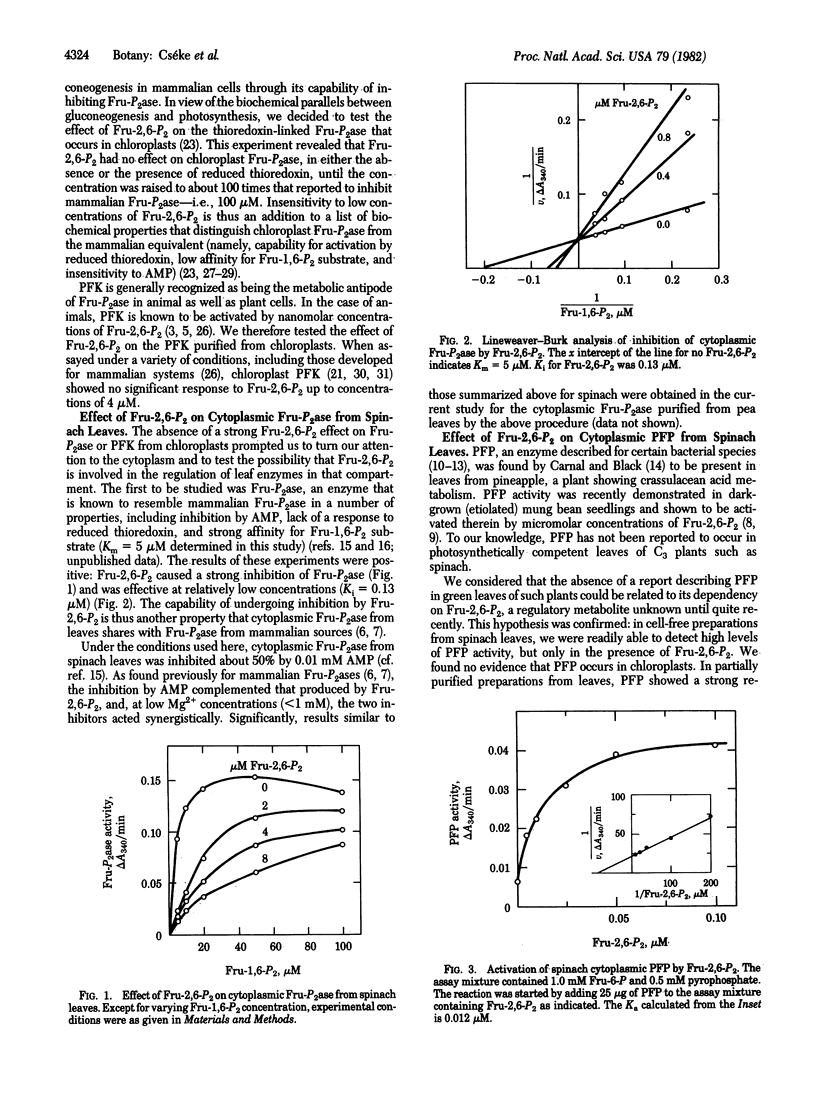
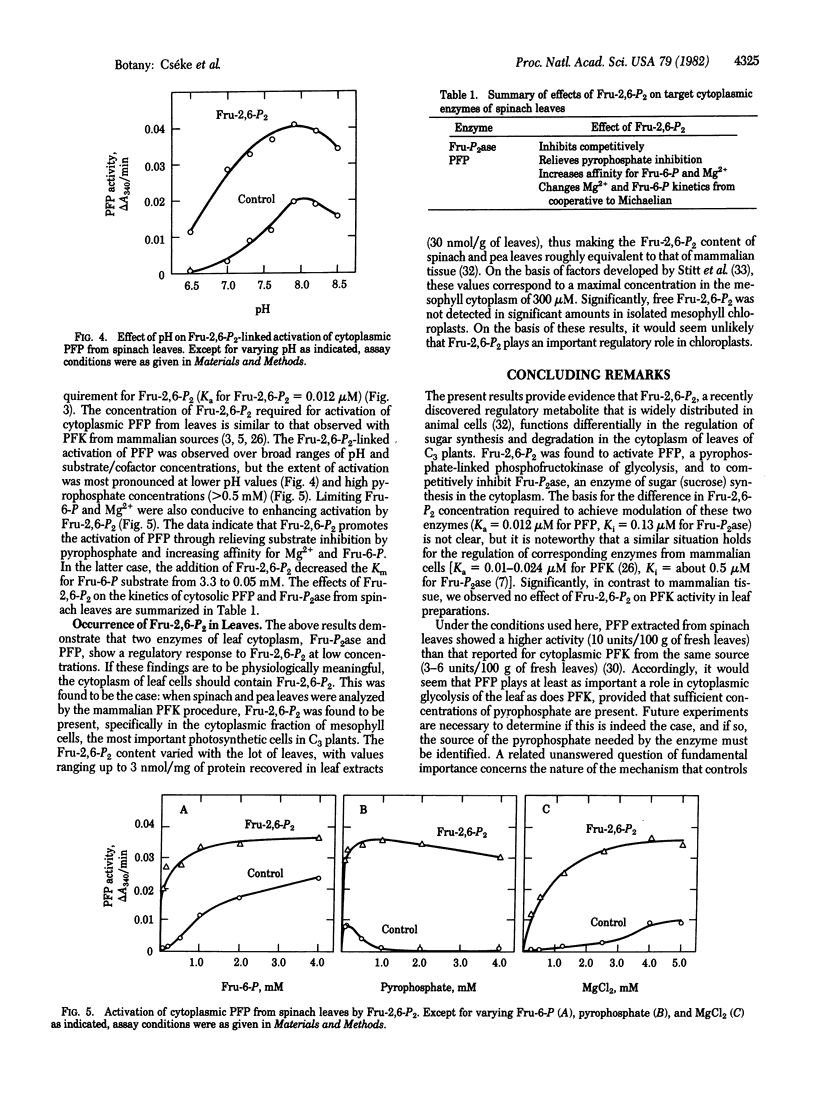
Abstract
Fructose 2,6-bisphosphate (Fru-2,6-P2), a regulatory metabolite discovered in animal cells and recently reported to occur in etiolated seedlings, was found to be present in the cytoplasmic fraction of leaves of spinach and peas (typical C3 plants, in which a three-carbon carboxylic acid is a major early photosynthetic product). At concentrations approximating those calculated to occur physiologically, Fru-2,6-P2 modulated two enzymes of the leaf cytoplasm: (i) Fructose-1,6-bisphosphatase (EC 3.1.3.11), a key enzyme of sugar synthesis, was competitively inhibited by Fru-2,6-P2, and (ii) pyrophosphate-linked phosphofructokinase (inorganic pyrophosphate-D-fructose-6-phosphate 1-phosphotransferase, EC 2.7.1.90), a cytoplasmic enzyme that now seems important in glycolysis of C3 plants, was activated by Fru-2,6-P2. There was no indication of a role for Fru-2,6-P2 in photosynthesis of either chloroplasts or oxygenic prokaryotes. The results suggest that Fru-2,6-P2 functions in the regulation of glycolysis and gluconeogenesis (carbohydrate synthesis) in the cytoplasm of leaves of C3 plants.
Keywords: fructose 2,6-bisphosphate; sucrose synthesis; fructose bisphosphatase; phosphofructokinase; glycolysis
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Carnal N. W., Black C. C. Pyrophosphate-dependent 6-phosphofructokinase, a new glycolytic enzyme in pineapple leaves. Biochem Biophys Res Commun. 1979 Jan 15;86(1):20–26. doi: 10.1016/0006-291x(79)90376-0. [DOI] [PubMed] [Google Scholar]
- Furuya E., Uyeda K. An activation factor of liver phosphofructokinase. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5861–5864. doi: 10.1073/pnas.77.10.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya E., Yokoyama M., Uyeda K. Regulation of fructose-6-phosphate 2-kinase by phosphorylation and dephosphorylation: possible mechanism for coordinated control of glycolysis and glycogenolysis. Proc Natl Acad Sci U S A. 1982 Jan;79(2):325–329. doi: 10.1073/pnas.79.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbron S., Foyer C., Walker D. The purification and properties of sucrose-phosphate synthetase from spinach leaves: the involvement of this enzyme and fructose bisphosphatase in the regulation of sucrose biosynthesis. Arch Biochem Biophys. 1981 Nov;212(1):237–246. doi: 10.1016/0003-9861(81)90363-5. [DOI] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Latzko E. Chloroplast Phosphofructokinase: II. Partial Purification, Kinetic and Regulatory Properties. Plant Physiol. 1977 Aug;60(2):295–299. doi: 10.1104/pp.60.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Latzko E. Chloroplast phosphofructokinase: I. Proof of phosphofructokinase activity in chloroplasts. Plant Physiol. 1977 Aug;60(2):290–294. doi: 10.1104/pp.60.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M., Uyeda K. The tissue distribution of fructose-2,6-p2 and fructose-6-P,2-kinase in rats and the effect of starvation diabetes and hypoglycemia on hepatic fructose-2,6-P2 and fructose-6-P,2-kinase. Biochem Biophys Res Commun. 1982 Jan 15;104(1):84–88. doi: 10.1016/0006-291x(82)91943-x. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Macy J. M., Ljungdahl L. G., Gottschalk G. Pathway of succinate and propionate formation in Bacteroides fragilis. J Bacteriol. 1978 Apr;134(1):84–91. doi: 10.1128/jb.134.1.84-91.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. H., Cumming D. A. Fructose 2,6-bisphosphate. A new activator of phosphofructokinase. J Biol Chem. 1981 Apr 10;256(7):3171–3174. [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-bisphosphate. J Biol Chem. 1981 Apr 25;256(8):3619–3622. [PubMed] [Google Scholar]
- Preiss J., Biggs M. L., Greenberg E. The effect of magnesium ion concentration on the pH optimum of the spinach leaf alkaline fructose diphosphatase. J Biol Chem. 1967 May 10;242(9):2292–2294. [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. D-Fructose 2,6-bisphosphate: a naturally occurring activator for inorganic pyrophosphate:D-fructose-6-phosphate 1-phosphotransferase in plants. Biochem Biophys Res Commun. 1981 Dec 15;103(3):848–855. doi: 10.1016/0006-291x(81)90888-3. [DOI] [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. Inorganic pyrophosphate: D-fructose-6-phosphate 1-phosphotransferase in mung beans and its activation by D-fructose 1,6-bisphosphate and D-glucose 1, 6-bisphosphate. Biochem Biophys Res Commun. 1981 Jun;100(4):1423–1429. doi: 10.1016/0006-291x(81)90677-x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Wirtz W., Heldt H. W. Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim Biophys Acta. 1980 Nov 5;593(1):85–102. doi: 10.1016/0005-2728(80)90010-9. [DOI] [PubMed] [Google Scholar]
- Uyeda K., Furuya E., Luby L. J. The effect of natural and synthetic D-fructose 2,6-bisphosphate on the regulatory kinetic properties of liver and muscle phosphofructokinases. J Biol Chem. 1981 Aug 25;256(16):8394–8399. [PubMed] [Google Scholar]
- Uyeda K., Furuya E., Sherry A. D. The structure of "activation factor" for phosphofructokinase. J Biol Chem. 1981 Aug 25;256(16):8679–8684. [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc Natl Acad Sci U S A. 1981 May;78(5):2861–2863. doi: 10.1073/pnas.78.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Phosphofructokinase 2: the enzyme that forms fructose 2,6-bisphosphate from fructose 6-phosphate and ATP. Biochem Biophys Res Commun. 1981 Aug 14;101(3):1078–1084. doi: 10.1016/0006-291x(81)91859-3. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Control of the fructose-6-phosphate/fructose 1,6-bisphosphate cycle in isolated hepatocytes by glucose and glucagon. Role of a low-molecular-weight stimulator of phosphofructokinase. Biochem J. 1980 Dec 15;192(3):887–895. doi: 10.1042/bj1920887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Fructose 2,6-bisphosphate, the probably structure of the glucose- and glucagon-sensitive stimulator of phosphofructokinase. Biochem J. 1980 Dec 15;192(3):897–901. doi: 10.1042/bj1920897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H. W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980 Jul;66(1):187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., O'brien W. E., Micheales G. Properties of carboxytransphosphorylase; pyruvate, phosphate dikinase; pyrophosphate-phosphofructikinase and pyrophosphate-acetate kinase and their roles in the metabolism of inorganic pyrophosphate. Adv Enzymol Relat Areas Mol Biol. 1977;45:85–155. doi: 10.1002/9780470122907.ch2. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase. Eur J Biochem. 1976 Nov 15;70(2):361–367. doi: 10.1111/j.1432-1033.1976.tb11025.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Purification and properties of spinach leaf cytoplasmic fructose-1,6-bisphosphatase. J Biol Chem. 1978 Sep 10;253(17):5952–5956. [PubMed] [Google Scholar]
- el-Maghrabi M. R., Claus T. H., Pilkis J., Pilkis S. J. Regulation of 6-phosphofructo-2-kinase activity by cyclic AMP-dependent phosphorylation. Proc Natl Acad Sci U S A. 1982 Jan;79(2):315–319. doi: 10.1073/pnas.79.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]


