Abstract
Objective:
New preclinical Alzheimer disease (AD) diagnostic criteria have been developed using biomarkers in cognitively normal (CN) adults. We implemented these criteria using an MRI biomarker previously associated with AD dementia, testing the hypothesis that individuals at high risk for preclinical AD would be at elevated risk for cognitive decline.
Methods:
The Alzheimer's Disease Neuroimaging Initiative database was interrogated for CN individuals. MRI data were processed using a published set of a priori regions of interest to derive a single measure known as the AD signature (ADsig). Each individual was classified as ADsig-low (≥1 SD below the mean: high risk for preclinical AD), ADsig-average (within 1 SD of mean), or ADsig-high (≥1 SD above mean). A 3-year cognitive decline outcome was defined a priori using change in Clinical Dementia Rating sum of boxes and selected neuropsychological measures.
Results:
Individuals at high risk for preclinical AD were more likely to experience cognitive decline, which developed in 21% compared with 7% of ADsig-average and 0% of ADsig-high groups (p = 0.03). Logistic regression demonstrated that every 1 SD of cortical thinning was associated with a nearly tripled risk of cognitive decline (p = 0.02). Of those for whom baseline CSF data were available, 60% of the high risk for preclinical AD group had CSF characteristics consistent with AD while 36% of the ADsig-average and 19% of the ADsig-high groups had such CSF characteristics (p = 0.1).
Conclusions:
This approach to the detection of individuals at high risk for preclinical AD—identified in single CN individuals using this quantitative ADsig MRI biomarker—may provide investigators with a population enriched for AD pathobiology and with a relatively high likelihood of imminent cognitive decline consistent with prodromal AD.
Although it has been known for years that Alzheimer disease (AD) neuropathology may be present in individuals who were cognitively normal (CN) prior to death,1–6 this population is receiving increasing recent attention. In 2010–2011 expert groups published diagnostic criteria for “preclinical” AD that hinge upon biological markers, calling for longitudinal study of individuals who meet criteria.7,8
Although the core of preclinical AD criteria is molecular biomarker evidence of amyloid-β deposition, abnormalities of brain structure or function topographically consistent with AD-related neurodegeneration are also considered supportive preclinical AD biomarkers.
Here we performed a hypothesis-driven analysis to answer this question: if CN older adults harbor an MRI biomarker suggestive of early AD neurodegeneration, are they more likely to develop cognitive decline than CN individuals lacking this marker?
Based on previous studies, this analysis employed strong a priori hypotheses to define both the imaging biomarker and the cognitive decline outcome. The imaging biomarker used here was the AD signature (ADsig) of regional cortical thinning, a reliable and valid marker of mild AD dementia,9 useful for prediction of AD dementia in individuals with mild cognitive impairment (MCI),10 and detectable in CN individuals with brain amyloid-β measured with PET.9 We also recently found that it is detectable in CN adults who later develop AD dementia; individuals who express this marker have a 3-fold increased risk for AD dementia over the next decade.11 Here we used the previously published cutoff to operationalize this MRI biomarker in a manner consistent with subtle neurodegeneration predicted to occur in preclinical AD.
METHODS
Participants.
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). The ADNI was launched in 2003 by the National Institute on Aging, National Institute of Biomedical Imaging and Bioengineering, Food and Drug Administration, private pharmaceutical companies, and nonprofit organizations, as a $60-million, 5-year public–private partnership. The primary goal of ADNI has been to test whether imaging measures, biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.
For the current analysis, we selected CN individuals (controls) at baseline with a 1.5-T MRI of adequate quality to obtain morphometric measures (n = 159). Detailed diagnostic, inclusion, and exclusion criteria are described on the ADNI Web site (http://www.adni-info.org/).
Standard protocol approvals, registrations, and patient consents.
Each participant gave written informed consent in accordance with institutional Human Subjects Research Committee guidelines.
MRI and analysis.
MRI scans were collected on a 1.5-T scanner using a standardized magnetization-prepared rapid gradient echo protocol: sagittal plane, repetition time/echo time/inversion time, 2,400/3/1,000 msec, flip angle 8°, 24 cm field of view, 192 × 192 in-plane matrix, 1.2-mm slice thickness.12
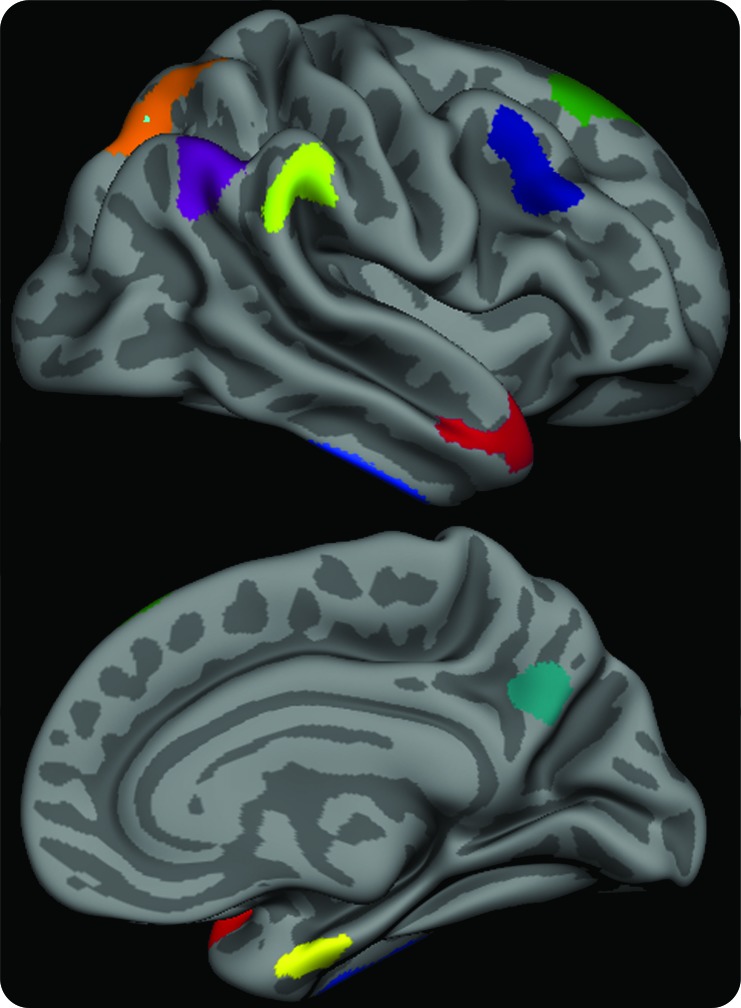
T1 image volumes were examined quantitatively by a cortical surface-based reconstruction and analysis of cortical thickness, using a hypothesis-driven approach as described in multiple previous publications.9,10,13,14 Briefly, we utilized 9 regions of interest (ROIs; see figure 1) previously determined to be associated with AD, the “cortical signature” of AD.9,10
Figure 1. The cortical signature of Alzheimer disease (AD).
A priori regions of interest composing the “AD signature” in which consistent thinning has been previously observed in multiple samples of patients with mild AD dementia. The MRI biomarker used in the present study is an average of the thickness of the cerebral cortex in all 9 of these regions of interest, obtained from each individual subject.
For the purposes of this study, our primary diagnostic biomarker was a single summary “AD-signature measure,” the average thickness of all 9 ROIs. We calculated individual Z scores as follows where x is AD-signature thickness for each individual, μsample is the sample mean, and σsample is the sample SD:
Using this measure, each individual was classified as “ADsig-low” (≥1 SD below the mean), “ADsig-average” (within 1 SD of the mean), or “ADsig-high” (≥1 SD above the mean). Individuals in the former group, ADsig-low, were considered to have evidence of early neurodegeneration consistent with AD, and were thus classified as high risk for preclinical AD. Since the new diagnostic criteria7 require the presence of a marker of amyloid-β to define stage 1 preclinical AD and a structural or functional neuroimaging marker suggestive of AD in the setting of evidence of amyloid-β to define stage 2 preclinical AD, these individuals would not fulfill preclinical AD criteria since we do not know their amyloid- β status.
In addition, for comparison purposes, we analyzed entorhinal cortical thickness using the measure provided by the automated parcellation from FreeSurfer; this is meant to provide a relatively widely used MRI biomarker thought to be sensitive to early AD pathology for comparison with the AD-signature biomarker.
Baseline neuropsychological, genetic, and CSF measures.
The groups were compared with respect to performance on neuropsychological tests and frequency of the APOE ϵ4 allele (carriers). Also, the frequencies of individuals with CSF amyloid-β values consistent with those of autopsy-proven AD (<19215) were compared between the groups.
Three-year longitudinal cognitive outcomes.
ADNI participants were followed longitudinally for at least 3 years. Since CN subjects are expected to have relatively subtle if any cognitive decline over 3 years, we defined a 3-year cognitive decline outcome measure using a method similar to one we have used in previous independent studies which is sensitive to subtle early symptoms and signs of cognitive decline consistent with AD.16–18 Specifically, we operationalized cognitive decline as a significant decline in both Clinical Dementia Rating–sum of boxes (CDR-SB) and psychometric performance, classifying individuals who met these criteria at 3 years as CN-decliners and those who did not as CN-stable. Significant decline in CDR-SB, representing the development of new cognitive symptoms in daily life in comparison to the individual's previous baseline,19 was defined as an increase of ≥1.0 on CDR-SB, a criterion we have used previously.16–18 Significant decline in psychometric test performance was defined as a decline of ≥1.0 SD on at least 1 of 3 neuropsychological measures that we and others have previously shown to be sensitive to presymptomatic and prodromal AD16,20,21: 1) Total Word List Learning (Rey Auditory Verbal Learning Test [AVLT]), 2) 30-minute AVLT Delayed Free Recall, and 3) Trail Making Test Part B (time in seconds). These change measures were obtained by calculating a Z score for each individual at baseline and a separate Z score at 3-year follow-up as follows where x is test performance for an individual, μsample is the sample mean from the same timepoint, and σsample is the sample SD:
For each test for each individual, the change score was calculated as the difference between the 2 Z scores.
The primary outcome measure used in the present analysis was cognitive decline as operationalized above.
Statistical analysis.
Tests of group differences were performed using χ2 analysis (for frequencies) or analysis of variance (for continuous measures) with post hoc pairwise comparisons where relevant; α = 0.05. Since effect sizes were expected to be subtle and strong a priori hypotheses were being tested, no multiple comparisons correction procedures were performed. Cohen d effect sizes were calculated using the standard approach. In addition to these analyses comparing groups, the impact of the AD-signature MRI biomarker on clinical outcome was analyzed using a logistic regression model. The model was constructed using the dichotomous cognitive decline outcome measure as the dependent variable. Age, gender, education, and APOE genotype (ϵ4 carrier vs noncarrier) were entered into the model and, in a second block, the effect of the AD-signature MRI measure (the full continuous measure without categorization) was tested. Statistical analyses were performed using SPSS 16.0 (Chicago, IL).
RESULTS
Baseline clinical characteristics of entire sample.
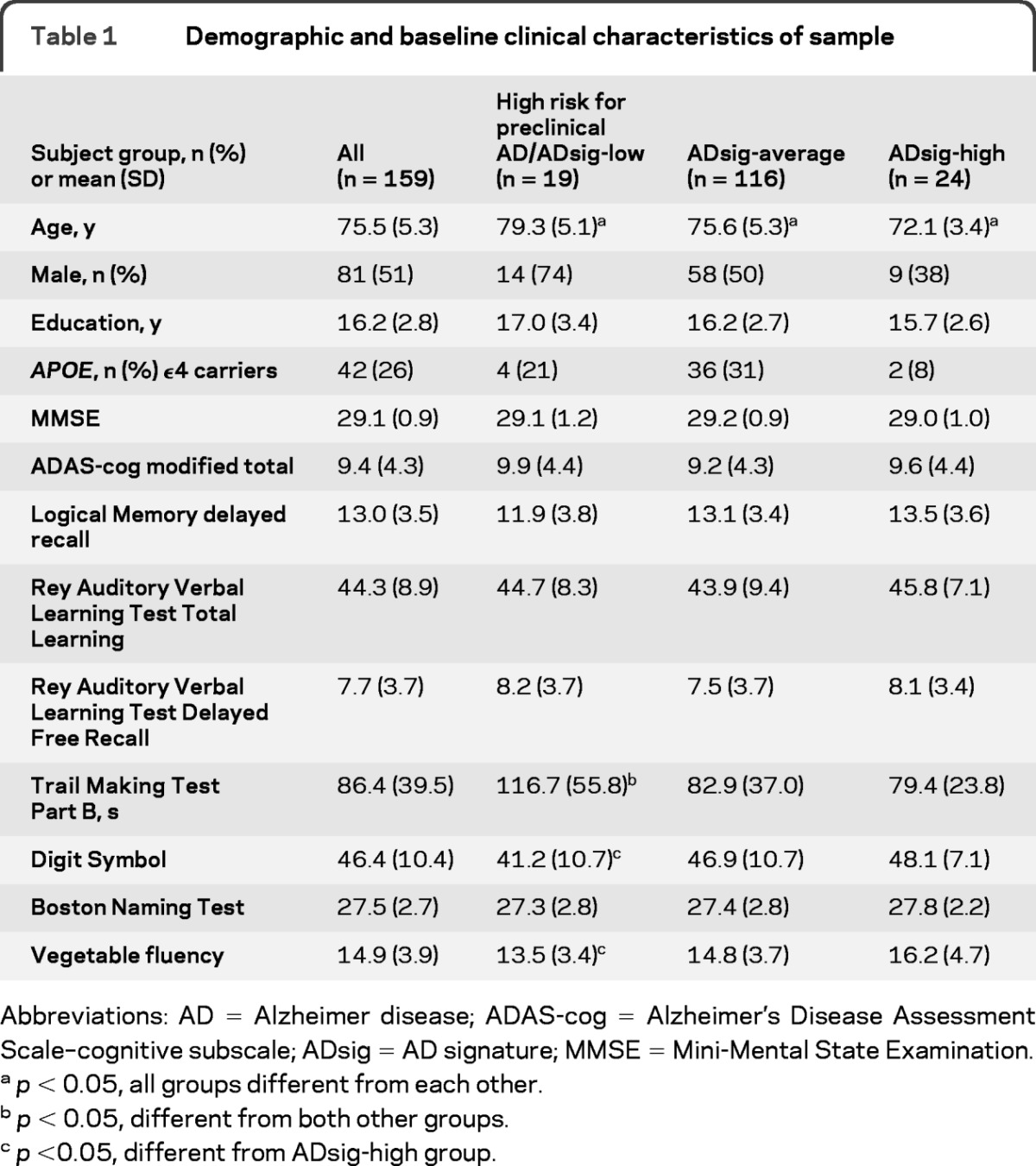
At baseline evaluation, the clinical and neuropsychological characteristics of the sample were consistent with those of a well-educated group of CN older adults (mean age = 75.5, see table 1). Mean baseline Mini-Mental State Examination was 29.1 with only 7 of the 159 individuals scoring below 28 on this global cognitive test.
Table 1.
Demographic and baseline clinical characteristics of sample
Abbreviations: AD = Alzheimer disease; ADAS-cog = Alzheimer's Disease Assessment Scale–cognitive subscale; ADsig = AD signature; MMSE = Mini-Mental State Examination.
p < 0.05, all groups different from each other.
p < 0.05, different from both other groups.
p <0.05, different from ADsig-high group.
Baseline characteristics of CN individuals classified as high risk for preclinical AD.
Of the 159 CN individuals in this sample, 19 were classified as high risk for preclinical AD using the MRI biomarker described above. Of the remaining individuals, 116 were classified as ADsig-average and 24 as ADsig-high. The groups demonstrated small differences in age, but measures of global cognition at baseline (Alzheimer's Disease Assessment Scale–cognitive subscale modified total, Mini-Mental State Examination, CDR-SB) did not differ (p > 0.1). As for the neuropsychological measures examined, the only difference was for Trails B, with the high risk for preclinical AD group performing more slowly than the other 2 groups (p < 0.005). There were similar trends (p < 0.1) for Digit Symbol and Verbal Fluency (for vegetables). Of the episodic memory performance scores that were examined, none differed (p > 0.1, see table 1).
High risk for preclinical AD individuals are more likely to develop cognitive decline.
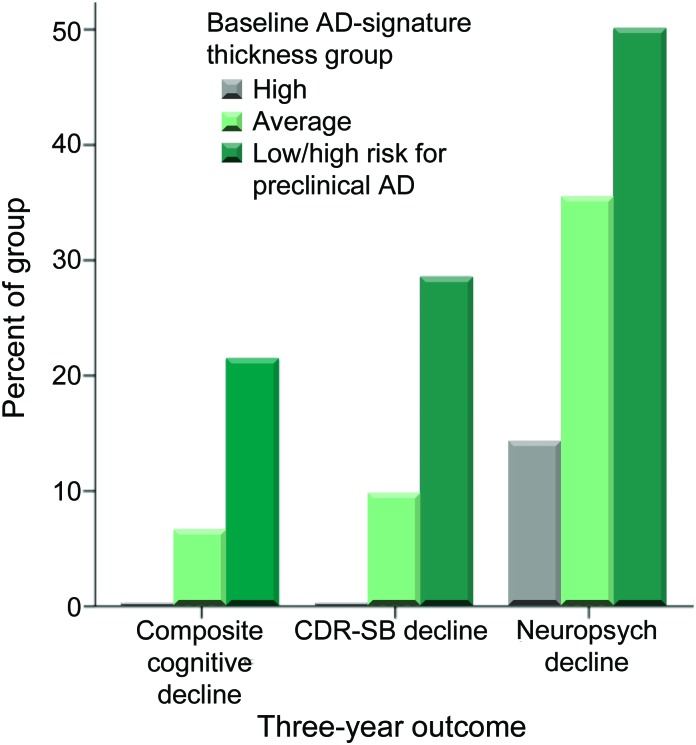
Of the entire sample of 159 CN individuals, 125 had 3-year outcome data available for both CDR and neuropsychological testing (table 2). Nine individuals met the composite cognitive decline outcome measure (CN-decliners: CDR-SB increase ≥1 and decline in performance of ≥1.0 SD on at least 1 of the 3 neuropsychological tests). Comparing the 3 baseline imaging biomarker groups, cognitive decline developed in 21.4% (3/14) of the high risk for preclinical AD group, 6.6% (6/90) of the ADsig-average group, and 0% (0/21) of the ADsig-high group (n = 125, χ2 = 6.8, p = 0.03), as shown in figure 2. The AD-signature cortical thickness of CN-decliners (mean = 2.41 mm, SD = 0.15) was significantly (0.87 SD) smaller than that of the CN-stable group (mean = 2.56 mm, SD = 0.16); Cohen d effect size was large at 0.95. For comparison purposes, entorhinal thickness showed a trend-level difference (3.32 mm [0.51] vs 3.50 mm [0.28]) with a small-to-medium Cohen d effect size of 0.43.
Table 2.
Primary outcome measures: 3-year change from baseline
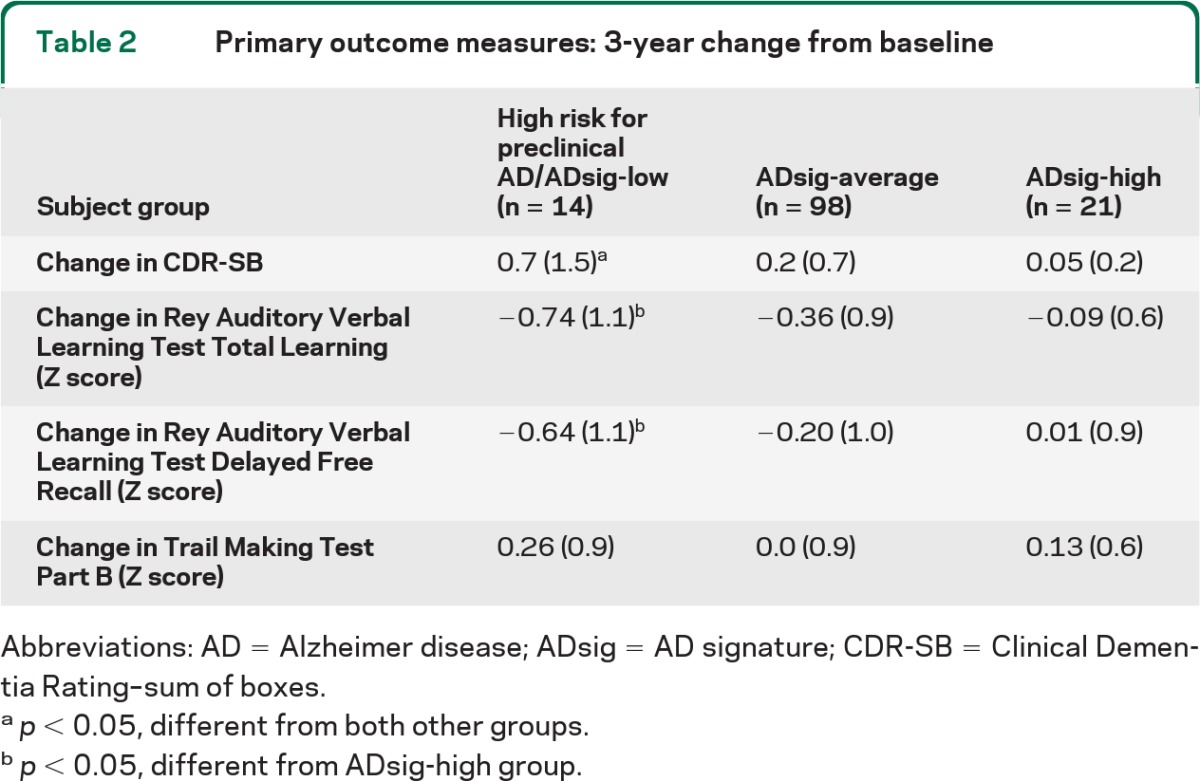
Abbreviations: AD = Alzheimer disease; ADsig = AD signature; CDR-SB = Clinical Dementia Rating–sum of boxes.
p < 0.05, different from both other groups.
p < 0.05, different from ADsig-high group.
Figure 2. Expression of cortical signature of Alzheimer disease (AD) is associated with future cognitive decline.
Participants who were cognitively normal at baseline but classified as high risk for preclinical AD on the basis of having low AD-signature cortical thickness were at markedly elevated risk of meeting the 3-year cognitive decline outcome (composite cognitive decline) as compared with participants with average or high AD-signature cortical thickness. Similar findings were present when the individual CDR–sum of boxes (CDR-SB) decline outcome or the neuropsychological performance decline outcome were examined.
In addition to the primary clinical outcome measure, we also investigated 2 secondary outcomes. Using the criterion of a CDR-SB increase ≥1, decline developed in 28.5% of the high risk for preclinical AD group, 9.7% of the ADsig-average group, and 0% of the ADsig-high group (n = 125, χ2 = 7.5, p = 0.02). Based on a decline in at least 1 of the 3 neuropsychologic tests of ≥1.0 SD, cognitive decline developed in 50%/35.4%/14.2% of the 3 groups (n = 131, χ2 = 5.3, p = 0.07).
Similarly, the results of the logistic regression model demonstrated that the value of the baseline ADsig MRI biomarker was strongly associated with the likelihood of cognitive decline. After adjustment for covariates, the likelihood of cognitive decline was nearly tripled with each 1 SD decrease of baseline ADsig cortical thickness (odds ratio = 2.95, 95% confidence interval 1.2–7.5, p = 0.02). Gender, education, and APOE genotype did not contribute to the model (p > 0.1), while age showed a trend-level effect (odds ratio = 1.09, 95% confidence interval 0.94–1.3, p = 0.06). A similar model for entorhinal cortex did not reach significance (p = 0.1).
AD-like CSF is more common in MRI-defined high risk for preclinical AD group.
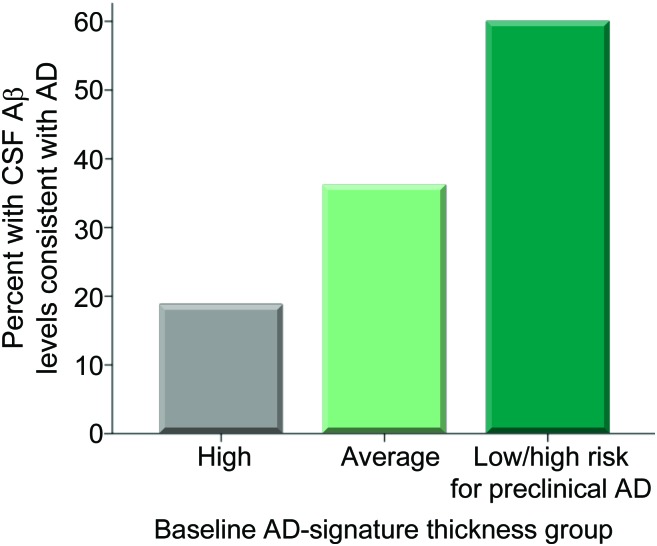
Of the 84 individuals with CSF available, the 3 groups differed in CSF amyloid-β1–42 levels (F = 5.8, p = 0.004), with the high risk for preclinical AD group exhibiting lower levels (mean = 168.2, SD = 46.0, p = 0.01) than the other 2 groups (ADsig-average group mean = 213.2, SD = 53.1; ADsig-high group mean = 236.3, SD = 36.0). When the amyloid-β1–42 measure was dichotomized in a manner consistent with the presence of AD pathology (<192),15 60% of the high risk for preclinical AD group exhibited CSF characteristics consistent with AD while 36.2% of the ADsig-average and 18.8% of the ADsig-high groups have such CSF characteristics, a trend toward an overall difference (n = 84, χ2 = 4.6, p = 0.1; linear-by-linear association χ2 = 4.5, p = 0.03), as shown in figure 3.
Figure 3. Expression of cortical signature of Alzheimer disease (AD) is associated with AD-like spinal fluid.
Cognitively normal participants who were classified as high risk for preclinical AD on the basis of having low AD-signature cortical thickness showed a trend toward being more likely to harbor abnormally low amyloid-β levels in CSF compared to participants with average or high AD-signature cortical thickness.
DISCUSSION
For the designation of preclinical AD to have utility, biomarkers used to identify this state need to be linked to a clinical outcome of cognitive decline and, ultimately, AD dementia. Studies of the full course of this process will likely take longitudinal follow-up of 5–10 years, possibly more. In the meantime, we can employ intermediate clinical markers that have been established as strong indicators of future AD dementia. This was the approach we took in the present analysis, which demonstrated that individuals classified as high risk for preclinical AD on the basis of a quantitative MRI biomarker suggestive of subtle neurodegenerative pathology were more likely than other older adults to develop clinically significant cognitive decline over the ensuing 3 years. We also showed that individuals with this MRI biomarker suggestive of preclinical AD were more likely to harbor abnormal CSF amyloid-β levels, an independent marker of AD pathobiology.
Taken together, the present data support our hypothesis that the identification of individuals expressing this quantitative ADsig MRI biomarker may provide investigators with a CN population enriched for AD pathobiology and at relatively high risk for imminent cognitive decline consistent with prodromal AD. We will now consider these findings in relation to prior literature and discuss their implications for further research and clinical practice.
A few previous studies have scanned individuals who are CN and followed them over time to investigate whether the subgroup that demonstrates cognitive decline could be, in retrospect, differentiated from those who remain stable on the basis of baseline MRI measures. Several of these studies have determined that, compared to the subgroup remaining CN over follow-up, the subgroup of CN-decliners had smaller baseline brain structures, including hippocampal formation and temporoparietal cortical regions.22–26 A few other investigations have reported MTL and whole brain atrophy in the presymptomatic stage of genetically determined early-onset familial AD, again supporting the concept that atrophy as measured on MRI, while often subtle, may be present for a number of years prior to the development of AD-related symptoms.27–29 Yet at least one similarly designed study found that ventricular volume was predictive of decline, but hippocampal, entorhinal, or whole brain volume were not.30
None of these prior longitudinal studies investigated other biomarkers of AD pathobiology, in part because many were carried out before the technologies to measure brain amyloid-β via imaging or CSF were widely available. One recent study followed CN older adults for an average of 2.4 years and determined that the subgroup exhibiting cognitive decline also demonstrated imaging evidence for greater fibrillar brain amyloid-β at baseline assessment as well as parahippocampal cortical atrophy.31 We have previously demonstrated that older CN adults with brain amyloid-β have subtle ADsig cortical thinning compared to CN older adults without brain amyloid-β, but these individuals had not yet been followed longitudinally9; other investigators have reported smaller whole brain volume in CN older adults with brain amyloid-β compared to those without.32
An important strength of the present study is that, rather than performing a retrospective analysis comparing subgroups to each other to demonstrate differences between the groups of participants, we employed the conceptual approach of the newly proposed diagnostic criteria for preclinical AD and applied it a priori to each individual in the present sample. That is, using an a priori MRI biomarker suggestive of early neurodegeneration consistent with preclinical AD (one element defining stage 2 according to the National Institute on Aging–Alzheimer's Association criteria7), we classified each individual CN subject in a manner similar to a hypothetical design of a prospective clinical research study or intervention trial, and then investigated the likelihood of subsequent cognitive decline.
To our knowledge, only one other recent study has used a similar conceptual approach, employing baseline CSF amyloid-β to identify CN individuals with low levels suggestive of preclinical AD. This group of individuals with low CSF amyloid-β had higher rates of whole brain atrophy, hippocampal atrophy, and ventricular expansion than the group of individuals with high CSF amyloid-β.33
Here we used a relatively new technique for quantitative neuroanatomic measurement (cortical thickness analysis) and employed it using a novel a priori approach focusing on brain regions known to be consistently affected early in AD from prior studies. More importantly, these measures are possible to obtain reliably from single individuals, thus making it feasible to use Z scores to compare the size of a CN individual's ADsig cortical regions to a normative sample. We previously demonstrated in 2 independent samples of CN adults followed over nearly a decade that such a measure is useful at the individual level in the assessment of risk of AD dementia.11 In each sample, a few individuals developed AD dementia over the follow-up period of 7–12 years. The magnitude of the difference in the thickness of the cerebral cortex in the ADsig regions at baseline in CN-AD converters compared to those remaining stable was remarkably similar in both samples, about a 0.2 mm difference (p < 0.05). Despite this small absolute difference, Cohen d effect sizes for these differences were very large (>1). When those 2 samples in the prior study were pooled together, 55% of the 11 CN individuals with baseline low ADsig thickness (≥1 SD below cohort mean) developed AD dementia over nearly the next decade, while none of the 9 high ADsig thickness individuals (≥1 SD above mean) developed AD dementia. This specific finding motivated us to conduct the present analysis in a strictly a priori hypothesis-driven fashion, based on the methods and cutoffs previously identified. Here the ADsig cortical thickness measure differed by 0.15 mm between the CN-decliners and CN-stables (Cohen d = 0.95), remarkably similar to our prior results. In the previous study, the ADsig imaging biomarker predicted time to diagnosis of AD dementia in a Cox hazards model (HR = 3.4, p < 0.0005); 1 SD of thinning increased dementia risk by 3.4, very similar to the nearly tripled risk of cognitive decline we found in the present analysis for those with similarly reduced thickness. The Cox regression model results from the previous study are powerful in that they augment the likelihood estimate with an estimate of the potential time to event (in this case, AD dementia). Estimates of timing may be of particular importance in the preclinical phase of disease given the potential for a significant lag between the development of biomarker change, such as CSF amyloid-β, and clinical symptoms, almost certainly having a major impact on decisions to initiate therapies. Based on the results of the prior study and those presented here, it appears that this approach may be useful in a generalizable manner. The present dataset was underpowered for analyses directly comparing MRI markers to CSF markers; thus, we were not able to formally define stage 2 preclinical AD for this analysis. Further work will be required to determine how this MRI measure of AD-related neurodegeneration could be used in combination with measures indicative of cerebral amyloidosis, an important topic for future research.
The major limitations of the present study are the relatively small numbers of subjects and the short follow-up interval. The apparently low sensitivity of the present MRI biomarker to cognitive decline is likely related in part to the short follow-up interval. These limitations are also present in prior studies that resemble the present one. The field is now primed for larger, prospectively designed studies of this sort, although many challenges remain in making such cumbersome investigations as efficient as possible. The development of more sensitive methods to detect very early indicators of the development of AD-related symptoms seems of particular relevance to increase the efficiency of such studies.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Michael Brickhouse for his assistance in data management and analysis.
GLOSSARY
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- ADsig
AD signature
- AVLT
Auditory Verbal Learning Test
- CDR-SB
Clinical Dementia Rating–sum of boxes
- CN
cognitively normal
- MCI
mild cognitive impairment
- ROI
region of interest.
Footnotes
Editorial, page 80
Supplemental data at www.neurology.org
Alzheimer's Disease Neuroimaging Initiative Coinvestigators are listed on the Neurology® Web site at www.neurology.org.
AUTHOR CONTRIBUTIONS
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu\ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators is available at http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Authorship_List.pdf. Dr. Dickerson: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis, study supervision. Dr. Wolk: drafting/revising the manuscript, study concept or design, analysis or interpretation of data.
Study Funding
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., as well as nonprofit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the NIH (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation. This analysis was also supported by grants from the NIA R01-AG29411, R21-AG29840, P50-AG005134, K23-AG028018, P30AG010124 and the Alzheimer's Association.
DISCLOSURE
Dr. Dickerson serves on the editorial board of Hippocampus; serves as a consultant for Pfizer Inc; and receives research support from the NIH and the Alzheimer's Association. Dr. Wolk serves as a consultant for GE Healthcare and receives research support from GE Healthcare, Pfizer Inc, the NIH, and the PA Department of Health.
REFERENCES
- 1. Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006; 66: 1837– 1844 [DOI] [PubMed] [Google Scholar]
- 2. Morris JC, Storandt M, McKeel DW, Jr, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer's disease. Neurology 1996; 46: 707– 719 [DOI] [PubMed] [Google Scholar]
- 3. Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging 1991; 12: 295– 312 [DOI] [PubMed] [Google Scholar]
- 4. Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol 2001; 58: 1395– 1402 [DOI] [PubMed] [Google Scholar]
- 5. Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 2009; 30: 1026– 1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crystal H, Dickson D, Fuld P, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology 1988; 38: 1682– 1687 [DOI] [PubMed] [Google Scholar]
- 7. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement 2011; 7: 280– 292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol 2010; 9: 1118– 1127 [DOI] [PubMed] [Google Scholar]
- 9. Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex 2009; 19: 497– 510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology 2009; 72: 1048– 1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dickerson BC, Stoub T, Shah RC, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 2011; 76: 1395– 1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008; 27: 685– 691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolk DA, Dickerson BC, Alzheimer's Disease Neuroimaging Initiative, et al. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci USA 2010; 107: 10256– 10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dickerson BC, Wolk DA, the Alzheimer's Disease Neuroimaging Initiative Dysexecutive versus amnesic phenotypes of very mild Alzheimer's disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry 2011; 82: 45– 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 2009; 65: 403– 413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry 2007; 64: 1443– 1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry 2008; 79: 630– 635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol 2004; 56: 27– 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412– 2414 [DOI] [PubMed] [Google Scholar]
- 20. Blacker D, Lee H, Muzikansky A, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol 2007; 64: 862– 871 [DOI] [PubMed] [Google Scholar]
- 21. Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc 2001; 7: 631– 639 [DOI] [PubMed] [Google Scholar]
- 22. Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology 1997; 48: 1297– 1304 [DOI] [PubMed] [Google Scholar]
- 23. Smith CD, Chebrolu H, Wekstein DR, et al. Brain structural alterations before mild cognitive impairment. Neurology 2007; 68: 1268– 1273 [DOI] [PubMed] [Google Scholar]
- 24. Martin SB, Smith CD, Collins HR, Schmitt FA, Gold BT. Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol Aging; 31: 1099– 1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiol Aging 2010; 31: 1077– 1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry 2006; 63: 57– 62 [DOI] [PubMed] [Google Scholar]
- 27. Schott JM, Fox NC, Frost C, et al. Assessing the onset of structural change in familial Alzheimer's disease. Ann Neurol 2003; 53: 181– 188 [DOI] [PubMed] [Google Scholar]
- 28. Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease: a longitudinal MRI study. Brain 1996; 119: 2001– 2007 [DOI] [PubMed] [Google Scholar]
- 29. Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet 2001; 358: 201– 205 [DOI] [PubMed] [Google Scholar]
- 30. Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 2005; 65: 1227– 1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 2009; 66: 1469– 1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol 2009; 65: 176– 183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schott JM, Bartlett JW, Fox NC, Barnes J, for the Alzheimer's Disease Neuroimaging Initiative Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Abeta1–42. Ann Neurol 2010; 68: 825– 834 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.