Abstract
Elevated intracranial pressure (ICP) is a significant problem in several forms of ischemic brain injury including stroke, traumatic brain injury and cardiac arrest. This elevation may result in further neurological injury, in the form of transtentorial herniation1,2,3,4, midbrain compression, neurological deficit or increased cerebral infarct2,4. Current therapies are often inadequate to control elevated ICP in the clinical setting5,6,7 . Thus there is a need for accurate methods of ICP measurement in animal models to further our understanding of the basic mechanisms and to develop new treatments for elevated ICP.
In both the clinical and experimental setting ICP cannot be estimated without direct measurement. Several methods of ICP catheter insertion currently exist. Of these the intraventricular catheter has become the clinical 'gold standard' of ICP measurement in humans8. This method involves the partial removal of skull and the instrumentation of the catheter through brain tissue. Consequently, intraventricular catheters have an infection rate of 6-11%9. For this reason, subdural and epidural cannulations have become the preferred methods in animal models of ischemic injury.
Various ICP measurement techniques have been adapted for animal models, and of these, fluid-filled telemetry catheters10 and solid state catheters are the most frequently used11,12,13,14,15. The fluid-filled systems are prone to developing air bubbles in the line, resulting in false ICP readings. Solid state probes avoid this problem (Figure 1). An additional problem is fitting catheters under the skull or into the ventricles without causing any brain injury that might alter the experimental outcomes. Therefore, we have developed a method that places an ICP catheter contiguous with the epidural space, but avoids the need to insert it between skull and brain.
An optic fibre pressure catheter (420LP, SAMBA Sensors, Sweden) was used to measure ICP at the epidural location because the location of the pressure sensor (at the very tip of the catheter) was found to produce a high fidelity ICP signal in this model. There are other manufacturers of similar optic fibre technologies13 that may be used with our methodology. Alternative solid state catheters, which have the pressure sensor located at the side of the catheter tip, would not be appropriate for this model as the signal would be dampened by the presence of the monitoring screw.
Here, we present a relatively simple and accurate method to measure ICP. This method can be used across a wide range of ICP related animal models.
Keywords: Medicine, Issue 62, Neuroscience, brain, rat, intracranial pressure, epidural, fibre-optic transducer, ischemic injury
Protocol
1. Skull Penetration
Anaesthetize rat with isoflurane (5% induction, 1.5-2% maintenance) in 70% N2 and 30% O2. Following induction of anaesthesia, place the rat prone on a warming plate, positioning the rat's nose in an anaesthetic nose cone.
Whilst maintaining anaesthesia, secure the head in a stereotaxic frame, inserting the ear-bars until head is stabilised. Ensure breathing is not impaired. (Figure 2-A).
Inject scalp subcutaneously with long lasting local anaesthetic, Bupivacaine 0.3 ml 0.5% (Pfizer, Australia) before making a 1.5 cm skin midline head incision. (Sterile instruments and gloves should be used.)
Blunt dissect the soft tissue and surrounding muscles to clearly locate Lambda and Bregma. Retract the skin and connective tissue.
Stem any bleeding by applying pressure to the exposed skull. Excessive skull bleeding may be cauterized.
Using a dental drill with a 1 mm tip burr, burr a hole 2 mm wide into the right parietal bone. Burr the hole 2 mm lateral and 2 mm posterior from Bregma to avoid the superior sagittal sinus and ensure the placement of the ICP sensor is over the ischemic territory, for stroke studies. Alternative locations would be equally suitable for other applications. Burr the hole to a depth where the skull above the dura becomes translucent. (Figure 2-B).
Replace the burr with a 0.5 mm tip burr to remove the skull at the base of hole.
When the skull begins to crack, use 45° forceps to remove all remaining skull, ensuring the base of the hole is cleared of debris. (Figure 3).
2. Screw Modification and Insertion
Drill a 0.7 mm hole through a hexagonal-headed screw using a lathe and 0.7 mm drill bit.
Insert the monitoring screw into the hole by turning it approximately 1.5 turns (use the minimum amount of turns needed to secure screw in skull so as not to damage underlying tissue). (Figure 2-C and Figure 4).
Burr a second hole for an anchoring screw in the left parietal bone, 2 mm lateral and 2 mm posterior from Bregma. This hole does not require complete penetration of the skull, so the 1 mm tip burr is used to thin the skull for screw insertion.
Insert a 2 x 4 mm hexagonal-headed screw into the second hole. This screw helps to anchor the dental cement and hence the monitoring screw to the skull.
Use a transfer pipette to mix and apply dental cement monomer and polymer to the base of the head of the screws.
Allow dental cement to dry for at least 10 minutes.
3. Intracranial Pressure Transducer Insertion
Using white correction fluid, mark the fibre-optic sensor 4 mm from the tip.
Fill the hole of the monitoring screw with sterile saline (0.9%) and ensure no air bubbles are present within the screw.
Insert the ICP probe 4 mm into the screw so that the tip of the probe is level with the end of the screw. Ensure the tip does not pierce the dura.
Adjust the tip of the probe within the screw until an ICP trace reflecting ventilation and blood pressure pulse waves can be observed. (Figure 5).
4. Forming an Airtight Seal
An airtight seal is imperative to an accurate ICP reading. Mix a viscous biocompatible caulking material monomer and polymer in the ratio of 1:1. Because the pressure sensor is at the tip of the probe, and this is within the hollowed out screw, application of caulking material to the shaft of the fibre-optic probe has no effect on pressure sensitivity of the sensor.
Apply a thin layer around the probe and the head of the monitoring screw. Avoid displacing the ICP probe.
Allow to set for 5 minutes.
Apply a second layer of caulking material around the entire monitoring screw and probe. Ensure that no liquid is leaking from any crevasses in the caulking material. (Figure 2-D).
Remove ear-bars.
The rat may remain in the prone position, or be carefully rotated into the supine position during ICP monitoring.
A schematic of the completed procedure is depicted in (Figure 6).
5. Intracranial Pressure Transducer Removal and Reinsertion
At the completion ICP monitoring, the ICP sensor may be removed by gently pulling the catheter from the screw and caulking material.
The SAMBA sensor should be immediately place into 1 % Terg-A-Zyme solution to prevent tip corrosion.
The hole remaining in the caulking material should be covered with an additional layer of caulking material. (Rat may be woken at this stage).
To reinsert the SAMBA catheter for additional monitoring, slice the caulking material at the level of the head of the screw.
Repeat steps 3.2 - 5.3.
6. Representative Results
Figure 5 is a representation of ICP readings over ten seconds. At baseline, the average ICP in a Wistar rat is 6 mmHg. The events of shorter periodicity depicted in Figure 5 reflect blood pressure pulse waves. The events of longer periodicity show ventilation events. Note that the SAMBA sensor reflects a ventilation amplitude of 3-4 mmHg and pulse amplitude of 1-2 mmHg.
To validate the position of the SAMBA sensor in each experiment, ICP traces should be tested for responsiveness to abdominal compressions and respiratory events, such as periods of apnoea. An abdominal compression is depicted in Figure 7.
Periods of apnoea (illustrated in Figure 8) are observed in most experiments involving spontaneously breathing animals. These events are identified on physiological records by an absence of respiratory deflections on respiratory (diaphragm transducer) and arterial pressure traces. An equivalent alteration in the ICP trace validates the ICP probe positioning.
Figure 9 depicts a typical ICP trace after the removal of the ear-bars (step 4.6). The insertion of the ear-bars in step 1.2 results in a slight compression of the skull and consequent disruption in intracranial volume and hence an increased ICP. If the sensor is positioned correctly, ICP will drop at least 4 - 5 mmHg with the removal of the ear-bars.
Histological analysis may be used to check for any damage to the cortical area immediately under the pressure sensor and screw. An example of a traumatic and non-traumatic screw insertion is depicted in Figure 4.
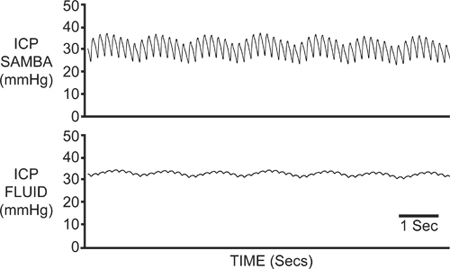 Figure 1. Fluid Filled Vs SAMBA ICP Traces. ICP was recorded simultaneously via the SAMBA fibre-optic catheter (top) and a fluid filled catheter (bottom). The mean ICP values were similar in both traces, however the fluid filled catheter signal was notably dampened compared to the clear respiratory and arterial pressure waveforms seen with the fibre-optic catheter.
Figure 1. Fluid Filled Vs SAMBA ICP Traces. ICP was recorded simultaneously via the SAMBA fibre-optic catheter (top) and a fluid filled catheter (bottom). The mean ICP values were similar in both traces, however the fluid filled catheter signal was notably dampened compared to the clear respiratory and arterial pressure waveforms seen with the fibre-optic catheter.
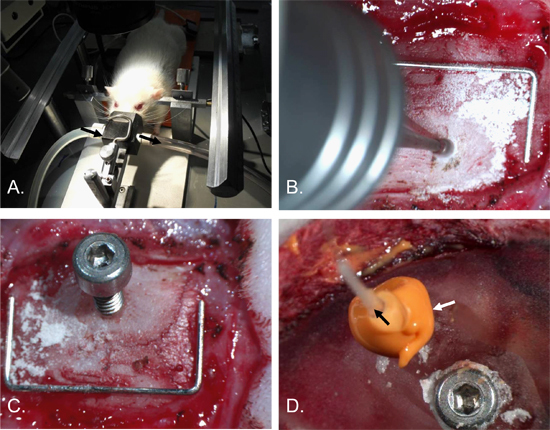 Figure 2. Intracranial Pressure Catheter Insertion Procedure. The rat's head was secured in a stereotaxic frame with ear-bars and an anaesthetic nose cone [A]. A hole, approximately 2 mm in diameter, was drilled into the right parietal bone [B]. A 2 x 4 mm screw with a 0.7 mm hole in the shaft was inserted [C]. An anchoring screw was inserted into the left parietal bone and the skull and surgical site covered in dental cement. The ICP catheter (black arrow) was then inserted into the screw-hole and an airtight seal made with the caulking material (white arrow) [D]. Staple (for scale) = 12 mm x 5 mm.
Figure 2. Intracranial Pressure Catheter Insertion Procedure. The rat's head was secured in a stereotaxic frame with ear-bars and an anaesthetic nose cone [A]. A hole, approximately 2 mm in diameter, was drilled into the right parietal bone [B]. A 2 x 4 mm screw with a 0.7 mm hole in the shaft was inserted [C]. An anchoring screw was inserted into the left parietal bone and the skull and surgical site covered in dental cement. The ICP catheter (black arrow) was then inserted into the screw-hole and an airtight seal made with the caulking material (white arrow) [D]. Staple (for scale) = 12 mm x 5 mm.
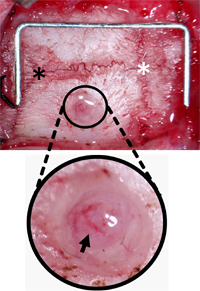 Figure 3. Monitoring Screw Burr Hole Orientation. The skull was cleared of connective tissue to locate Lambda (black asterisk) and Bregma (white asterisk) and the hole drilled 2 mm lateral and 2 mm posterior from Bregma. The hole was cleared of debris leaving the dura and pial vessels (black arrow) intact. Staple (for scale) = 12 mm x 5 mm.
Figure 3. Monitoring Screw Burr Hole Orientation. The skull was cleared of connective tissue to locate Lambda (black asterisk) and Bregma (white asterisk) and the hole drilled 2 mm lateral and 2 mm posterior from Bregma. The hole was cleared of debris leaving the dura and pial vessels (black arrow) intact. Staple (for scale) = 12 mm x 5 mm.
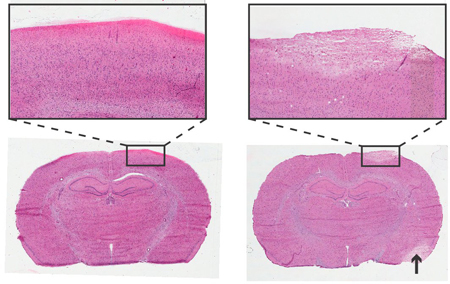 Figure 4. Histology of Rat Brain 24 Hours after Instrumentation of ICP Monitoring Screw. Haemotoxylin and Eosin staining, 6 μm coronal sections. Left: Non-traumatic screw insertion. Right: Traumatic screw insertion, area of pallor depicts damaged tissue with similar cellular morphology to stroke damaged area (arrow). Inserts at 4x objective.
Figure 4. Histology of Rat Brain 24 Hours after Instrumentation of ICP Monitoring Screw. Haemotoxylin and Eosin staining, 6 μm coronal sections. Left: Non-traumatic screw insertion. Right: Traumatic screw insertion, area of pallor depicts damaged tissue with similar cellular morphology to stroke damaged area (arrow). Inserts at 4x objective.
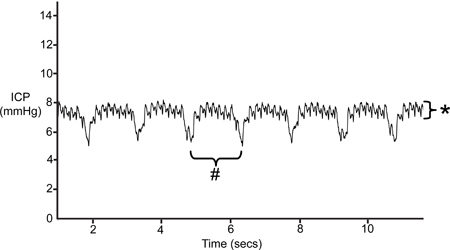 Figure 5. Typical ICP Trace. Pulse pressure waves are depicted by events of smaller amplitude (*). Ventilation is reflected by events of longer periodicity (#).
Figure 5. Typical ICP Trace. Pulse pressure waves are depicted by events of smaller amplitude (*). Ventilation is reflected by events of longer periodicity (#).
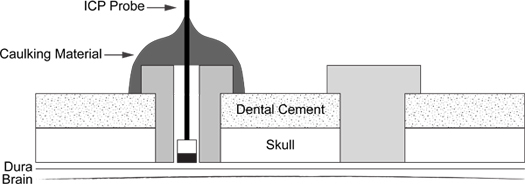 Figure 6. ICP Probe Insertion Schematic. Diagram illustrates placement of support screw (right) and caulking material coated ICP probe into screw (left).
Figure 6. ICP Probe Insertion Schematic. Diagram illustrates placement of support screw (right) and caulking material coated ICP probe into screw (left).
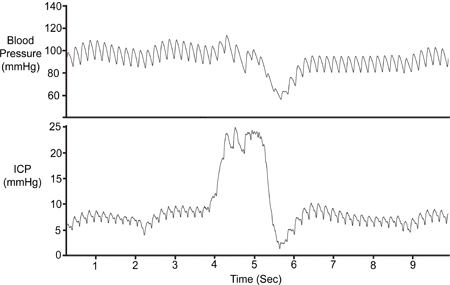 Figure 7. Abdominal Compression. The abdomen was temporarily compressed (~1 sec) to validate the viability of the ICP signal. Compression results in reduced cerebral venous return, increasing intracranial volume and thus increasing ICP. Arterial pressure (Pa) dropped only after the initial ICP rise.
Figure 7. Abdominal Compression. The abdomen was temporarily compressed (~1 sec) to validate the viability of the ICP signal. Compression results in reduced cerebral venous return, increasing intracranial volume and thus increasing ICP. Arterial pressure (Pa) dropped only after the initial ICP rise.
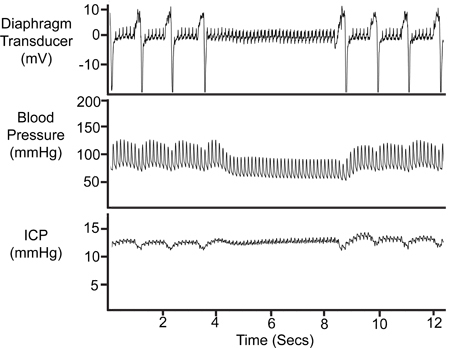 Figure 8. Period of Apnoea. The temporary cessation of breathing is reflected in the diaphragm transducer trace, the arterial pressure (Pa) trace and the ICP trace.
Figure 8. Period of Apnoea. The temporary cessation of breathing is reflected in the diaphragm transducer trace, the arterial pressure (Pa) trace and the ICP trace.
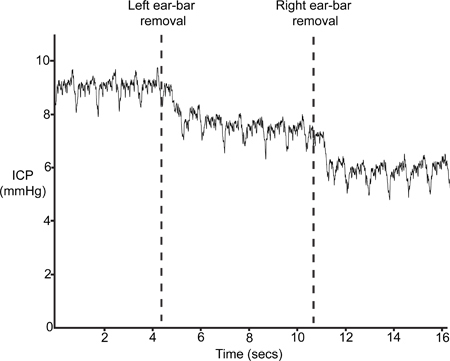 Figure 9. Ear-Bar Removal. ICP should drop with the removal of the stereotaxic frame ear-bars.
Figure 9. Ear-Bar Removal. ICP should drop with the removal of the stereotaxic frame ear-bars.
Discussion
The procedure presented here enables a very sensitive and accurate recording of intracranial pressure. This minimally invasive technique avoids significant brain trauma by positioning the pressure sensor in the epidural space and not the brain tissue or ventricles.
The critical steps Include: 1) drilling through the skull – care must be taken not to pierce the dura or damage underlying brain tissue; 2) ensuring a tight seal with the caulking material – if there is any leak, the ICP trace will not be reliable. When the ICP sensor is appropriately positioned, the reading will give an accurate trace of not only ICP, but also respiratory and heart rate. With inspiration, the more negative intrathoracic pressure reduces downstream intravascular pressure, creating a greater pressure gradient and increasing cerebral venous return. The subsequent reduction in cerebral blood volume results in a decrease in ICP. Conversely, expiration increases the downstream venous pressure and increases ICP. Brief, one second abdominal compressions can be performed in every experimental animal to simulate a stimulus similar to a Valsalva manoeuvre. When applied, this physiological stimulus is known to reduce cerebral venous return and result in a transient rise in ICP. A lack of response to abdominal compression (no rise in ICP) suggests a leak in the airtight seal or blockage of the hollow screw. If a leak is apparent, a third layer of caulking material may be applied around the sensor to obtain an airtight seal. Note that the caulking material will not compress the optic fibre, so an additional layer will only ensure an adequate seal. If the screw is blocked, remove the caulking material and sensor, flush the screw gently with sterile saline and repeat steps 3.2 – 4.5. If the ICP trace is still weak, the fibre optic cable must be checked. The SAMBA optic fibre cable can tolerate a bend radius of 10 cm, if this is exceeded the ICP trace will be compromised.
The dura is in very close proximity to the skull, and therefore utmost care must be taken when removing the skull in step 1.8. When learning this technique, the dura may be accidently pierced and cerebrospinal fluid (CSF) will leak into the epidural space and into the monitoring screw. A knick in the dura, however, will not affect the ICP measurement because the cranial vault is sealed.
This method is suitable for use in animals under anaesthetic, however it is readily modifiable to perform recordings using a tether system in awake animals. The technique described has the potential to be used in many models of ICP measurement. The optic fibre used in this method is insensitive to any form of electro-magnetic fields and is therefore compatible with imaging technologies such as MR, CT, PET and SPECT. The quality of the recordings and reliability of measurements over time is superior to those obtained using commercially available fluid-filled catheter systems.
Disclosures
We have nothing to disclose.
Acknowledgments
This project was funded by the National Stroke Foundation, Hunter Medical Research Institute (HMRI) and National Health and Medical Research Council (NH&MRC), Australia. Special thanks to the Faculty of Health Workshop staff at The University of Newcastle for their technical expertise.
References
- Ng LK, Nimmannitya J. Massive cerebral infarction with severe brain swelling: a clinicopathological study. Stroke. 1970;1:158–163. doi: 10.1161/01.str.1.3.158. [DOI] [PubMed] [Google Scholar]
- Plum F. Brain swelling and edema in cerebral vascular disease. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1966;41:318–348. [PubMed] [Google Scholar]
- Ropper AH, Shafran B. Brain edema after stroke. Clinical syndrome and intracranial pressure. Arch. Neurol. 1984;41:26–29. doi: 10.1001/archneur.1984.04050130032017. [DOI] [PubMed] [Google Scholar]
- Silver FL, Norris JW, Lewis AJ, Hachinski VC. Early mortality following stroke: a prospective review. Stroke. 1984;15:492–496. doi: 10.1161/01.str.15.3.492. [DOI] [PubMed] [Google Scholar]
- Geraci EB, Geraci TA. Hyperventilation and head injury: controversies and concerns. J. Neurosci. Nurs. 1996;28:381–387. doi: 10.1097/01376517-199612000-00005. [DOI] [PubMed] [Google Scholar]
- Schwab S, Aschoff A, Spranger M, Albert F, Hacke W. The value of intracranial pressure monitoring in acute hemispheric stroke. Neurology. 1996;47:393–398. doi: 10.1212/wnl.47.2.393. [DOI] [PubMed] [Google Scholar]
- Adams HP. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–1083. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- Zhong J. Advances in ICP monitoring techniques. Neurol. Res. 2003;25:339–350. doi: 10.1179/016164103101201661. [DOI] [PubMed] [Google Scholar]
- Aucoin PJ. Intracranial pressure monitors. Epidemiologic study of risk factors and infections. Am. J. Med. 1986;80:369–376. doi: 10.1016/0002-9343(86)90708-4. [DOI] [PubMed] [Google Scholar]
- Silasi G, MacLellan CL, Colbourne F. Use of telemetry blood pressure transmitters to measure intracranial pressure (ICP) in freely moving rats. Curr. Neurovasc. Res. 2009;6:62–69. doi: 10.2174/156720209787466046. [DOI] [PubMed] [Google Scholar]
- Crutchfield JS, Narayan RK, Robertson CS, Michael LH. Evaluation of a fiberoptic intracranial pressure monitor. J. Neurosurg. 1990;72:482–487. doi: 10.3171/jns.1990.72.3.0482. [DOI] [PubMed] [Google Scholar]
- Bolander R, Mathie B, Bir C, Ritzel D, Vandevord P. Skull Flexure as a Contributing Factor in the Mechanism of Injury in the Rat when Exposed to a Shock Wave. Ann. Biomed. Eng. 2011. [DOI] [PubMed]
- Chavko M, Koller WA, Prusaczyk WK, McCarron RM. Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. J. Neurosci. Methods. 2007;159:277–281. doi: 10.1016/j.jneumeth.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Chavko M. Relationship between orientation to a blast and pressure wave propagation inside the rat brain. J. Neurosci. Methods. 2011;195:61–66. doi: 10.1016/j.jneumeth.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Leonardi AD, Bir CA, Ritzel DV, VandeVord PJ. Intracranial pressure increases during exposure to a shock wave. J. Neurotrauma. 2011;28:85–94. doi: 10.1089/neu.2010.1324. [DOI] [PubMed] [Google Scholar]


