overall p Value |
Hazard Ratio for Death |
95% CI | p | Overall p Value |
| Age | 0.84 | 0.06 | ||||||
| <65 yr | 1.02 | 0.87–1.20 | 1.23 | 0.99–1.53 | ||||
| ≥65 yr | Reference | Reference | ||||||
| Gender | 0.02 | 0.04 | ||||||
| Male | Reference | Reference | ||||||
| Female | 0.84 | 0.72–0.98 | 0.85 | 0.72–0.99 | ||||
| Race | 0.53 | 0.94 | ||||||
| White | Reference | Reference | ||||||
| Non-white | 1.05 | 0.90–1.22 | 0.99 | 0.85–1.16 | ||||
| Insurance type | <0.001 | 0.23 | ||||||
| No insurance | 1.38 | 1.14–1.67 | <0.001 | 1.08 | 0.89–1.32 | 0.44 | ||
| Medicaid | 1.21 | 0.87–1.68 | 0.28 | 1.18 | 0.84–1.65 | 0.33 | ||
| Medicare | 1.25 | 1.02–1.54 | 0.04 | 1.29 | 1.00–1.66 | 0.05 | ||
| Private insurance | Reference | Reference | ||||||
| Year of diagnosis | 0.45 | 0.33 | ||||||
| 2000–2003 | Reference | Reference | ||||||
| 2004–2007 | 1.06 | 0.91–1.24 | 1.08 | 0.93–1.27 | ||||
| Chemotherapy administration | <0.001 | <0.001 | ||||||
| Yes | Reference | Reference | ||||||
| No | 2.64 | 2.26–3.08 | 2.81 | 2.38–3.31 | ||||
CI, confidence interval.
FIGURE 2.
Association between chemotherapy administration and survival.
DISCUSSION
To our knowledge, this is the first study to examine chemotherapy use for advanced NSCLC in a contemporary population encompassing the full age range of this disease. In this setting, approximately half of all patients received chemotherapy, more than twice the rate reported in some earlier series.7 Chemotherapy administration was associated with patient age and insurance status. Specifically, younger patients and those with private health insurance were more likely to receive chemotherapy. Median overall survival was fourfold longer among individuals who received chemotherapy.
Whether the higher rate of chemotherapy administration in this cohort reflects the inclusion of all patient ages, the contemporary era of the study, the particular practice patterns of clinicians at our institution, or other factors is not clear. The mean age in our study sample was 60 years, a full decade younger than the mean age of NSCLC diagnosis in the United States. That stated, more than 40% of patients aged 65 years and older received chemotherapy, a rate still considerably higher than that reported in prior studies. Because tumor registry data at UT Southwestern is limited before 2000, the impact of study era on our findings is not readily determined.
Although higher than previously reported, the 49% treatment rate in this series of patients with advanced NSCLC is still lower than treatment rates for other common malignancies. For example, a recent population-based study in Canada found that 63% of patients with metastatic colon cancer receive chemotherapy13; at least 70 to 80% of patients with breast and prostate cancer receive systemic therapy.14 Several factors may explain the lower likelihood of lung cancer patients receiving treatment. With a median age at diagnosis of 71 years, they are older than patients with breast (median 61 years) and prostate (median 68 years) cancer.10 In contrast to breast and prostate cancer, there are no hormonal therapeutic options, which are generally more easily administered and better tolerated than conventional cytotoxic chemotherapy. Furthermore, many physicians may not be aware of the growing evidence in support of chemotherapy for advanced NSCLC. Wassenaar et al.9 found that primary care physicians are less likely to refer patients with advanced lung cancer to an oncologist, compared with patients with advanced breast cancer. In that study, only 31% of primary care physicians were aware that chemotherapy improves survival in advanced lung cancer. Once NSCLC patients are referred to cancer specialists for treatment, relatively low response rates may limit recommendations for chemotherapy. In two landmark phase III trials comparing various chemotherapy regimens for advanced NSCLC, the highest response rates ranged from 22 to 35%.15,16 In analogous trials for other malignancies, chemotherapy response rates were 61 to 74% for breast cancer17–19 and 45 to 53% for colon cancer.20–23
In this study, chemotherapy administration was significantly associated with patient age and insurance type. Forty-one percent of patients aged 65 years and older received chemotherapy, compared with 53% of patients younger than 65 years. When one considers that older patients are more likely to have comorbidities and/or poor performance status, this finding is not surprising. Indeed, it was recently shown that older patients with advanced NSCLC are more likely to suffer chemotherapy-associated adverse events, despite a lower likelihood of receiving (potentially more toxic) platinum-based regimens.24
To our knowledge, the lower rate of chemotherapy receipt among underinsured patients with advanced NSCLC has not been previously reported but is consistent with a number of earlier observations in this disease. It has been shown that insured patients with NSCLC are more likely to be diagnosed with early-stage disease,25 to have surgery for early-stage disease,26 and to receive timely treatment after diagnosis.27 Furthermore, patients with stages I to IIIA NSCLC who were dually eligible for Medicare and Medicaid (a socioeconomically disadvantaged group) had inferior survival compared with patients with Medicare.28 Thus, socioeconomic status can have far-reaching effects throughout the disease course of patients with NSCLC, a finding also noted in other malignancies, including breast and colorectal cancer.29,30
In our cohort, explanations for this association are not clear. Although we previously reported longer diagnostic and treatment intervals for stage I to III NSCLC in our safety-net medical system, these intervals were not associated with clinical outcomes.27 Indeed, it seems unlikely that treatment selection would differ by medical facility within our institution, because the same clinicians care for lung cancer patients at all sites. Furthermore, the various facilities have similar treatment options, including state-of-the art chemotherapy, biologic agents, and supportive medications, and these are available to patients regardless of insurance status through safety-net medical programs. However, as we have previously reported,27 other aspects of care, such as treatment delays, do differ among UT Southwestern-associated facilities, and these factors may ultimately impact the administration of chemotherapy. It has also been shown that there are wide variations in attitudes toward chemotherapy among patients with NSCLC, although the association between these attitudes and demographic characteristics such as socioeconomic status remains unclear.31
Although we presumed that treatment of advanced NSCLC may be increasing over time, within the time period of our study, chemotherapy administration was not significantly associated with the year of diagnosis. Nevertheless, as shown in Figure 1, 2 of the 3 years in which chemotherapy was given to more than 50% of patients were the last 2 years of the study period (2006 and 2007), which may suggest a recent upward trend. The last 10 years have seen substantial advances in the medical management of advanced NSCLC. In the area of supportive care, darbepoetin (Food and Drug Administration approved in 2001) and PEG-filgrastim (approved in 2002) have markedly simplified the administration of hematopoietic growth factors, and aprepitant (approved in 2003) has improved prevention of nausea and vomiting. Since 2004, the anticancer therapies bevacizumab, erlotinib, and pemetrexed have been approved for advanced NSCLC, drugs notable for their relative tolerability. Indeed, from 1997 to 2002, chemotherapy administration for patients aged 65 years and older with advanced NSCLC increased from 28 to 36%.32 Whether the developments in the 2000s will lead to a further increase in treatment rates is not yet known.
Median survival in this study was 5.0 months, and receipt of chemotherapy was the only factor associated with survival in multivariate analysis. The median survival for patients treated with chemotherapy was 9.2 months, similar to other studies.5,16 However, at 2.3 months, median survival for the 51% of patients who did not receive chemotherapy was considerably lower than that in previous reports. In pivotal phase III clinical trials demonstrating the benefit of chemotherapy for advanced NSCLC, the median survival of patients randomized to best supportive care alone ranged from 4.0 to 5.7 months.1,3 Our observational, nonrandomized study permits no conclusions to be drawn about the effectiveness of chemotherapy, because the patients who did and did not receive chemotherapy differed in several measured and unmeasured ways.
Nevertheless, our outcome data may provide insight into reasons why patients did not receive chemotherapy. That overall survival among chemotherapy-treated patients in our series approximates survival reported in prospective clinical trials suggests that the performance status and organ function of these individuals may be roughly similar to those of trial participants. By contrast, the particularly poor survival among untreated patients in our cohort suggests that these individuals differ from patients who were eligible for the trials of chemotherapy versus supportive care; they are likely to have a worse performance status, more comorbidities, and impaired organ function. Thus, although there are several reasons why patients may not receive chemotherapy, including patient preference, access to care, and provider preference, it seems reasonable to conclude that insufficient medical fitness was a driving factor in our study. Accordingly, it is possible that insurance type, a widely used socioeconomic marker, is also serving as a surrogate for overall medical condition. Separately, the finding that overall survival seems shorter in multivariate analysis for younger patients in this cohort (hazard ratio for death = 1.23; p = 0.06) merits comment. Although this study offers no certain explanation, we believe that this observation may reflect the characteristics of our particular lung cancer patient population in which younger individuals are more likely to come from socioeconomically disadvantaged groups.27
This study has a number of limitations. Perhaps most importantly, the patient sample is drawn from a single academic medical center, limiting the generalizability of our findings. That stated, because of our geographic setting and the variety of UT Southwestern-associated clinical facilities, our patient cohort is racially and socioeconomically diverse. Second, this is a retrospective analysis and therefore subject to bias from incomplete data availability. However, as evidenced by follow-up through death for more than 95% of patients in the cohort, it seems that the tumor registries have successfully captured the clinical and therapeutic course of these patients. As mentioned, this study does not provide specific reasons why chemotherapy was not administered to half of all patients, and this remains a critical question for lung cancer clinicians and researchers alike. In addition, we do not have data on which treatments were administered. Finally, because of the process by which cases are coded by the tumor registries, our cohort does not include patients with recurrent, metastatic disease after treatment for early-stage NSCLC or patients with malignant pleural effusions, who are typically treated with an advanced disease paradigm but until recently were categorized as stage IIIB.
In conclusion, in this contemporary, diverse cohort of patients with advanced NSCLC, approximately half of all patients receive chemotherapy. Chemotherapy administration is associated with patient age and insurance type, a relationship that may reflect patients’ underlying medical condition. Among patients who did not receive chemotherapy—most likely because of lack of medical fitness for treatment—overall survival was approximately 2 months. It is hoped that future developments in this field increase not only treatment efficacy but also the proportion of patients able to benefit from them.
ACKNOWLEDGMENTS
The authors thank Alejandra Madrigales from the UT Southwestern tumor registry, Joan Cox from the Parkland Health and Hospital System tumor registry, and Eileen Marley, PharmD, from the Parkland Health and Hospital System oncology pharmacy.
Disclosure: Supported by an American Cancer Society and Simmons Cancer Center Grant ACS-IRG-02-196 and the North and the Central Texas Clinical and Translational Research Initiative (KL2RR024983) (to D.E.G.).
REFERENCES
- 1.Cartei G, Cartei F, Cantone A, et al. Cisplatin-cyclophosphamide-mitomycin combination chemotherapy with supportive care versus supportive care alone for treatment of metastatic non-small-cell lung cancer. J Natl Cancer Inst. 1993;85:794–800. doi: 10.1093/jnci/85.10.794. [DOI] [PubMed] [Google Scholar]
- 2.Quoix E, Dietemann A, Charbonneau J, et al. Is chemotherapy with cisplatin useful in non small cell bronchial cancer at staging IV? Results of a randomized study. Bull Cancer. 1991;78:341–346. [PubMed] [Google Scholar]
- 3.Spiro SG, Rudd RM, Souhami RL, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax. 2004;59:828–836. doi: 10.1136/thx.2003.020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 5.Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 7.Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117:1239–1246. doi: 10.1378/chest.117.5.1239. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey SD, Howlader N, Etzioni RD, et al. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results-Medicare. J Clin Oncol. 2004;22:4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Wassenaar TR, Eickhoff JC, Jarzemsky DR, et al. Differences in primary care clinicians’ approach to non-small cell lung cancer patients compared with breast cancer. J Thorac Oncol. 2007;2:722–728. doi: 10.1097/JTO.0b013e3180cc2599. [DOI] [PubMed] [Google Scholar]
- 10.SEER Cancer Statistics Review. National Cancer Institute, 1975–2006. [Accessed March 13, 2010]; Available at: http://seer.cancer.gov/csr/1975_2006/browse_csr.php.
- 11.Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5:29–33. doi: 10.1097/JTO.0b013e3181c5920c. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Census State and County. Dallas County, TX: QuickFacts; [Accessed March 13, 2010]. Available at: http://quickfacts.census.gov/qfd/states/48/48113.html. [Google Scholar]
- 13.Renouf D, Kennecke H, Gill S. Trends in chemotherapy utilization for colorectal cancer. Clin Colorectal Cancer. 2008;7:386–389. doi: 10.3816/CCC.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Database Public Benchmark Reports. [Accessed March 13, 2010]; Available at: http://cromwell.facs.org/BMarks/BMPub/Ver10/bm_reports.cfm.
- 15.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 16.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 17.Alba E, Martin M, Ramos M, et al. Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first-line treatment of metastatic breast cancer: a Spanish Breast Cancer Research Group (GEICAM-9903) phase III study. J Clin Oncol. 2004;22:2587–2593. doi: 10.1200/JCO.2004.08.125. [DOI] [PubMed] [Google Scholar]
- 18.Pegram M, Forbes J, Pienkowski T, et al. BCIRG 007: first overall survival analysis of randomized phase III trial of trastuzumab plus docetaxel with or without carboplatin as first line therapy in HER2 amplified metastatic breast cancer. J Clin Oncol. 2007;25 doi: 10.1200/JCO.2010.28.6450. LBA1008. [DOI] [PubMed] [Google Scholar]
- 19.Soto CTT, Reyes S, Ramirez M, et al. Capecitabine and taxanes in patients with anthracycline-pretreated metastatic breast cancer: sequential vs. combined therapy results from a MOSG randomized phase III trial. J Clin Oncol. 2006;24:570. [Google Scholar]
- 20.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 21.Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 24.Chrischilles EA, Pendergast JF, Kahn KL, et al. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:620–627. doi: 10.1200/JCO.2009.23.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg ER, Chute CG, Stukel T, et al. Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. N Engl J Med. 1988;318:612–617. doi: 10.1056/NEJM198803103181006. [DOI] [PubMed] [Google Scholar]
- 27.Yorio JT, Xie Y, Yan J, et al. Lung cancer diagnostic and treatment intervals in the United States: a health care disparity? J Thorac Oncol. 2009;4:1322–1330. doi: 10.1097/JTO.0b013e3181bbb130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley CJ, Dahman B, Given CW. Treatment and survival differences in older Medicare patients with lung cancer as compared with those who are dually eligible for Medicare and Medicaid. J Clin Oncol. 2008;26:5067–5073. doi: 10.1200/JCO.2008.16.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayanian JZ, Kohler BA, Abe T, et al. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329:326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 30.Robbins AS, Pavluck AL, Fedewa SA, et al. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. 2009;27:3627–3633. doi: 10.1200/JCO.2008.20.8025. [DOI] [PubMed] [Google Scholar]
- 31.Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. BMJ. 1998;317:771–775. doi: 10.1136/bmj.317.7161.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang K, Marciniak MD, Faries D, et al. Trends and predictors of first-line chemotherapy use among elderly patients with advanced non-small cell lung cancer in the United States. Lung Cancer. 2009;63:264–270. doi: 10.1016/j.lungcan.2008.05.003. [DOI] [PubMed] [Google Scholar]