Abstract
Objective:
To describe the phenotype of levodopa-induced “on” freezing of gait (FOG) in Parkinson disease (PD).
Methods:
We present a diagnostic approach to separate “on” FOG (deterioration during the “on state”) from other FOG forms. Four patients with PD with suspected “on” FOG were examined in the “off state” (>12 hours after last medication intake), “on state” (peak effect of usual medication), and “supra-on” state (after intake of at least twice the usual dose).
Results:
Patients showed clear “on” FOG, which worsened in a dose-dependent fashion from the “on” to the “supra-on” state. Two patients also demonstrated FOG during the “off state,” of lesser magnitude than during “on.” In addition, levodopa produced motor blocks in hand and feet movements, while other parkinsonian features improved. None of the patients had cognitive impairment or a predating “off” FOG.
Conclusions:
True “on” FOG exists as a rare phenotype in PD, unassociated with cognitive impairment or a predating “off” FOG. Distinguishing the different FOG subtypes requires a comprehensive motor assessment in at least 3 medication states.
Freezing of gait (FOG) is common in Parkinson disease (PD). FOG refers to sudden, relatively brief episodes of gait arrest, experienced subjectively by patients as their feet being “glued to the floor.”1 The relationship between FOG and dopaminergic medication is complex. Most common is “off” FOG, which is relieved by dopaminergic medication.1 Less well recognized types include “unresponsive FOG,” which is indifferent to changes in dopaminergic medication2,3; “pseudo-on” FOG, seen during a seemingly optimal “on” state, but which nevertheless improves with stronger dopaminergic stimulation; and “on” FOG, induced by dopaminergic medication.4
Here, we describe the phenotype of “on” FOG, illustrated by 4 patients with PD. We also present a diagnostic approach to separate the various FOG subtypes, as a basis for understanding pathophysiology and for tailored therapeutic intervention.
METHODS
Four patients (ages 59–82, disease duration 6–20 years) with a history of deteriorating FOG during “on” are described in appendix e-1 on the Neurology® Web site at www.neurology.org. Subjects were tested in 3 states: 1) “practically defined off” state, after 12 hours without medication; 2) “on” state, 45–60 minutes after intake of regular levodopa doses; and 3) “supra-on” state, after at least twice the regular dose. The Institutional Review Board approved the study protocol and written informed consent was obtained. These tests were done on 1 day, in a fixed order, without blinding. Subject 3 was also tested 1 month later in the “supra-off” state, after 72 hours without any medication. Motor evaluations included the Unified Parkinson's Disease Rating Scale (UPDRS)–III subscale (table e-1) and timed gait tasks (gait initiation, straight walking with 2 180-degree turns, and 1 360-degree turn in each direction). Determination of FOG appearance was based on patient and investigator judgment.
Standard protocol approvals and patient consents.
Subjects provided informed consent to participate in this study protocol, according to the recommendations of the Declaration of Helsinki.
RESULTS
Effect of levodopa.
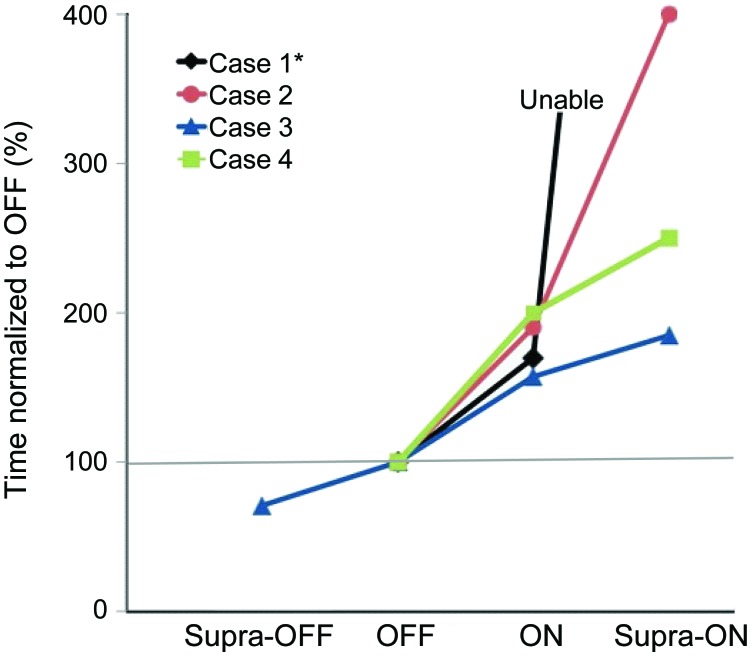
All subjects had gait worsening in the “on” state, which deteriorated further in the “supra-on” state (figure 1). Overall motor UPDRS scores either did not improve or worsened in 3 of 4 patients due to the appearance of severe FOG and associated postural impairment, in the absence of substantial improvements in bradykinesia or rigidity to compensate for such deterioration. In the “off” state, only subject 1 and 3 showed FOG, although substantially less compared to the “on” state. The other subjects exhibited mainly hypokinetic gait, with small steps and shuffling, but no FOG. Timed gait tests are available in table e-2. After 3 days without medication, subject 3 experienced gait ignition problems, but without associated trembling or subjective gluing, which were typical for his FOG in the “on” state. Probably, this represented profound general akinesia, instead of episodic FOG. In the “on” state, all subjects developed FOG. In the “supra-on” state, subjects showed severely disabling FOG, being almost completely unable to walk. Consequently, timed tasks took longer to complete from “off” to “on” to “supra-on.” Video documentation of gait for subject 2 is available online at www.neurology.org.
Figure 1. The 360-degree turn task.
Subjects performed a 360-degree turn into each direction in each medication state. The figure shows the time normalized with respect to baseline performance (“off ”). For each subject, only the worst performance (with regard to the right/left direction) is displayed; case 1 was unable to complete the 360-degree-turn task and therefore the straight walking task is shown (the same subject was unable to complete the task in the “supra-on” condition).
Other levodopa side effects.
Subjects 2 and 3 showed peak-dose dyskinesias. Subjects 2 and 4 experienced nausea, yawning, and orthostatic hypotension during the “supra-on” state. None of these side effects interfered with gait.
Phenotype of “on” FOG.
Periods of FOG were sudden and never preceded by “festination” (rapid increase in cadence at the expense of shortening stride length), and were more common during gait ignition and turns. Subjects 1, 3, and 4 also displayed marked FOG during straight walking. FOG was almost as severe during straight walking as during 180-degree turns in all subjects, and during 360-degree turns in subject 4 (figure 1). Most episodes had the trembling-in-place phenotype, although there were some akinetic episodes, especially in the “supra-on” phase. During the “on” phase, FOG sometimes led to near falls (subjects 1, 3, and 4).
Concurrently with progressive FOG in the “on” and “supra-on” states, there was corresponding deterioration of rapid alternating pronation and supination of the hands resembling freezing. These movements were relatively fast and of amplitude. In contrast, other parkinsonian features (speech, handwriting, rigidity) improved with levodopa.
DISCUSSION
This article addresses 2 issues. First, we propose a test protocol to unveil the complex relationship between medication effects and FOG, using both supratherapeutic medication doses and a practically defined off state. Second, we preselected 4 patients with PD with self-reported levodopa-induced worsening of gait, to report the phenotype of “on” FOG. Several common characteristics emerged: 1) a trembling FOG type which worsened with increasing levodopa doses; 2) debut of “on” FOG without predating “off” FOG, after a short disease duration and brief dopaminergic treatment; 3) concurrent worsening (freezing) of repetitive alternating hand movements in the “on” state; 4) good levodopa responsiveness of other parkinsonian features; and 5) preserved cognition, unlike the frontal cognitive impairment associated with “off” FOG.5 In 3 patients FOG affected open space walking as much as turning and gait initiation, unlike classic “off” FOG, where turning is much more affected compared to straight walking.1 These features point to a different pathophysiology underlying “on” and “off” FOG, and suggest that “on” FOG does not result from an effect of dopaminergic drugs on alertness, from orthostatic hypotension, or from isolated D2 agonism in the absence of D1 stimulation.6 Perhaps “on” FOG is related to dopaminergic disruption in rhythm generation of alternating hand and feet movements.7 Alternatively, dopamine regulation or nondopaminergic neurotransmitter systems may be changed by levodopa use, as occurs in levodopa-induced dyskinesias.8
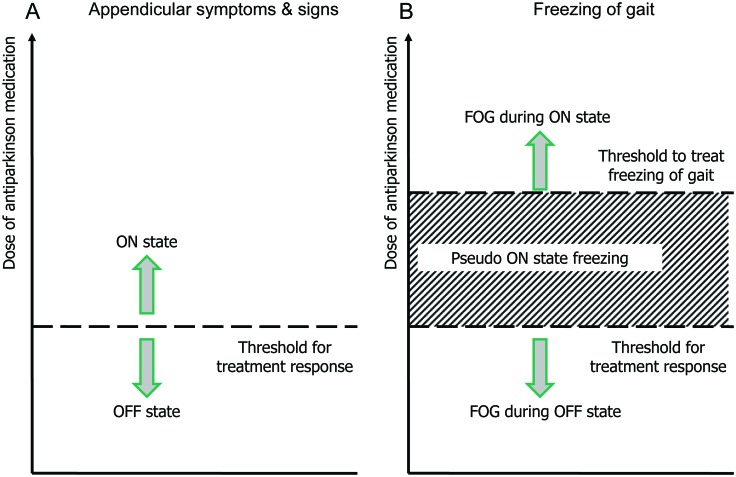
Based on prior evidence and the observations reported here, 4 FOG types can be recognized (figure 2): 1) the most common, “off” FOG, which diminishes or disappears in the “on” state; 2) “pseudo-on” FOG, due to insufficient dopaminergic stimulation (probably representing “off” FOG); 3) “unresponsive” FOG, where FOG is present in “off” and “on” states, and which is not influenced by medication; and 4) “on” FOG, reported here, where FOG is absent during “off” periods (e.g., immediately after waking up) and emerges after the first medication dose.
Figure 2. Types of freezing of gait.
The theoretical “threshold” (or rather, the dose range) for motor improvement (and also the threshold for improvement of “off” freezing of gait [FOG]) may be lower for appendicular manifestations of Parkinson disease (A) vs freezing of gait (B). “Off” state FOG occurs below the threshold for motor benefits. In this case a dose increase above such threshold is expected to eliminate the FOG. “Pseudo-on” FOG is an intermediate FOG level, occurring at doses where patients appear to otherwise respond well to medication. Although counterintuitive, a tolerable increase in the dose of antiparkinsonian medication in these patients is also expected to eliminate the FOG. Finally, “on” state FOG reported here may develop or worsen with higher l-dopa doses. For these patients, gait only improves after l-dopa dose reduction (adapted from Grimbergen et al.9).
Appropriate distinction between “on” and “off” FOG may be difficult during routine clinical visits, where patients typically present in their usual “wearing off” or “subjectively good on” state (table 1). To identify the FOG subtypes with more certainty, we recommend testing patients after supratherapeutic doses of dopaminergic agents and, in selected cases, after medication withdrawal for >12 hours (“practically defined off” state). Using these 2 extreme medication conditions, we showed that our patients worsened in a dose-dependent fashion, from “off” to “on” to “supra-on.” This protocol thus eliminated the possibility of “pseudo-on” FOG and “off” FOG.
Table 1.
“Off” and “on” FOG behavior according to medication condition

Abbreviations: FOG = freezing of gait; “off” = after an overnight withdrawal, with testing about 12 hours after the last dose of dopaminergic medication; “on” = approximately 45 minutes after a dose of l-dopa, once a subject recognizes his or her best response; “supra-off” = after more than 70 hours without any medication; “supra-on” = 45 to 60 minutes after intake of 200% of the regular or “therapeutic” l-dopa dose.
Most FOG during the “on” state may actually represent patients who are relatively undertreated, because the “threshold” (dose range) to improve FOG is higher than the threshold to improve appendicular motor signs.9 The term “on” FOG should be reserved for FOG that is truly levodopa-induced, as in our cases. They did not have “unresponsive” (or nondopaminergic) FOG, because their gait improved and FOG disappeared when levodopa doses were reduced, and did not even return during a profound off state
“On” FOG was first observed at the time when levodopa was introduced.4 Symptoms disappeared when levodopa dosage was reduced. Further studies will be needed to determine whether supratherapeutic levodopa doses have similarly deleterious effects in patients without overt “on” freezing. Also, the prevalence of true “on” FOG with the current medication regimen remains to be determined.
“On” FOG presents a serious therapeutic dilemma. Dopaminergic treatment should be reduced to alleviate FOG, but this may lead to unacceptable worsening of other parkinsonian features. Subthalamic deep brain stimulation is not a good option, because this is generally reserved for levodopa-responsive features, and not for medication-resistant features. However, its dopa-sparing effect may alleviate “on” FOG. Finally, external cueing may be considered, which was indeed effective in 3 of our subjects. However, others have reported less favorable results of visual cueing for patients with PD with “on” FOG.10
Supplementary Material
GLOSSARY
- FOG
freezing of gait
- PD
Parkinson disease
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Editorial, page 446
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Espay: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data. Dr. Fasano: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data. Dr. van Nuenen: drafting/revising the manuscript, acquisition of data. M.M. Payne: analysis or interpretation of data, acquisition of data, study supervision. Dr. Snijders: drafting/revising the manuscript, analysis or interpretation of data. Dr. Bloem: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision.
DISCLOSURE
Dr. Espay is supported by the KL2 Research Scholars mentored career development award through the NIH Institutional Clinical and Translational Science Award (RR026315–02); serves on scientific advisory boards for Solvay Pharmaceuticals, Inc. and Abbott; has received honoraria from Novartis, the American Academy of Neurology, and the Movement Disorders Society; serves on the editorial advisory board for The European Neurological Journal; served on the speakers' bureau for Novartis; and receives research support from Medtronic, Inc., CleveMed, the Davis Phinney Foundation, and the Michael J Fox Foundation. Dr. Fasano serves on the editorial board of Movement Disorders; serves as a consultant for Chiesi Pharmaceuticals Inc and Metronics, Inc.; and received research support from Chiesi Pharmaceuticals Inc, AFaR-Fatebenefratelli Association for Biomedical Research (Rome), and Fondazione Neureca Onlus (Milan). Dr. van Nuenen and M.M. Payne report no disclosures. Dr. Snijders received research support from the Netherlands Organization for Scientific Research. Dr. Bloem served on the editorial board of Movement Disorders and currently serves on the editorial board of Physiotherapy Canada, as Associate Editor for the Journal of Parkinson's Disease, and as Editor-in-Chief for Tijdschrift voor Neurologie & Neurochirurgie; has served on scientific advisory boards for Boehringer Ingelheim, Teva Pharmaceutical Industries Ltd., GlaxoSmithKline, and Novartis; and received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, the Prinses Beatrix Foundation, the Stichting Internationaal Parkinson Fonds, the Alkemade Keuls fonds, ZonMw, BrainGain, and MijnZorgNet.
REFERENCES
- 1. Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease. Eur J Neurol 2003; 10: 391– 398 [DOI] [PubMed] [Google Scholar]
- 2. Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord 2004; 19: 871– 884 [DOI] [PubMed] [Google Scholar]
- 3. Narabayashi H. Clinical analysis of akinesia. J Neural Transm Suppl 1980; 129– 136 [DOI] [PubMed] [Google Scholar]
- 4. Ambani LM, Van Woert MH. Start hesitation: a side effect of long-term levodopa therapy. N Engl J Med 1973; 288: 1113– 1115 [DOI] [PubMed] [Google Scholar]
- 5. Amboni M, Barone P, Picillo M, et al. A two-year follow-up study of executive dysfunctions in parkinsonian patients with freezing of gait at on-state. Mov Disord 2010; 25: 793– 795 [DOI] [PubMed] [Google Scholar]
- 6. Ahlskog JE, Muenter MD, Bailey PA, Stevens PM. Dopamine agonist treatment of fluctuating parkinsonism. D-2 (controlled-release MK-458) vs combined D-1 and D-2 (pergolide). Arch Neurol 1992; 49: 560– 568 [DOI] [PubMed] [Google Scholar]
- 7. Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swinnen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson's disease. Eur J Neurosci 2009; 29: 1422– 1430 [DOI] [PubMed] [Google Scholar]
- 8. Calabresi P, Di FM, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with Parkinson's disease: filling the bench-to-bedside gap. Lancet Neurol 2010; 9: 1106– 1117 [DOI] [PubMed] [Google Scholar]
- 9. Grimbergen YAM, Speelman AD, van der Marck MA, Schoon Y, Bloem BR. Gait, postural instability and freezing. In: Lang AE, Olanow CW, Stocchi F. eds. The Non-motor and Non-dopaminergic Features of Parkinson's Disease. Hoboken, NJ: Blackwell; 2010 [Google Scholar]
- 10. Kompoliti K, Goetz CG, Leurgans S, Morrissey M, Siegel IM. “On” freezing in Parkinson's disease: resistance to visual cue walking devices. Mov Disord 2000; 15: 309– 312 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.