Abstract
G protein-gated inward rectifier K+ (GIRK) channels function as cellular mediators of a wide range of hormones and neurotransmitters and are expressed in the brain, heart, skeletal muscle and endocrine tissue1,2. GIRK channels become activated following the binding of ligands (neurotransmitters, hormones, drugs, etc.) to their plasma membrane-bound, G protein-coupled receptors (GPCRs). This binding causes the stimulation of G proteins (Gi and Go) which subsequently bind to and activate the GIRK channel. Once opened the GIRK channel allows the movement of K+ out of the cell causing the resting membrane potential to become more negative. As a consequence, GIRK channel activation in neurons decreases spontaneous action potential formation and inhibits the release of excitatory neurotransmitters. In the heart, activation of the GIRK channel inhibits pacemaker activity thereby slowing the heart rate.
GIRK channels represent novel targets for the development of new therapeutic agents for the treatment neuropathic pain, drug addiction, cardiac arrhythmias and other disorders3. However, the pharmacology of these channels remains largely unexplored. Although a number of drugs including anti-arrhythmic agents, antipsychotic drugs and antidepressants block the GIRK channel, this inhibition is not selective and occurs at relatively high drug concentrations3.
Here, we describe a real-time screening assay for identifying new modulators of GIRK channels. In this assay, neuronal AtT20 cells, expressing GIRK channels, are loaded with membrane potential-sensitive fluorescent dyes such as bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3)] or HLB 021-152 (Figure 1). The dye molecules become strongly fluorescent following uptake into the cells (Figure 1). Treatment of the cells with GPCR ligands stimulates the GIRK channels to open. The resulting K+ efflux out of the cell causes the membrane potential to become more negative and the fluorescent signal to decrease (Figure 1). Thus, drugs that modulate K+ efflux through the GIRK channel can be assayed using a fluorescent plate reader. Unlike other ion channel screening assays, such atomic absorption spectrometry4 or radiotracer analysis5, the GIRK channel fluorescent assay provides a fast, real-time and inexpensive screening procedure.
Keywords: Medicine, Issue 62, G protein-gated inward rectifier K+ (GIRK) channels, clonal cell lines, drug screening, fluorescent dyes, K+ channel modulators, Pharmacology
Protocol
1. Preparation of Cells
Grow pituitary AtT20 cells in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% horse serum and maintain cultures at 37 °C in a humidified atmosphere of 5% CO2.
Maintain the culture by subculturing the cells every 5 to 7 days using a standard trypsinization procedure.
Coat the wells of black, clear bottom, 96-well plates with 50 μL of poly-l-lysine. Allow the wells to dry for 30 min in the incubator.
Plate the cells in the 96-well plates containing 200 μL media at density of 30,000 cells per well and store the cells in the incubator for 3-4 days.
Use an aspiration setup, consisting of a stoppered 4 liter jar connected to a vacuum on one end and a 200 μL pipette tip at the other end, to slowly aspirate the culture medium from the cell monolayer.
Wash the cells with 200 μL of normal buffer solution (132 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM dextrose, 5 mM HEPES, pH 7.4 with NaOH).
Add 200 μL of buffer solution containing the membrane potential-sensitive dye (MPD) DiBAC4(3) or HLB 021-152 (5 μM) to each well and incubate the cells for 40 min at 37 °C.
2. Preparation of Test Compounds and Cell Treatment
Prepare a serial dilution of test compounds (stock = 10 mM in DMSO) from a compound library (in 96-well format) using a Versette (ThermoFisher) automated liquid handling system.
Remove the 96-well plate, containing the AtT20 cells, from the incubator and allow the plate to return to room temperature. Aspirate off the old buffer and apply fresh buffer solution containing the MPD to the cells.
Using the liquid handling system apply various concentrations (10 nM to 10 μM) of the test compounds (2 to 20 μL) and control solutions (0.1% DMSO) (in buffer solution containing the MPD) to the 96-well plate containing the cells.
Incubate the cells for 5 min with test compounds prior to the fluorescent measurements.
3. Activation of GIRK Channels, Fluorescent Measurement and Analysis
Insert the 96-well plate into a Biotek Synergy2 fluorescent plate reader and obtain the fluorescent background signal at excitation and emission wavelengths of 520 and 560 nm, respectively.
Apply the GPCR ligands (somatostatin = 200 nM or carbachol = 10 μM) or control solution (buffer alone) (20 μL) to the wells (total volume = 220 μL) to activate the GIRK channels using the Synergy2 injector system. Collect data points at 10 s intervals over a 300 s sampling period at excitation and emission wavelengths of 520 and 560 nm, respectively.
Obtain the time course and peak amplitude of the fluorescent change using Biotek Gen5 software and export the data to Excel for further analysis.
Calculate the Z'-factor to determine the reliability of the assay. Plates used for Z'-factor determination contained 2 rows each of positive (GPCR ligand) and negative (buffer alone) controls. The Z'-factor is defined as: Z' = 1 - (3σP + 3σN) / | μP - μN |, where μP & μN are the means of the positive control and negative control signals, and σP & σN are the standard deviations of the positive control and negative control signals, respectively 6. Z'-factors in the range of 0.5 to 1.0 indicate that the quality of the assay is excellent 6. Plates with Z'-factors below 0.5 are excluded from the analysis.
Compounds that modulate the fluorescent signal are retested in the presence of Ba2+ or tertiapin-Q to confirm inward rectifier K+ channel specificity.
4. Representative Results
An example of the fluorescence signal measured using the GIRK channel assay is shown in Figure 2. Addition of the GPCR ligand somatostatin (200 nM) to the AtT20 cells caused a rapid, time-dependent decrease in the HLB 021-152 fluorescent signal (Figure 2). In contrast, addition of control solution produced a small instantaneous rise in the fluorescence that with time returned to the baseline (Figure 2). Comparison of peak fluorescent values in 96-well plates injected with control and somatostatin solutions gave a Z'-factors in the range of 0.5 to 0.7. For further quantification, the control record was subtracted from the somatostatin record and the resulting somatostatin-sensitive signal analyzed (Figure 2).
The assay provides a method for quickly identifying drugs that modulate GIRK channels. For example, drugs that inhibit the GIRK channel should reduce GPCR ligand-mediated decreases in fluorescence by preventing K+ efflux from the cells. As shown in Figure 3, treatment of the cells with tertiapin-Q, a toxin that blocks GIRK channels7, produced an inhibition of the somatostatin-mediated fluorescent change. Propafenone, an anti-arrhythmic drug that blocks GIRK channels in the heart8, also inhibited the fluorescent change (Figure 4). The assay might also be useful for identifying activators of the GIRK channel. However, application of ethanol (100 & 200 mM), an activator of the GIRK channel2,3, caused no significant change in the fluorescent signal when compared to control solution (p > 0.5).
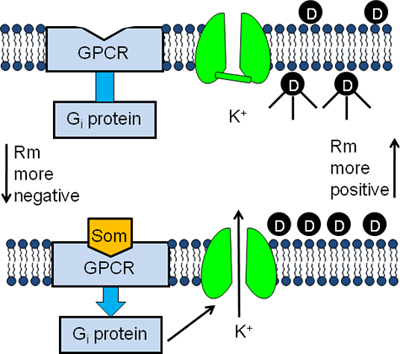 Figure 1. Experimental design of the GIRK channel fluorescent assay. AtT20 cells are incubated in buffer containing a membrane potential-sensitive fluorescent dye (D). The dye molecules enter into the cells and become fluorescent upon binding to intracellular proteins (top panel). Binding of somatostatin (Som) to its GPCR stimulates the G inhibitory protein (Gi) causing the activation of the GIRK channel (bottom panel). The subsequent efflux of K+ out of the cells causes the membrane potential to become more negative and the dye molecules to exit the cells. As a result the fluorescent signal decreases (bottom panel).
Figure 1. Experimental design of the GIRK channel fluorescent assay. AtT20 cells are incubated in buffer containing a membrane potential-sensitive fluorescent dye (D). The dye molecules enter into the cells and become fluorescent upon binding to intracellular proteins (top panel). Binding of somatostatin (Som) to its GPCR stimulates the G inhibitory protein (Gi) causing the activation of the GIRK channel (bottom panel). The subsequent efflux of K+ out of the cells causes the membrane potential to become more negative and the dye molecules to exit the cells. As a result the fluorescent signal decreases (bottom panel).
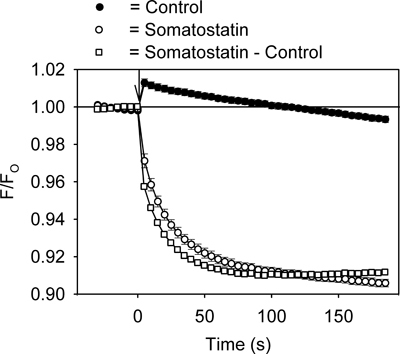 Figure 2. Representative HLB 021-152 fluorescent signal measured over time in the AtT20 cells during addition of somatostatin or control solution. The ratio of the fluorescent intensity (F/FO) was calculated by dividing the signal in the presence (F) of somatostatin (or control solution) by the baseline signal measured before (FO) addition of somatostatin (or control solution). Each point represents the mean ± SE obtained in 5-6 wells. Somatostatin or control solution was added at time zero (↓). Addition of the control solution caused a small transient increase in the fluorescent signal. This resulted from a temperature change caused by injection of the solution.
Figure 2. Representative HLB 021-152 fluorescent signal measured over time in the AtT20 cells during addition of somatostatin or control solution. The ratio of the fluorescent intensity (F/FO) was calculated by dividing the signal in the presence (F) of somatostatin (or control solution) by the baseline signal measured before (FO) addition of somatostatin (or control solution). Each point represents the mean ± SE obtained in 5-6 wells. Somatostatin or control solution was added at time zero (↓). Addition of the control solution caused a small transient increase in the fluorescent signal. This resulted from a temperature change caused by injection of the solution.
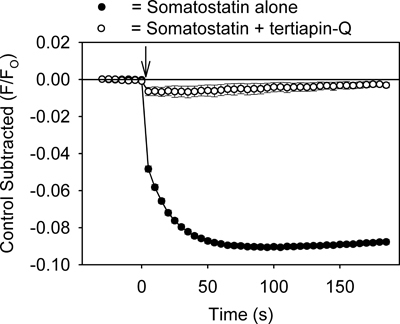 Figure 3. Treatment of the AtT20 cells with tertiapin-Q (500 nM) inhibits the somatostatin-mediated decrease in the fluorescent signal by binding to and blocking the GIRK channels. Each point represents the mean ± SE obtained in 4-6 wells. Somatostatin was added at time zero (↓).
Figure 3. Treatment of the AtT20 cells with tertiapin-Q (500 nM) inhibits the somatostatin-mediated decrease in the fluorescent signal by binding to and blocking the GIRK channels. Each point represents the mean ± SE obtained in 4-6 wells. Somatostatin was added at time zero (↓).
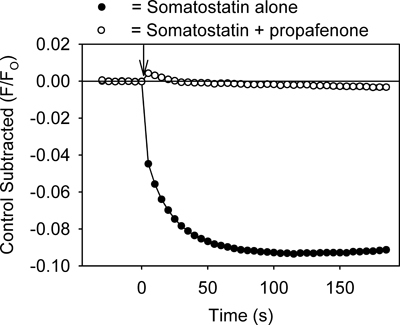 Figure 4. Treatment of the AtT20 cells with propafenone (20 μM) also inhibits the somatostatin-mediated decrease in the fluorescent signal. Each point represents the mean ± SE obtained in 5-6 wells. Somatostatin was added at time zero (↓).
Figure 4. Treatment of the AtT20 cells with propafenone (20 μM) also inhibits the somatostatin-mediated decrease in the fluorescent signal. Each point represents the mean ± SE obtained in 5-6 wells. Somatostatin was added at time zero (↓).
Discussion
While membrane potential-sensitive fluorescent dyes have been used to identify drugs that modulate ion channels9,10, this is the first report of their application for neuronal GIRK channel drug discovery. The GIRK channel fluorescent assay presented here provides a fast, reliable and real-time method for the screening of ligand-gated K+ channels. The assay can be modified for use with a wide range of cells including immortalized cells lines (HEK293, CHO, etc.) expressing an exogenous recombinant GIRK channel11 or clonal cell lines (AtT20, PC-12) expressing an endogenous channel. In addition, the assay may be adapted for screening drugs in embryonic stem cells and primary cell cultures. As used with the AtT20 cells, the assay also provides the extra benefit of allowing the study of multiple GPCR ligands (somatostatin and carbachol) on the GIRK channel. As with any fluorescent assay, control experiments must be carried out to eliminate artifacts due to compound auto-fluorescence and to identify errors resulting from direct interactions of the compounds with the dye molecules. Alternate approaches such as the thallium influx assay11 and automated patch clamp procedure12 should also be considered.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by US Public Health Service award NS-071530.
References
- Hibino H. Inward rectifying potassium channels: their structure, function and physiological roles. Physiol. Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Lusscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K. G protein-activated inwardly rectifying potassium channels as potential therapeutic targets. Cur. Pharm. Des. 2006;12:4513–4523. doi: 10.2174/138161206779010468. [DOI] [PubMed] [Google Scholar]
- Terstappen GC. Functional analysis of native and recombinant ion channels using a high-capacity nonradioactive rubidium efflux assay. Anal. Biochem. 1999;272:149–155. doi: 10.1006/abio.1999.4179. [DOI] [PubMed] [Google Scholar]
- Cheng CS. A high-throughput HERG potassium channel function assay: an old assay with a new look. Drug Dev. Ind. Pharm. 2002;28:177–191. doi: 10.1081/ddc-120002451. [DOI] [PubMed] [Google Scholar]
- Zhang J-H, Chung TDY, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Jin W, Lu Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochem. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- Inomata N. Anti-arrhythmic agents act differently on the activation phase of the Ach-response in guinea pig atrial myocytes. Br. J. Pharmacol. 1993;108:111–116. doi: 10.1111/j.1476-5381.1993.tb13448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. Development and evaluation of high throughput functional assay methods for HERG potassium channel. J. Biomol. Screen. 2001;6:325–331. doi: 10.1177/108705710100600506. [DOI] [PubMed] [Google Scholar]
- Wolff C, Fuks B, Chatelain P. Comparative study of membrane potential-sensitive fluorescent probes and their use in ion channel screening assays. J. Biomol. Screen. 2003;8:533–513. doi: 10.1177/1087057103257806. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Luo Q, Avala JE, Kim C, Conn PJ, Weaver CD. A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of group III metabotropic glutamate receptors. Mol. Pharm. 2008;73:1213–1224. doi: 10.1124/mol.107.041053. [DOI] [PubMed] [Google Scholar]
- Bridal TR, Marquilis M, Wang X, Donio M, Sorota S. Comparison of human Ether-á-go-go related gene screening assays based on IonWorks Quattro and thallium flux. Assay Drug Dev. Technol. 2010;8:755–765. doi: 10.1089/adt.2010.0267. [DOI] [PubMed] [Google Scholar]


