Abstract
Objective:
Lacunar infarctions are mainly due to 2 microvascular pathologies: lipohyalinosis and microatheroma. Little is known about risk factor differences for these subtypes. We hypothesized that diabetes and glycated hemoglobin (HbA1c) would be related preferentially to the lipohyalinotic subtype.
Methods:
We performed a cross-section analysis of the brain MRI data from 1,827 participants in the Atherosclerosis Risk in Communities study. We divided subcortical lesions ≤20 mm in diameter into those ≤7 mm (of probable lipohyalinotic etiology) and 8–20 mm (probably due to microatheroma) and used Poisson regression to investigate associations with the number of each type of lesion. Unlike previous studies, we also fitted a model involving lesions <3 mm.
Results:
Age (prevalence ratio [PR] 1.11 per year; 95% confidence interval [CI] 1.08–1.14), black ethnicity (vs white, PR 1.66; 95% CI 1.27–2.16), hypertension (PR 2.12; 95% CI 1.61–2.79), diabetes (PR 1.42; 95% CI 1.08–1.87), and ever-smoking (PR 1.34; 95% CI 1.04–1.74) were significantly associated with lesions ≤7 mm. Findings were similar for lesions <3 mm. HbA1c, substituted for diabetes, was also associated with smaller lesions. Significantly associated with 8–20 mm lesions were age (PR 1.14; 95% CI 1.09–1.20), hypertension (PR 1.79; 95% CI 1.14–2.83), ever-smoking (PR 2.66; 95% CI 1.63–4.34), and low-density lipoprotein (LDL) cholesterol (PR 1.27 per SD; 95% CI 1.06–1.52). When we analyzed only participants with lesions, history of smoking (PR 1.99; 95% CI 1.23–3.20) and LDL (PR 1.33 per SD; 95% CI 1.08–1.65) were associated with lesions 8–20 mm.
Conclusions:
Smaller lacunes (even those <3 mm) were associated with diabetes and HbA1c, and larger lacunes associated with LDL cholesterol, differences which support long-held theories relating to their underlying pathology. The findings may contribute to broader understanding of cerebral microvascular disease.
Lacunar infarcts are small CSF-filled cavities caused by the occlusion of small arteries in the brain. When symptomatic, they may be evident as lacunar strokes.1
The pathogenesis of lacunes has not been fully clarified, and there is pathologic and clinical evidence of distinct types of lacunar entities.2–4 Fisher described 2 main vascular culprit lesions: 1) lipohyalinosis, usually with 1 or more small lacunar infarcts (up to 7 mm) and rare symptoms; and 2) microatheromata, usually linked to larger (5–20 mm), more often symptomatic lacunes.4,5 This pathologic distinction remains unchallenged. Although contemporary authors describe several types of small-vessel disease associated with lacunes,5–7 we will, for convenience, consistently use only the term “lipohyalinosis” in this article to refer to the type of arteriolar lesion underlying lesions ≤7 mm, without intending to support any of the term's more specific pathogenic implications.
The differentiation between these 2 entities could be of clinical importance, as they might have distinct etiologies, consequences, and management. However, despite the long time since their initial description, knowledge of risk factor patterns for these 2 types of lacunes is still limited.
We hypothesized that lacunes classified by their presumed underlying pathology (lipohyalinosis vs microatheroma) would be associated with different risk factors. We examined the question using data from the Atherosclerosis Risk in Communities (ARIC) study. Since treatment of hyperglycemia appears to more successfully reduce microvascular disease than disease due to large-artery atherosclerosis,8 we expected that diabetes and impaired glucose metabolism measured by glycated hemoglobin (HbA1c) would be more strongly associated with the lipohyalinotic subtype of lacunar infarction than with lacunes caused by microatheroma. Other risk factors were evaluated without specific a priori hypotheses.
METHODS
Study population.
ARIC is a community-based prospective cohort study of 15,792 middle-aged adults from 4 US communities who were aged 45–64 years at baseline. The first examination of participants (visit 1) was performed during the 1987–1989 period, with 3 follow-up visits taking place every 3 years.9 During 1993–1995 (ARIC visit 3) a sample of cohort members who were ≥55 years old at Forsyth County, NC, and Jackson, MS, were screened for eligibility for a cerebral MRI examination. Only black subjects were recruited from Jackson whereas Forsyth County included both black and white subjects. Approximately 2% of participants were excluded on the basis of criteria regarding participant safety: 1) having had surgery for brain aneurysm; 2) having metal fragments in the eyes, brain, or spinal cord; 3) having a valvular prosthesis, a cardiac pacemaker, cochlear implant, spinal cord stimulator, or other internal electrical device; 4) being pregnant; or 5) having occupational exposure to metal fragments. Of those meeting inclusion criteria, 27.6% declined to undergo the cerebral MRI examinations.10,11 Compared with participants who did not undergo MRI, study participants were more likely to be black, older, thinner, and more educated, and to have higher levels of high-density lipoprotein cholesterol. They were also less likely to have diabetes, to have congestive heart disease, or to smoke cigarettes.10 This study includes the subjects who completed the MRI examination.
Standard protocol approvals, registrations, and patient consents.
Institutional review boards at each clinical site approved the study protocol, and written informed consent was obtained from all participants.
MRI variables.
Five-millimeter contiguous axial T1, T2, and proton density–weighted images were acquired through the whole brain on 1.5-T scanners. Infarct-like lesions (ILL) were defined as focal lesions hyperintense to gray matter on both proton density and T2-weighted images. To be considered an ILL in cerebral white matter, lesions were required to be hypointense on T1-weighted images, similar to CSF.10 The dimensions of ILLs ≥3 mm were recorded using an electronic cursor, recording maximal right-to-left and anterior-to-posterior dimension of each lesion. The superior-to-inferior dimension was calculated from the number of 5-mm axial sections on which the lesion appeared. Lesions with right-to-left or anterior-to-posterior measurements <3 mm were recorded simply as “less than 3 mm.” Each scan was scored independently by 2 neuroradiologists and if there was disagreement over the presence or absence of ILLs <3 mm or ≥3 mm, the scan was subject to an adjudication review and a final scoring was performed by consensus of at least 3 readers. Lesions <3 mm were counted as “0,” “1–2,” or “>2,” but for the purposes of the analyses in this article they were considered as “0,” “1,” and “2,” respectively. The interreader and intrareader κ statistics for presence of lesions <3 mm in maximal diameter were 0.25 and 0.54, respectively. The kappa values for the presence of ILLs ≥3 mm were 0.52 and 0.78 for interreader and intrareader agreement, respectively.10
Lesions were assigned to 1 or more of 23 anatomic regions defined by anatomic and vascular characteristics. Since we are limiting our study to lacunar disease, we considered only noncortical lesions of less than or equal to 20 mm in maximum dimension located in the caudate, lenticular nucleus, internal capsule, thalamus, brainstem, deep cerebellar white matter, centrum semiovale, or corona radiata.
Covariates.
ARIC examinations included blood sampling12–14 and standardized interview.15 Cigarette smoking was ascertained from interview. Blood pressure was measured using a random-zero sphygmomanometer, and the mean of the last 2 measurements was calculated. Hypertension was defined as systolic blood pressure of 140 mm Hg or greater, diastolic blood pressure of 90 mm Hg or greater, or use of antihypertensive medication during the previous 2 weeks. Diabetes mellitus was defined as a fasting glucose level of at least 126 mg/dL (7.0 mmol/L), a nonfasting glucose level of at least 200 mg/dL (11.1 mmol/L), or a self-reported history of physician-diagnosed diabetes or treatment for diabetes. Assays of total cholesterol, high-density lipoprotein cholesterol, calculation of low-density lipoprotein (LDL) cholesterol, and glucose levels are described elsewhere.15 Frozen whole-blood samples obtained at the second ARIC visit were thawed and assayed for HbA1c with Tosoh HPLC instruments (Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer in 2003–2004 and the Tosoh G7 in 2007–2008, Tosoh Corp.; both instruments were DCCT-aligned).16 Left ventricular hypertrophy was evaluated on EKG as previously described.15 The LDL and HbA1c values used in this study were from visit 2, and the other variables were from visit 3. Of the 1,930 participants who underwent MRI, 1,827 were not missing variables of interest and made up our final sample.
Statistical analysis.
Since lipohyalinotic lesions are assumed to occur up to 7 mm5, we divided the ILLs into 2 strata by maximum diameter: ≤7 mm (more likely due to lipohyalinosis) and 8–20 mm (probably due to microatheromata). The baseline characteristics of 4 participant groups were described: those without ILLs, with ILLs between 8 and 20 mm only, with ILLs ≤7 mm only, and with both types of lesions.
Our primary analysis involved Poisson regression models using the number of ILLs ≤7 mm or 8–20 mm as dependent variables, in order to capture dose-response effects. Therefore, when ILLs ≤7 mm were used as a response variable, lesions in the 8–20 mm range were counted as “0” and vice versa.
The models included adjustment for demographic and known cerebrovascular risk factors (age: per year; gender: male vs female; race: blacks vs whites; hypertension; ever-smoking; and LDL: per SD mg/dL). For each lesion size, we fitted alternate models using either diabetes or HbA1c (per SD %) as a covariate. Previous studies tended to exclude lesions <3 mm from outcome measures, supposing that they were more likely to represent enlarged perivascular spaces, and thus not related to risk factors.17,18 Therefore, we decided to examine this by also fitting a model including only this lesion size as a dependent variable. In order to clarify risk factor differences by lesion type, we fitted models excluding people without ILL with alternately small, then large lacunes as endpoints. Three more sensitivity analyses were performed: the same Poisson models were fitted after excluding people with previous stroke; after excluding people with diabetes; and all models were examined using binomial logistic regressions using each lesion size category (present vs absent) as dependent variables.
All analyses were performed using the software package R, version 2.10.1.19 Poisson models were evaluated for overdispersion with the Poisson goodness-of-fit test available in the epicalc library.20 p Values < 0.05 were considered significant. All tests were 2-sided.
Role of the funding source.
The National Heart, Lung, and Blood Institute participates in the ARIC steering committee but had no role in data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Approximately 13% of the 1,827 subjects had noncortical ILLs in the lacunar size range: 1,586 participants without ILLs, 37 with ILLs 8–20 mm only, 169 with ILLs ≤7 mm only, and 35 with both lesion sizes. Characteristics of the study population are shown in table 1. As compared to participants with ILLs 8–20 mm only, those with ILLs <7 mm only had a higher proportion of black race, diabetes, and hypertension, and smaller proportion of ever-smoking and lower mean LDL level. The characteristics of subjects with both lesion types seemed mixed, with higher LDL levels and higher frequency of ever-smoking than the other groups, for example.
Table 1.
Baseline characteristics of participants stratified by lesion size: Atherosclerosis Risk in Communities men and women
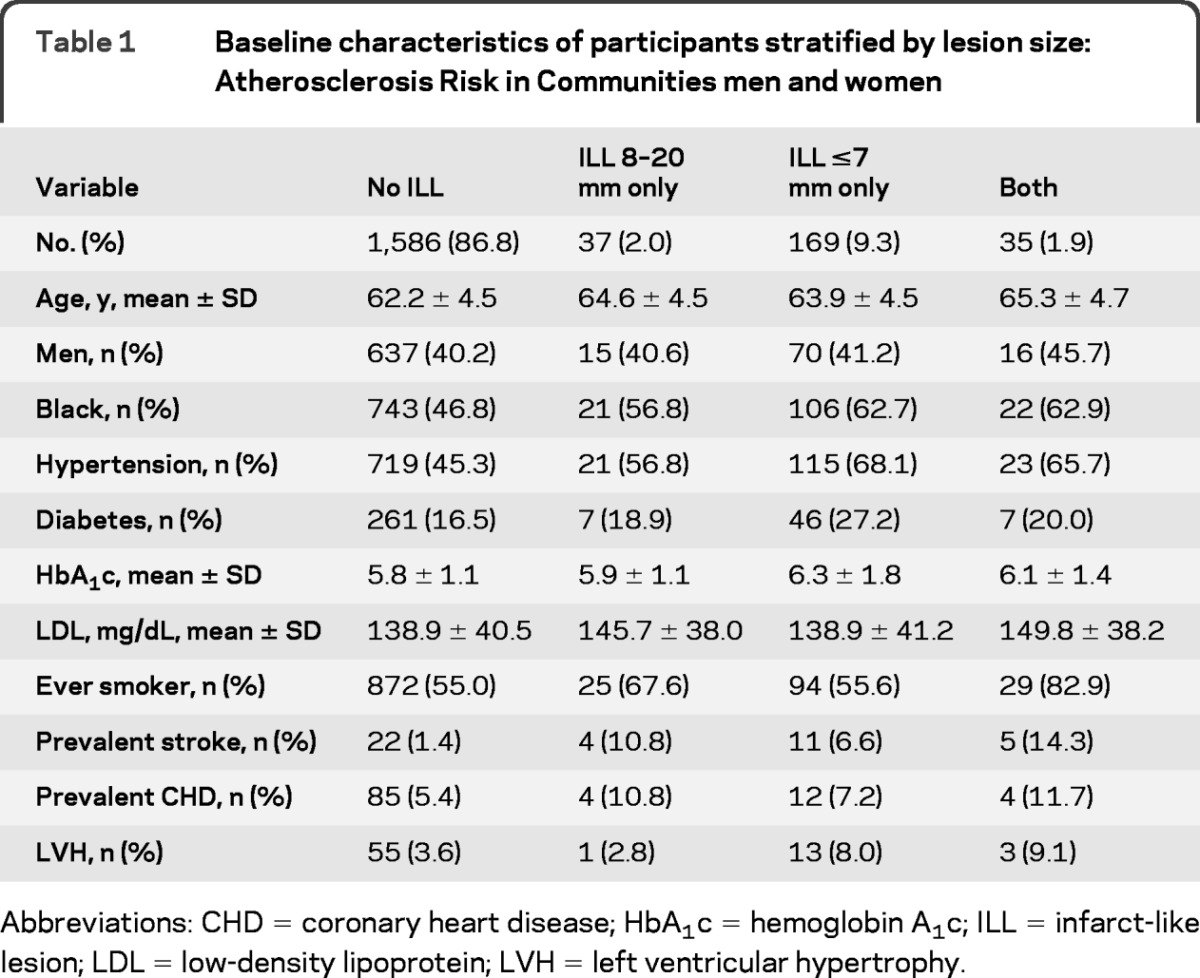
Abbreviations: CHD = coronary heart disease; HbA1c = hemoglobin A1c; ILL = infarct-like lesion; LDL = low-density lipoprotein; LVH = left ventricular hypertrophy.
The number of lesions per participant followed roughly a Poisson distribution. Seventy-six percent of the 8–20 mm ILLs and 73% of the ILLs ≤7 mm occurred singly (table e-1 on the Neurology® Web site at www.neurology.org). Poisson models seemed appropriate, with no evidence of overdispersion in the models.
In the Poisson model (PRs pertain to each additional lesion), age (PR 1.11 per year; 95% CI 1.08–1.14), black ethnicity (PR 1.66; 95% CI 1.27–2.16), hypertension (PR 2.12; 95% CI 1.61–2.79), diabetes (PR 1.42; 95% CI 1.08–1.87), and ever-smoking (PR 1.34; 95% CI 1.04–1.74) were significantly associated with the number of ILLs ≤7 mm (table 2, model 1). The association with LDL was not significant (PR 1.03 per SD, 95% CI 0.92–1.16). Similar results were found for HbA1c, when it was used as a covariate instead of diabetes (table 2, model 2). However, the HbA1c association was no longer significant when it was analyzed only in nondiabetic subjects (not shown).
Table 2.
Poisson regression model with either the number of ILLs ≤7 mm or 8–20 mm as dependent variable
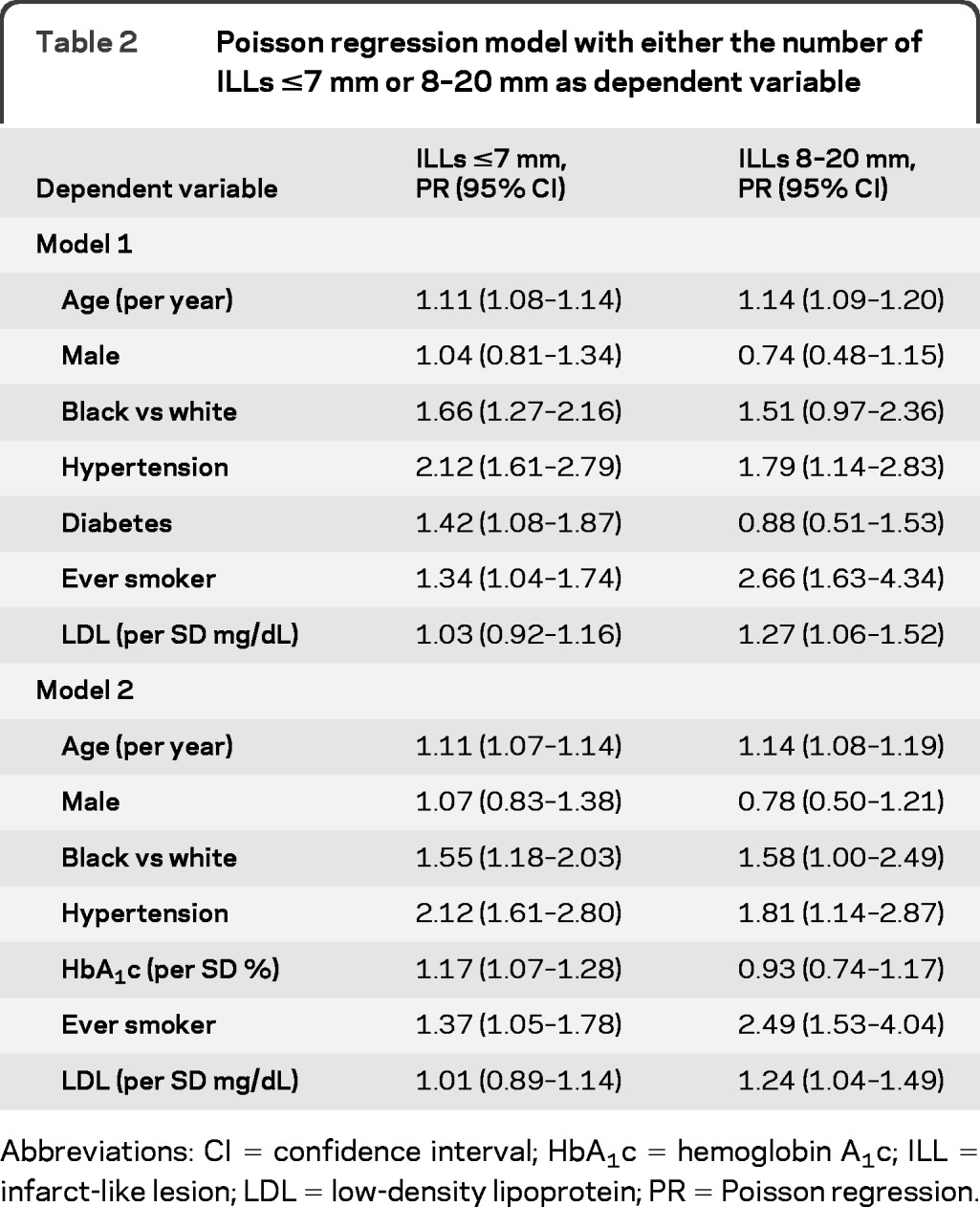
Abbreviations: CI = confidence interval; HbA1c = hemoglobin A1c; ILL = infarct-like lesion; LDL = low-density lipoprotein; PR = Poisson regression.
Significantly associated with the number of ILLs 8–20 mm were age (PR 1.14 per year; 95% CI 1.09–1.20), hypertension (PR 1.79; 95% CI 1.14–2.83), ever-smoking (PR 2.66; 95% CI 1.63–4.34), and LDL (per SD mg/dL; PR 1.27 per SD; 95% CI 1.06–1.52), but not diabetes (table 2, model 1). HbA1c, like diabetes, also showed no association with the larger ILLs (table 2, model 2). We observed similar associations in the logistic regression models using either the presence of ILLs ≤7 mm or 8–20 mm ILLs as response variables (not shown), but with less precision, as expected.
Table 3 shows the Poisson model with lesions <3 mm as the dependent variable. Risk factor associations were similar to those for ILLs ≤7 mm. Although ever-smoking was no longer significant, its magnitude of association was similar to what was found for the ≤7-mm ILL endpoint. We also fitted a model involving only lesions 3–7 mm as a sensitivity analysis, and the associations were also similar (table 3). Although diabetes was not significantly associated with the number of lesions 3–7 mm, HbA1c levels remained significant.
Table 3.
Poisson regression model with either the number of ILLs <3 mm or 3–7 mm as a dependent variable
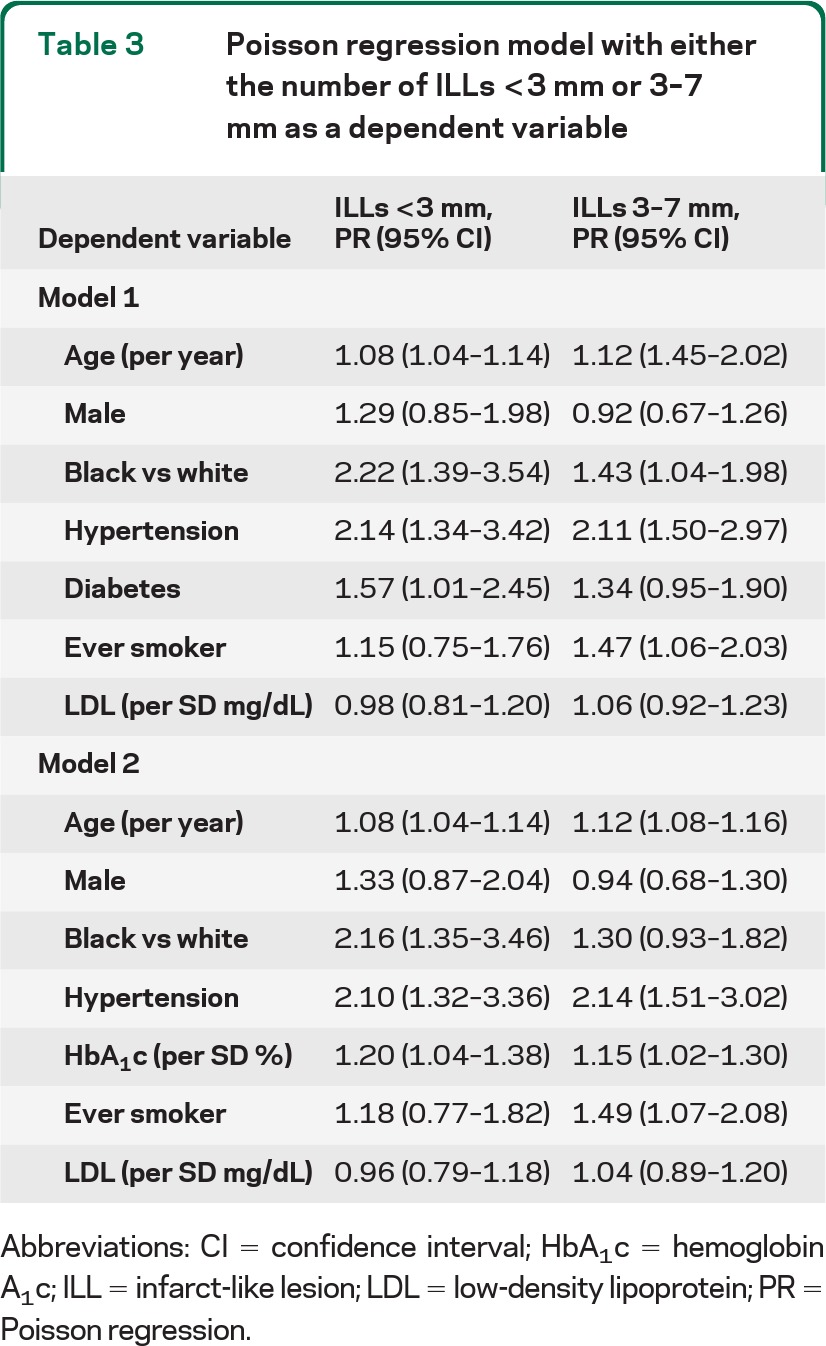
Abbreviations: CI = confidence interval; HbA1c = hemoglobin A1c; ILL = infarct-like lesion; LDL = low-density lipoprotein; PR = Poisson regression.
In the model excluding people without ILLs and using the number of lesions ≤7 mm as a dependent variable, no variable was significant (not shown). Conversely, in the model using ILLs 8–20 mm as a dependent variable, ever-smoking (PR 1.99; 95% CI 1.23–3.20) and LDL levels (per SD mg/dL; PR 1.33; 95% CI 1.08–1.65) were significant predictors (not shown).
Finally, after excluding people with previous symptomatic stroke, the magnitude of the effects was similar for both lesion sizes but the associations with hypertension and LDL were no longer significant for larger lesions (table e-2).
DISCUSSION
In this cohort, smaller and larger ILLs were associated with different risk factor profiles, supporting the view that lacunes arise from 2 distinct vascular pathologies. As we hypothesized, diabetes and HbA1c, consistently shown to be risk factors for small-vessel disease in the literature, were significantly associated with smaller ILLs but not with larger ones. Other risk factors associated with smaller lesions were age, nonwhite ethnicity, hypertension, and ever-smoking, but not LDL (table 2). Conversely, LDL levels, age, hypertension, and smoking were positively associated with larger lacunar lesions thought to be caused by microatheromata (table 2). When we limited our analysis to participants with lacunes, LDL levels and history of smoking were linked to ILLs 8–20 mm.
These results shed light on the controversy regarding the differences in risk factor profile between lacunar and nonlacunar strokes. Although treatment of hyperglycemia appears to more successfully reduce microvascular disease than disease due to large-artery atherosclerosis,8 in a recent systematic review diabetes was equally associated with lacunar and nonlacunar ischemic stroke.21 However, in all studies included in this review, lacunar infarction was defined clinically, requiring the presence of lacunar symptoms. Therefore, the lacunes studied tend to represent Fisher's microatheromatous lacunar subtype, which we believe is more similar than smaller lacunes to large-artery cerebral infarctions.
As the lipohyalinotic subtype is more likely to be associated with multiple asymptomatic lacunes, rather than occurring as a single symptomatic lesion, some studies involving patients admitted for a clinical lacunar syndrome used multiplicity as a criterion for differentiating lacunar subtypes.2,3,22,23 An association of diabetes with the lipohyalinotic subtype defined in this way was also described by some,3 but not by others.2,3,22,23 However, as discussed above, since clinical recognition was an inclusion criterion for lacunar infarctions in these studies, most of the patients in both groups would tend to also have the microatheromatous subtype of lacunes, thus potentially weakening the association of risk factors such as diabetes with smaller lesions. Furthermore, a criterion based on multiple lesions would likely miss many lipohyalinotic lesions, thus reducing statistical power for detecting associations with diabetes. For instance, in our study, only 60 of the 215 lipohyalinotic subtype (ILLs ≤7 mm) occurred in participants with multiple lesions.
Studies involving asymptomatic populations should be more comparable to ours than those cited above. However, even in this setting, markers of impaired glucose metabolism were not consistently described in association with lacunes.24 Possible explanations of these negative findings would be the absence of differentiation between the size of lesions as indicators of the lacunar subtype; the inclusion of lesions of potential “nonlacunar” etiology,17 such as those extending to the cerebral cortex; and the exclusion of the smallest lesions, those <3 mm in diameter.17,18 Our data show that lesions <3 mm were significantly associated with diabetes and HbA1c, justifying their inclusion as a marker of lipohyalinotic disease (table 3). Although in our study there was poor agreement concerning the presence of these small lesions,10 our reported associations are likely to be valid, since the misclassification of these small lesions is nondifferential with respect to diabetes status.25 For example, even if our scans failed to detect 50% of the <3 mm diameter lesions, this would not bias the associations with diabetes or other risk factors unless our failure to detect those lesions depended in some way on the level of the risk factors, and we have no reason to believe that such would be the case. Our MRI definition of ILLs requiring hyperintensity on both spin-density and T2-weighed images tended to select ischemic lesions rather than perivascular spaces, though this criterion could be less reliable for smaller lesions.10,26 Most previous studies excluded lesions <3 mm with the concern that they could represent enlarged perivascular spaces, assuming that they may not be related to cardiovascular risk factors. However, emerging evidence indicates that enlarged Virchow-Robin spaces may also result from small-vessel disease.27,28 Lipohyalinosis is associated with increased blood–brain barrier permeability, leading to leakage of proteins, not only to the arterial wall (causing fibrinoid necrosis) but also to the perivascular brain parenchyma. This could result in protein accumulation, congestion of the extracellular fluid, and enlargement of Virchow-Robin spaces.7
Our findings may have important clinical implications. Each type of lacunar disease may imply a different approach to patient management. Lipohyalinosis is associated with fibrinoid necrosis,5–7 an acute lesion that could lead to vessel rupture and blood extravasation.5–7 Therefore, it seems reasonable to expect that this lacunar subtype would be associated with cerebral microbleeds, and an increased risk of intracerebral hemorrhage, either primarily or with the use of antithrombotic drugs.29 Leukoaraiosis, which is also believed to be more common in the lipohyalinotic subtype of lacunar infarction, has already been associated with an increased risk of intracranial bleeding following the use of anticoagulants30 and thrombolytics.31 Future studies might address the prognostic implications of our findings.3,22,23
Due to the cross-sectional nature of this investigation, we cannot determine the temporality of the observed associations. Another limitation of our study is the availability of only a single HbA1c measurement performed approximately 3 years before the MRI scanning, which might underestimate the exposure to increased glucose levels over time. Furthermore, only a few participants had larger lesions, limiting our statistical power for that group. Finally, despite the adjustment for known cardiovascular risk factors, we cannot rule out the possibility of residual confounding.
Strengths of this study are the large community-based population, rigorous measurement of cardiovascular risk factors, standardized and reliable methods to evaluate the outcomes, and the large number of African American participants.15
In summary, this study strongly supports the hypothesis of 2 types of lacunar infarctions, with noncortical lesions divided according to size class. ILLs ≤7 mm (of presumptive lipohyalinotic origin) were associated with diabetes and elevated HbA1c level, age, nonwhite ethnicity, hypertension, and history of smoking, and ILLs 8–20 mm (likely of microatheromatous etiology) were linked to LDL as well as age, hypertension, and smoking. Furthermore, we found that the exclusions of lesions <3 mm as previously done in imaging studies may lead to underestimation of the lacunar burden. These findings might have future impact on the classification of lacunar disease, prognosis, and clinical management of these patients.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff and participants of the ARIC study for their contributions. Dr. Daniel C. Bezerra thanks the European Neurological Society for supporting part of his fellowship in Cerebrovascular Diseases at the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, during 2005.
GLOSSARY
- ARIC
Atherosclerosis Risk in Communities
- CI
confidence interval
- HbA1c
glycated hemoglobin
- ILL
infarct-like lesion
- LDL
low-density lipoprotein
- PR
prevalence ratio
Footnotes
Editorial, page 82
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Bezerra designed the study, performed the statistical analysis, and wrote the manuscript. Dr. Sharrett helped design the study, provided input into the statistical analyses, and revised the manuscript. Dr. Matsushita helped design the study, provided input into the statistical analyses, and revised the manuscript. Dr. Gottesman helped design the study and revised the manuscript. Dr. Shibata offered suggestions and comments on the analysis and on draft versions of the manuscript. Dr. Mosley Jr. offered suggestions and comments on the analysis and on draft versions of the manuscript. Dr. Coresh provided input into the statistical analyses and revised the manuscript. Dr. Carvalho provided input into the statistical analyses and revised the manuscript. Dr. Szklo helped design the study, provided input into the statistical analyses, and revised the manuscript. Dr. Selvin helped design the study, provided input into the statistical analyses, and revised the manuscript. All authors reviewed, edited, and approved the final version of the manuscript.
DISCLOSURE
Dr. Bezerra reports no disclosures. Dr. Sharrett has received research support from the NIH (NHLBI, NINDS). Dr. Matsushita receives research support from the NIH (NHLBI, NIDDK) and the US National Kidney Foundation. Dr. Gottesman has received research support from the American Heart Association. Dr. Shibata and Dr. Mosley, Jr. report no disclosures. Dr. Coresh has received speaker honoraria from Amgen; serves as an Associate Editor for the American Journal of Epidemiology; is listed as author on a patent re: ABCG2 as a mechanism for gout treatment; and receives research support from the NIH (NHLBI, NIDDK, NCRR) and the CDC. Dr. Szklo serves as Editor-in-Chief of the American Journal of Epidemiology; receives publishing royalties for Epidemiology: Beyond the Basics, Second Edition (Jones and Bartlett Publishers, 2006, 2007); and has received research support from the NIH. Dr. Carvalho serves as an Associate Editor for PLOs Neglected Diseases; and has received research support from the Brazilian Health Ministry, Brazilian Research Council, and Rio de Janeiro State Research agency. Dr. Selvin serves on a scientific advisory board for Novartis; serves on the editorial board of Diabetes Care; serves as a consultant for Doctor Evidence, LLC; and has received research support from Asahi Kasei, GlycoMark, Roche, and the NIH (NIDDK).
REFERENCES
- 1. Wardlaw JM. What is a lacune? Stroke 2008;39:2921–2922 [DOI] [PubMed] [Google Scholar]
- 2. Boiten J, Lodder J, Kessels F. Two clinically distinct lacunar infarct entities? A hypothesis. Stroke 1993;24:652–656 [DOI] [PubMed] [Google Scholar]
- 3. Arauz A, Murillo L, Cantú C, Barinagarrementeria F, Higuera J. Prospective study of single and multiple lacunar infarcts using magnetic resonance imaging: risk factors, recurrence, and outcome in 175 consecutive cases. Stroke 2003;34:2453–2458 [DOI] [PubMed] [Google Scholar]
- 4. Fisher CM. Lacunar strokes and infarcts: a review. Neurology 1982;32:871–876 [DOI] [PubMed] [Google Scholar]
- 5. Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol 2002;12:358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenblum WI. Fibrinoid necrosis of small brain arteries and arterioles and miliary aneurysms as causes of hypertensive hemorrhage: a critical reappraisal. Acta Neuropathol 2008;116:361–369 [DOI] [PubMed] [Google Scholar]
- 7. Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol 2010;119:277–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 9. The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 10. Bryan RN, Cai J, Burke G, et al. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: the atherosclerosis risk in communities study. AJNR Am J Neuroradiol 1999;20:1273–1280 [PMC free article] [PubMed] [Google Scholar]
- 11. Liao D, Cooper L, Cai J, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control: The ARIC Study: Atherosclerosis Risk in Communities Study. Stroke 1996;27:2262–2270 [DOI] [PubMed] [Google Scholar]
- 12. Saito I, Folsom AR, Brancati FL, et al. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Intern Med 2000;133:81–91 [DOI] [PubMed] [Google Scholar]
- 13. Folsom AR, Wu KK, Rosamond WD, Sharrett AR, Chambless LE. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 1997;96:1102–1108 [DOI] [PubMed] [Google Scholar]
- 14. Folsom AR, Desvarieux M, Nieto FJ, et al. B vitamin status and inflammatory markers. Atherosclerosis 2003;169:169–174 [DOI] [PubMed] [Google Scholar]
- 15. National Heart, Lung and Blood Institute The ARIC Manuals of Operation. University of North Carolina School of Public Health ARIC Coordinating Center , ed. Chapel Hill: University of North Carolina; 1987 [Google Scholar]
- 16. Selvin E, Coresh J, Zhu H, Folsom A, Steffes MW. Measurement of HbA1c from stored whole blood samples in the Atherosclerosis Risk in Communities study. J Diabetes 2010;2:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Longstreth WT, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2002;33:2376–2382 [DOI] [PubMed] [Google Scholar]
- 18. Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MMB. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 2002;33:21–25 [DOI] [PubMed] [Google Scholar]
- 19. R Development Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. Available at: http://www.R-project.org [Google Scholar]
- 20. Chongsuvivatwong V. epicalc: Epidemiological Calculator. R package version 2.10.1.1; 2010. Available at: http://CRAN.R-project.org/package=epicalc Accessed March 18, 2011 [Google Scholar]
- 21. Jackson CA, Hutchison A, Dennis MS, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke 2010;41:624–629 [DOI] [PubMed] [Google Scholar]
- 22. de Jong G, Kessels F, Lodder J. Two types of lacunar infarcts: further arguments from a study on prognosis. Stroke 2002;33:2072–2076 [DOI] [PubMed] [Google Scholar]
- 23. Staals J, van Raak L, Hilton A, Lodder J. Differences in long-term survival in two lacunar stroke types: a 15-year follow-up study in 782 cerebral infarct patients. Cerebrovasc Dis 2008;25:26–31 [DOI] [PubMed] [Google Scholar]
- 24. Vermeer SE, Longstreth WT, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–619 [DOI] [PubMed] [Google Scholar]
- 25. Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 2nd ed. Sudbury, MA: Jones and Bartlett Publishers; 2006 [Google Scholar]
- 26. Pullicino PM, Miller LL, Alexandrov AV, Ostrow PT. Infraputaminal ‘lacunes': clinical and pathological correlations. Stroke 1995;26:1598–1602 [DOI] [PubMed] [Google Scholar]
- 27. Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010;41:450–454 [DOI] [PubMed] [Google Scholar]
- 28. Rouhl RPW, van Oostenbrugge RJ, Knottnerus ILH, Staals JEA, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol 2008;255:692–696 [DOI] [PubMed] [Google Scholar]
- 29. Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group A randomized trial of anticoagulants versus aspirin after cerebral ischemia of presumed arterial origin. Ann Neurol 1997;42:857–865 [DOI] [PubMed] [Google Scholar]
- 31. Palumbo V, Boulanger JM, Hill MD, Inzitari D, Buchan AM. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke. Neurology 2007;68:1020–1024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


