Abstract
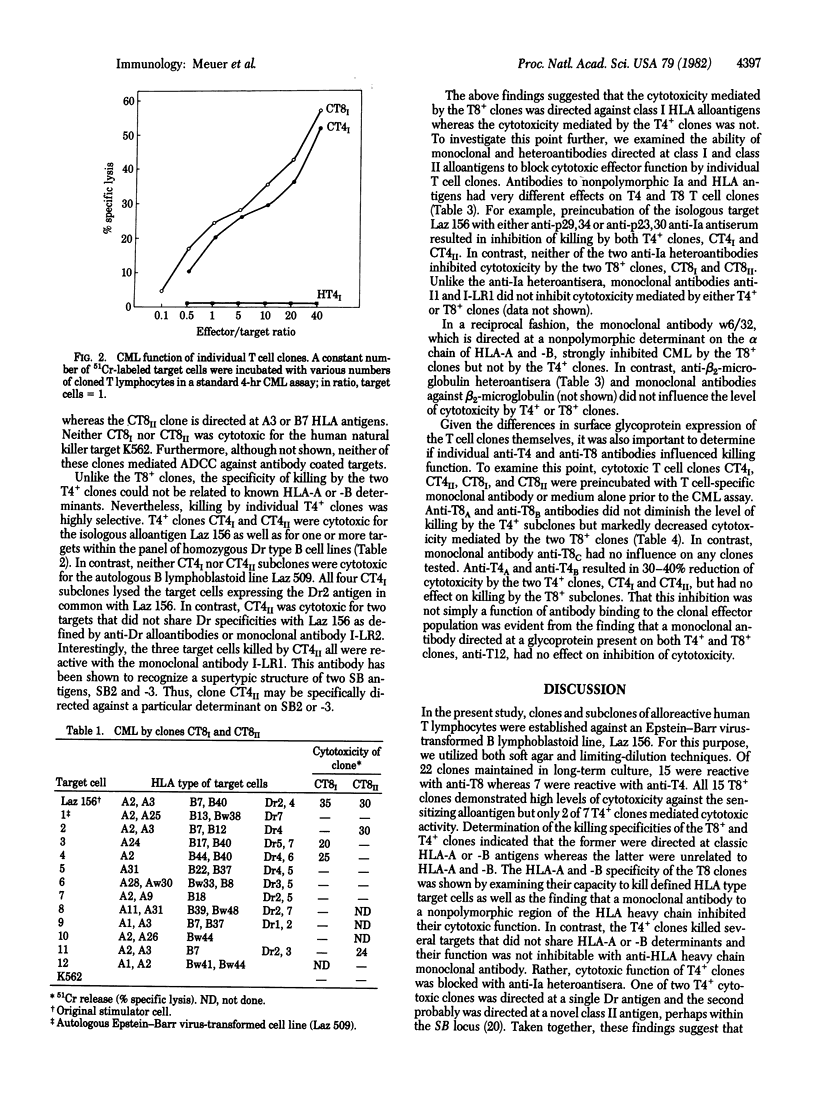
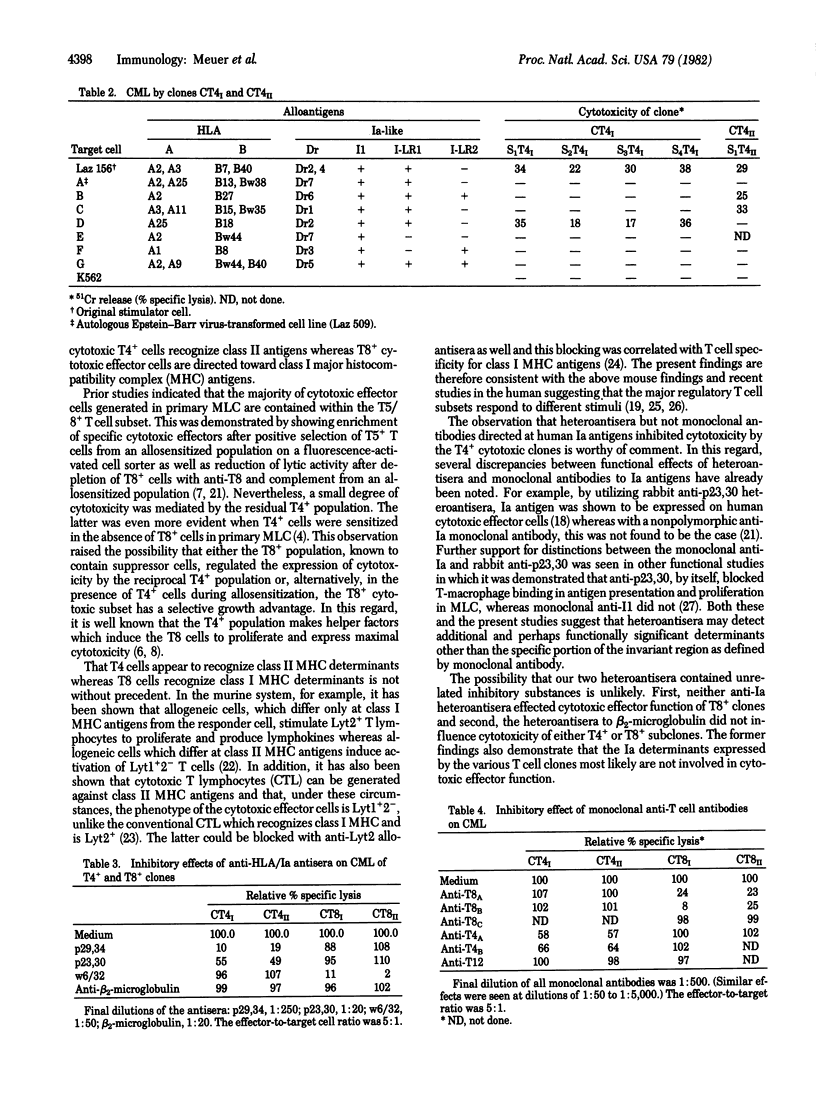
Alloreactive human T lymphocytes were cloned in soft agar or by limiting dilution and subsequently propagated with interleukin 2 and alloantigen for 8 months or more. By indirect immunofluorescence every clone was reactive with anti-Ia antibodies as well as the T cell-specific antibodies anti-T3 and anti-T11 and expressed either T4 or T8 antigens. All 15T8+ clones were highly cytotoxic for the sensitizing alloantigen. In contrast, only two of seven T4+ clones mediated cytotoxic effector function. The specificity of T4+ and T8+ clones and subclones was analyzed on a panel of typing cells and by antibody blocking studies of major histocompatibility complex (MHC) determinants on the stimulating alloantigen. It was found that T8+ clones killed targets that shared class I MHC antigens (HLA-A,B) with the original stimulator cells whereas cytotoxic T4+ clones were directed at class II MHC antigens (Ia-related). Preincubation of the allogeneic target cell with a monoclonal antibody to a nonpolymorphic HLA alpha-chain determinant inhibited killing by the T8+ clones but did not affect T4+ cytotoxic function. In a reciprocal fashion, anti-IA antibodies to common framework structures on the same target cell blocked killing by T4+ but not by T8+ clones. These results indicate that T4+ and T8+ T lymphocytes have receptors for different classes of MHC antigens and suggest tha cytotoxic T4+ subpopulations might be important in human transplantation and autoimmune disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreotti P. E., Apgar J. R., Cresswell P. HLA-A2 as a target for cell-mediated lympholysis: evidence from immunoselected HLA-A2 negative mutant cell lines. Hum Immunol. 1980 Jul;1(1):77–86. doi: 10.1016/0198-8859(80)90011-7. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Lazarus H., Penta A. C., Schlossman S. F. Two functionally distinct subpopulations of human T cells that collaborate in the generation of cytotoxic cells responsible for cell-mediated lympholysis. J Immunol. 1978 Apr;120(4):1423–1428. [PubMed] [Google Scholar]
- Friedman S. M., Hunter S. B., Irigoyen O. H., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. II. Collaborative T-T interactions in the generation of TNP-altered-self-reactive cytotoxic T lymphocytes. J Immunol. 1981 May;126(5):1702–1705. [PubMed] [Google Scholar]
- Gazit E., Terhorst C., Mahoney R. J., Yunis E. J. Alloantigens of the human T (HT) genetic region of the HLA linkage group. Hum Immunol. 1980 Sep;1(2):97–109. doi: 10.1016/0198-8859(80)90097-x. [DOI] [PubMed] [Google Scholar]
- Humphreys R. E., McCune J. M., Chess L., Herrman H. C., Malenka D. J., Mann D. L., Parham P., Schlossman S. F., Strominger J. L. Isolation and immunologic characterization of a human. B-lymphocyte-specific, cell surface antigen. J Exp Med. 1976 Jul 1;144(1):98–112. doi: 10.1084/jem.144.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. Lymphocyte transformation induced by autologous cells. IV. Human T-lymphocyte proliferation induced by autologous or allogeneic non-T lymphocytes. J Exp Med. 1976 May 1;143(5):1042–1054. doi: 10.1084/jem.143.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Henney C. S. The differentiation of cytotoxic T cells in vitro. II. Amplifying factor(s) produced in primary mixed lymphocyte cultures against K/D stimuli require the presence of Lyt 2+ cells but not Lyt 1+ cells. J Immunol. 1980 Jul;125(1):300–307. [PubMed] [Google Scholar]
- Opelz G., Kiuchi M., Takasugi M., Terasaki P. I. Autologous stimulation of human lymphocyte subpopulation. J Exp Med. 1975 Nov 1;142(5):1327–1333. doi: 10.1084/jem.142.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Hussey R. E., Fitzgerald K., Snow P., Terhorst C., Schlossman S. F. Antibody directed at a surface structure inhibits cytolytic but not suppressor function of human T lymphocytes. Nature. 1981 Nov 12;294(5837):168–170. doi: 10.1038/294168a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Hussey R. E., Schlossman S. F. Absence of expression of Ia antigen on human cytotoxic T cells. Immunogenetics. 1980;11(4):421–426. doi: 10.1007/BF01567810. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Breard J. M., Goldstein G., Schlossman S. F. T cell requirements for generation of helper factor(s) in man: analysis of the subsets involved. J Immunol. 1980 Apr;124(4):1883–1887. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Morimoto C., Fitzgerald K. A., Hussey R. E., Daley J. F., Schlossman S. F. Heterogeneity of human T4+ inducer T cells defined by a monoclonal antibody that delineates two functional subpopulations. J Immunol. 1982 Jan;128(1):463–468. [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Con A-inducible suppression of MLC: evidence for mediation by the TH2 + T cell subset in man. J Immunol. 1979 Apr;122(4):1335–1341. [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Current concepts in immunology: Regulation of the immune response--inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med. 1980 Aug 14;303(7):370–373. doi: 10.1056/NEJM198008143030704. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Shaw S., Kavathas P., Pollack M. S., Charmot D., Mawas C. Family studies define a new histocompatibility locus, SB, between HLA-DR and GLO. Nature. 1981 Oct 29;293(5835):745–747. doi: 10.1038/293745a0. [DOI] [PubMed] [Google Scholar]
- Smolen J. S., Luger T. A., Chused T. M., Steinberg A. D. Responder cells in the human autologous mixed lymphocyte reaction. J Clin Invest. 1981 Dec;68(6):1601–1604. doi: 10.1172/JCI110416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sredni B., Tse H. Y., Schwartz R. H. Direct cloning and extended culture of antigen-specific MHC-restricted, proliferating T lymphocytes. Nature. 1980 Feb 7;283(5747):581–583. doi: 10.1038/283581a0. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Dennert G., Wormsley S., Dutton R. W. The Lyt phenotype of a long-term allospecific T cell line. Both helper and killer activities to IA are mediated by Ly-1 cells. Eur J Immunol. 1981 Mar;11(3):175–180. doi: 10.1002/eji.1830110304. [DOI] [PubMed] [Google Scholar]
- Swain S. L. Significance of Lyt phenotypes: Lyt2 antibodies block activities of T cells that recognize class 1 major histocompatibility complex antigens regardless of their function. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7101–7105. doi: 10.1073/pnas.78.11.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Schlossman S. F. Human macrophage--lymphocyte interaction in proliferation to soluble antigen. II. Characterization of lymphocyte binding to antigen-pulsed macrophage monolayers. Cell Immunol. 1980 Nov;56(1):205–216. doi: 10.1016/0008-8749(80)90095-7. [DOI] [PubMed] [Google Scholar]



