Abstract
Sirtuins are members of the Sir2 (silent information regulator 2) family, a group of class III deacetylases. Mammals have seven different sirtuins, SIRT1–SIRT7. Among them, SIRT1, SIRT3 and SIRT6 are induced by calorie restriction conditions and are considered anti-aging molecules. SIRT1 has been the most extensively studied. SIRT1 deacetylates target proteins using the coenzyme NAD+ and is therefore linked to cellular energy metabolism and the redox state through multiple signalling and survival pathways. SIRT1 deficiency under various stress conditions, such as metabolic or oxidative stress or hypoxia, is implicated in the pathophysiologies of age-related diseases including diabetes, cardiovascular diseases, neurodegenerative disorders and renal diseases. In the kidneys, SIRT1 may inhibit renal cell apoptosis, inflammation and fibrosis, and may regulate lipid metabolism, autophagy, blood pressure and sodium balance. Therefore the activation of SIRT1 in the kidney may be a new therapeutic target to increase resistance to many causal factors in the development of renal diseases, including diabetic nephropathy. In addition, SIRT3 and SIRT6 are implicated in age-related disorders or longevity. In the present review, we discuss the protective functions of sirtuins and the association of sirtuins with the pathophysiology of renal diseases, including diabetic nephropathy.
Keywords: aging, diabetic nephropathy, sirtuin, SIRT1 (sirtuin 1)
Abbreviations: AGE, advanced glycation end-product; AMPK, AMP-activated protein kinase; AngII, angiotensin II; AT1R, angiotensin type 1 receptor; Atg, autophagy-related gene; BMAL1, brain and muscle ARNT (aryl hydrocarbon receptor nuclear translocator)-like 1; Bnip3, BCL2/adenovirus E1V 19-kDa interacting protein 3; CKD, chronic kidney disease; CR, calorie restriction; CRP, C-reactive protein; COX2, cyclo-oxygenase 2; α-ENaC, epithelial Na+ channel α-subunit; eNOS, endothelial NO synthase; ER, endoplasmic reticulum; FOXO, forkhead box O; FXR, farnesoid X receptor; H3K9, histone H3 Lys9; H3K9me3, H3K9 trimethylation; HIF, hypoxia-inducible factor; ICAM-1, intercellular adhesion molecule 1; Idh2, isocitrate dehydrogenase 2; IGF, insulin-like growth factor; IRS, insulin receptor substrate; LC3, light chain 3; LXR, liver X receptor; MCP-1, monocyte chemotactic protein-1; Mn-SOD, manganese superoxide dismutase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; PARP, poly(ADP-ribose) polymerase; PER2, Period 2; PGC-1α, PPAR-γ co-activator-1α; PKC, protein kinase C; PPAR, peroxisome-proliferator-activated receptor; PTP1B, protein tyrosine phosphatase 1B; RAS, renin–angiotensin system; ROS, reactive oxygen species; Sir2, silent information regulator 2; SIRT1 etc., sirtuin 1 etc., SNP, single nucleotide polymorphism; SREBP, sterol-regulatory-element-binding protein; TGF, transforming growth factor; TNF-α, tumour necrosis factor α; UUO, unilateral ureteral obstruction; VCAM-1, vascular cell adhesion protein 1; WFR, Wistar fatty diabetic rat
INTRODUCTION
The prevalence of diabetes mellitus has been increasing worldwide over recent years. Long-term diabetes results in vascular changes and dysfunction; diabetic complications are the major causes of morbidity and mortality in diabetic patients. Among diabetic vascular complications, nephropathy is recognized as not only a leading cause of end-stage renal disease, but also an independent risk factor for cardiovascular diseases.
Large clinical studies indicate that hyperglycaemia is a major contributing factor to the pathogenesis of diabetic vascular complications, including nephropathy [1]. Hyperglycaemia-mediated alterations of extra- and intra-cellular metabolism, such as AGEs (advanced glycation end-products), enhancement of diacylglycerol/PKC (protein kinase C) activity and increased flux through polyol and hexosamine pathways, constitute the classical pathogenesis of diabetic nephropathy [2,3]. In addition, there are convincing data that the RAS (renin–angiotensin system) is a major mediator of renal injuries. Blood pressure control using RAS inhibitors can reduce the progression of nephropathy [4]. However, it is not easy to control blood glucose, and treatment with RAS inhibitors may not completely prevent the progression of nephropathy. Therefore there is an urgent need to identify new therapeutic target molecules or cellular processes that underlie the pathogenesis of diabetic nephropathy to establish an additional therapeutic option, independent of glycaemic control and RAS inhibition [5].
Aging is a universal process that affects all organs. Age-related disruptions in cellular homoeostasis result in declines in organ functions and in the responsiveness to physiological stress. A gradual decline in renal function occurs in most healthy individuals as they age [6], and the amount of glomerular, vascular and interstitial scarring in the renal tissue of healthy adults increases with age [7]. In addition, aging is a risk factor for end-stage renal failure including diabetic nephropathy [8]. Therefore elucidating the process of renal aging might enable breakthroughs in the treatment of diabetic nephropathy.
CR (calorie restriction) promotes longevity and slows aging [9]. However, further restriction of food intake, leading to malnutrition, reduces the lifespan [9]. In addition, malnutrition is associated with inflammation in end-stage renal disease, and the so-called malnutrition–inflammation complex may also be a risk factor for cardiovascular death [10]. Therefore, although CR without malnutrition can extend lifespan in model organisms, further studies are required to determine when such CR can benefit humans.
One possible mechanism by which CR exerts such beneficial effects involves the actions of sirtuins, especially SIRT1. SIRT1 is associated with the regulation of a wide variety of cellular processes, such as apoptosis, metabolism, mitochondrial biogenesis and autophagy [11]. Therefore SIRT1 may improve or retard age-related disease processes, including diabetes, cardiovascular diseases, neurodegenerative disorders and renal diseases. In addition, SIRT3 [12] and SIRT6 have also been implicated as potential regulators of longevity. In the present review, we focus on the protective effects of sirtuins and discuss the potential of sirtuin-based therapeutics against diabetic nephropathy (Figure 1).
Figure 1. Therapeutic potential of sirtuins on diabetic nephropathy.
Sirtuin dysfunction associated with aging and NAD+ depletion may contribute to the initiation and progression of diabetic nephropathy, in addition to hyperglycaemia-mediated alterations of metabolism and haemodynamic changes, including RAS abnormality. The therapeutic activation of sirtuins could inhibit diabetic nephropathy. Genetic factors also may contribute to the activity of sirtuins.
Effects of CR on longevity and sirtuins
In 1935, McCay et al. [13] first reported that rats subjected to CR had longer median and maximum lifespans. After their discovery, numerous studies revealed that CR retards aging or extends the lifespans of yeast, worms, flies and rodents. Colman et al. [14] also reported that CR delayed the onset of age-associated pathologies, including diabetes, cancer, cardiovascular disease and brain atrophy, and decreased mortality in rhesus monkeys. In addition, Fontana et al. [15] showed that CR for an average of 6 years improved metabolism in humans, as measured by serum insulin, cholesterol, CRP (C-reactive protein) and TNF-α (tumour necrosis factor α) levels and by carotid intima media thickness. Fontana and co-workers [16] also observed that long-term CR ameliorated the decline in left ventricular diastolic function and decreased the levels of serum TGF (transforming growth factor)-β1, TNF-α and high-sensitivity CRP. Thus CR has a variety of beneficial effects on lifespan extension and delays the onset of age-related diseases such as cardiovascular diseases, neurodegenerative disorders and diabetes. CR is also accepted as the only established experimental anti-aging paradigm [9].
As one of the molecules through which CR improves lifespan extension or delays age-related diseases, Sir2 (silent information regulator 2), an NAD+-dependent deacetylase, was initially identified from studies of aging in yeast [17,18]. Homologues of Sir2 in higher eukaryotic organisms are known as sirtuins. SIRT1, the most closely related to Sir2, is one of the seven sirtuins in mammals. The beneficial effects of CR involve the function of the SIRT1, which is induced by CR in various tissues, including the kidney [19]. The significance of SIRT1 on the effects of CR has been shown in genetically altered mice. Bordne et al. [20] reported that Sirt1 transgenic mice exhibited a phenotype that resembles mice under CR, but they did not report on the longevity of those mice. However, in another study, Sirt1 deficiency in mice failed to extend lifespan under CR [21]. So far, it is unclear and controversial whether SIRT1 itself is crucial for CR-related lifespan extension; however, SIRT1 is associated with the regulation of a wide variety of cellular processes, from stress response, cell survival, mitochondrial biogenesis and metabolism in response to the cellular energy and redox status.
In addition, SIRT3 induced by CR exerts beneficial anti-aging effects. Mitochondrial function declines during aging, which is a major source of ROS (reactive oxygen species). CR reduces age-associated oxidative stress by reducing oxidative damage via enhancing antioxidant defences. Someya et al. [12] showed that SIRT3 mediates a reduction in oxidative damage under CR, leading to prevention of age-related hearing loss. SIRT3 deacetylates and activates mitochondrial Idh2 (isocitrate dehydrogenase 2) under CR, and enhances the glutathione antioxidant defence system.
SIRT6 has also been implicated in the regulation of longevity. Recently, Kanfi et al. [22] demonstrated that overexpression of SIRT6 extends the lifespan of male mice, is associated with decreased serum IGF (insulin-like growth factor)-1s and increased IGF-binding protein 1. In addition, Sirt6-deficient mice are small and have severe metabolic defects, and they develop abnormalities that are usually associated with aging [23]. Moreover, SIRT6 levels increase in rats exposed to CR [24], and transgenic mice that overexpress SIRT6 [25] are protected against some metabolic impairments, including triacylglycerol (triglyceride) and cholesterol accumulation in the serum, increased body fat and reduced glucose tolerance.
Thus the activation of sirtuins (especially SIRT1, SIRT3 and SIRT6) by CR might represent valuable therapeutic targets against aging and age-related diseases, including renal diseases.
Mammalian sirtuins: SIRT1–SIRT7
Table 1 lists some of the basic characteristics of the seven sirtuin genes and proteins in humans [26,27]. Phylogenetic analysis of 60 core domains from different eukaryotes and prokaryotes places the mammalian sirtuins into four different classes (I–IV). Mammalian sirtuins also differ in subcellular localization. SIRT1 and SIRT2 exist in the nucleus and cytoplasm (although SIRT1 is found predominantly in the nucleus, and SIRT2 resides most prominently in the cytoplasm) [28,29]. SIRT3, SIRT4 and SIRT5 localize to the mitochondria; however, SIRT3 is also found in the nucleus and cytoplasm [30,31]. SIRT6 and SIRT7 are nuclear sirtuins [31]. A large fraction of SIRT1 in the nucleus is associated with euchromatin, whereas SIRT6 associates with heterochromatin and SIRT7 is found in the nucleolus (Table 1).
Table 1. Characteristics of mammalian sirtuins.
| Sirtuin | Classification | Molecular mass (kDa) | Catalytic activity | Localization |
|---|---|---|---|---|
| SIRT1 | Class Ia | 120 | Deacetylase | Nucleus and cytoplasm |
| SIRT2 | Class Ib | 43 | Deacetylase | Cytoplasm and nucleus |
| SIRT3 | Class Ib | 28/44 | Deacetylase | Mitochondria, nucleus and cytoplasm |
| SIRT4 | Class II | 35 | ADP-ribosyl transferase | Mitochondria |
| SIRT5 | Class III | 34 | Deacetylase | Mitochondria |
| SIRT6 | Class IVa | 37 | Deacetylase and ADP-ribosyl transferase | Nucleus |
| SIRT7 | Class IVb | 45 | Deacetylase | Nucleus |
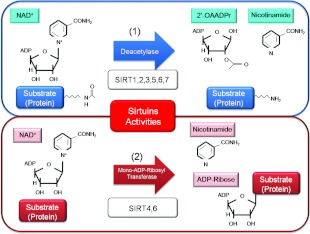
In terms of activity, sirtuins function as class III histone deacetylases, binding to NAD+ and acetyl-lysine within protein targets and generating lysine, 2′-O-acetyl-ADP-ribose and nicotinamide as enzymatic products (Figure 2). Nicotinamide acts as a negative-feedback inhibitor of SIRT1. SIRT1, SIRT2, SIRT3, SIRT5, SIRT6 and SIRT7 have NAD+-dependent deacetylase activity [11,32]. SIRT4 and SIRT6 also have ADP-ribosyl transferase activity [11].
Figure 2. Enzymatic activities of sirtuins.
(1) NAD+ is consumed as a substrate for the deacetylation of target proteins. The acetyl-lysine residues of the target protein serve as substrates for sirtuin deacetylation, which generate nicotinamide and 2′-O-acetyl-ADP-ribose (2′-OAADPr) as by-products. (2) NAD+ is also used as a substrate for the reaction of mono-ADP-ribosyl transferase with target proteins. ADP-ribose and nicotinamide are generated by the reaction.
BIOLOGICAL FUNCTIONS OF SIRTUINS
Mammalian SIRT1 promotes chromatin silencing and transcriptional repression through deacetylation of histone. Upon recruitment to chromatin, SIRT1 can directly deacetylate histones H4K16 (H4 Lys16), H3K9 (H3 Lys9), H3K14 (H3 Lys14) and H1K26 (H1 Lys26) [17,33]. These deacetylations promote hypoacetylation of nucleosomal histones and reduce transcriptional activity. Furthermore, histone acetylation and methylation are often co-ordinately regulated. Histone deacetylation by SIRT1 may also promote alterations in histone methylation. SIRT1 may enhance histone H4K20me (H4 Lys20 monomethylation) and H3K9me3 (H3K9 trimethylation), and may reduce H3K79me2 (H3 Lys79 dimethylation) [33]. There is still no clear understanding of the detailed mechanisms of SIRT1-induced histone methylation. However, Vaquero et al. [34] reported that SIRT1 can recruit histone methyltransferases such as SUV39H1 (suppressor of variegation 3-9 homologue 1) to target sites, thereby regulating both histone acetylation and methylation at these sites. SIRT1 deacetylates the catalytic domain of SUV39H1, which is the enzyme responsible for H3K9me3.
In addition, more than a dozen non-histone proteins, including transcription factors and transcriptional co-regulatory proteins, serve as substrates for SIRT1. For example, the involvement of NAD+ in the deacetylation reaction is thought to link sirtuin deacetylase activity to metabolism. SIRT1 regulates energy metabolism and mediates the longevity effect of CR by promoting gluconeogenesis and repressing glycolysis in the liver via deacetylation of PGC-1α [PPAR (peroxisome-proliferator-activated receptor)-γ coactivator-1α] [35,36], an important promoter of mitochondrial biogenesis. SIRT1 increases insulin sensitivity by modulating insulin signalling [18]. SIRT1 reduces the expression of PTP1B (protein tyrosine phosphatase 1B) [37], which is the tyrosine phosphatase for the insulin receptor. In addition, SIRT1 may regulate insulin-induced IRS-2 (insulin receptor substrate-2) tyrosine phosphorylation by regulating its acetylation level [38]. SIRT1 is also involved in insulin secretion. The overexpression of SIRT1 in pancreatic islet β-cells increases ATP production by repressing mitochondrial UCP2 (uncoupling protein 2) expression, thereby leading to the closing of ATP-sensitive K+ channels and to insulin secretion [39]. In addition, SIRT1 inhibits fat storage and increases fatty acid release in white adipose tissue via repression of PPAR-γ [40] and also regulates components of the circadian clock, such as BMAL1 [brain and muscle ARNT (aryl hydrocarbon receptor nuclear translocator)-like 1] [41] and PER2 (Period 2) [42]. These diverse functions underscore the interconnectedness of protein acetylation, metabolism, circadian rhythms and aging. SIRT1 is associated with lipid metabolism through the activation of nuclear receptors, including PPAR-α [43], LXR (liver X receptor), FXR (farnesoid X receptor) and negative regulation of SREBP (sterol-regulatory-element-binding protein) [44]. Furthermore, SIRT1 deacetylates transcription factors such as FOXO (forkhead box O) [45,46], p53 [47], PARP [poly(ADP-ribose) polymerase]-1 [48], HIF (hypoxia-inducible factor)-1α and HIF-2α [49,50], NF-κB (nuclear factor κB) [51], Atg (autophagy-related gene) 5, Atg7 and LC3 (light chain 3) [52] to mediate stress resistance, apoptosis, hypoxia, inflammatory signalling and autophagy as physiological responses to environmental toxicity. In addition, a recent study by Price et al. [53] demonstrated a direct link between SIRT1 and the metabolic benefits of resveratrol, a SIRT1 activator. They reported that a moderate dose of resveratrol first activates SIRT1, and then induces deacetylation of LKB1 (liver kinase B1) and the activation of AMPK (AMP-activated protein kinase), leading to increased mitochondrial biogenesis and function. AMPK is also required for SIRT1 activation. Moreover a high dose of resveratrol may directly activate AMPK independently of SIRT1. Thus the activation of SIRT1 may lead to the induction of gene silencing, reduction of apoptosis, enhanced mitochondrial biogenesis, inhibition of inflammation, regulation of glucose and lipid metabolism and circadian rhythm, induction of autophagy, and adaptation to cellular stress (Figure 3).
Figure 3. Biological functions of SIRT1.
SIRT1 promotes chromatin silencing through chromatin modification, mainly deacetylation, and regulates glucose and lipid metabolism, circadian rhythms, mitochondrial biogenesis, stress responses, apoptosis, inflammation and autophagy by deacetylating non-histone proteins, including transcription factors and transcriptional co-regulatory proteins.
SIRT3 exerts antioxidative effects through the deacetylation and activation of mitochondrial Idh2 and enhancement of the glutathione antioxidant defence system. SIRT3 also deacetylates Mn-SOD (manganese superoxide dismutase). In addition, SIRT3 is induced by CR in white adipose tissue, brown adipose tissue and skeletal muscle, and it regulates hepatic metabolic processes, including fatty acid oxidation, ketone body production and the urea cycle as adaptive changes [11].
SIRT6 is involved in DNA repair, telomere maintenance, genomic stability and cell senescence and may attenuate inflammation by modulating NF-κB signalling, as described below.
PROTECTIVE EFFECTS OF SIRTUINS IN THE KIDNEY (Figure 4)
Figure 4. Renoprotective effects of SIRT1.
SIRT1 exerts renoprotective effects by conferring resistance to cellular stresses such as hypoxia, reducing interstitial fibrosis, inhibiting tubular and glomerular cell apoptosis and inflammation, inducing autophagy, and regulating Na+-handling, blood pressure and renal lipid metabolism.
Renal aging and sirtuins (Table 2)
Table 2. Relationship between SIRT1 and pathophysiology on renal diseases.
Ace, acetylated; TG, transgenic; ↓, decreases; ↑, increases; ⊣, inhibits.
| Animals, tissue, cells or SIRT1 activator | Target for SIRT1 | Effect for pathophysiology of renal injuries | Reference(s) |
|---|---|---|---|
| Sirt1+/− mice | SIRT1↓ → Ace-HIF-2α (inactivation)↑ | Erythropoetin↓ | [50] |
| Medullary interstitial cells (Sirt1+/− mice) | SIRT1↓ → COX2↓ → PGE2↓ | Oxidative stress, apoptosis and fibrosis in UUO↑ | [64] |
| Resveratrol (proximal tubular cells) | SIRT1↑ → Ace-Smad3↓ | Fibrosis in UUO↓ | [65] |
| Resveratrol (proximal tubular cells) | SIRT1↑ → Ace-p53↓ | Cisplatin-induced apoptosis↓ | [68] |
| Proximal tubular cells (specific Sirt1-TG mice) | SIRT1↑ → Ace-FOXO3↓ | Catalase↑, oxidative stress↓, peroxisome function↑ and cisplatin-induced renal injuries↓ | [66,67] |
| Renal cortex (Wistar fatty rats) | SIRT1↓ → Ace-NF-κB (p65)↑ | Inflammation in diabetic nephropathy↑ | [79] |
| Proximal tubular cells (Sirt1+/− mice) (aging mice) | SIRT1↓ → Ace-FOXO3a↑ | Autophagy↓, abnormal mitochondria↑ and aging kidney ↑ | [46] |
| Mesangial cells | SIRT1⊣ Ace-p53 | Oxidative stress-induced apoptosis↓ | [47] |
| Mesangial cells | SIRT1⊣ Ace-Smad 7 | TGF-β-induced apoptosis↓ | [82] |
| Podocytes | SIRT1⊣ Ace-FOXO4 | AGE-induced apoptosis↓ | [83] |
| Endothelial cells | SIRT1⊣ Ace-eNOS | NO↑ | [85] |
| Collecting duct cells | SIRT1⊣ ENaC α-subunit | Na+ reabsorption↓ | [94] |
Aging causes progressive post-maturational deterioration of tissues and organs, leading to impaired tissue functioning, increased vulnerability to stress and death. The kidney is one of the typical target organs of age-associated tissue damage; the increased incidence of CKD (chronic kidney disease) in the elderly is a health problem worldwide [54]. Diabetic nephropathy is the most common result of CKD, and aging is a risk factor for the initiation and progression of CKD, including diabetic nephropathy [8].
Is SIRT1 associated with renal aging? We found that, compared with young mice, 24-month-old mice showed decreased SIRT1 expression in the kidney, which was associated with increased mitochondrial oxidative stress and morphological changes in mitochondria, such as swelling and disintegration of cristae [46]. Treatment with 40% CR for 12 months starting at 12 months of age improved all alterations observed in aging mice [46]. Moreover, in the kidneys of Sirt1+/− mice, mitochondrial morphological changes similar to those in 24-month-old wild-type mice were already observed at 12 months. CR in Sirt1+/− mice failed to attenuate the age-associated alterations in the kidney. Therefore mitochondrial damage in aged kidneys seems to be associated with SIRT1 deficiency.
Age-associated reduction in systemic NAD+ biosynthesis can reduce SIRT1 activity [55]. Braidy et al. [56] observed decreased SIRT1 activity in the heart, liver and kidneys of 12-month-old Wistar rats in parallel with decreased levels of NAD+, compared with 3-month-old rats. The depletion of cellular NAD+ stores attenuates SIRT1 activity, leading to hyperacetylation of p53 and consequently tilting the balance towards cell death via an apoptotic mechanism. NAD+ depletion is induced by the activation of PARP, which is activated by DNA damage due to age-related oxidative stress and consumes NAD+ as a substrate [56]. These findings suggest that adequate NAD+ concentrations associated with SIRT1 activity may be an important longevity assurance factor. In addition, studies in cardiomyocytes have shown that PARP-1 is activated by acetylation in response to cellular stress. SIRT1 directly binds to and deacetylates PARP-1, thereby inhibiting PARP-1 activity [48].
Hypoxia and sirtuins in the kidney (Table 2)
Chronic hypoxia is the final major pathway leading to end-stage renal failure. Ischaemia of the kidney is induced by the loss of peritubular capillaries in the tubule interstitium in the late stage of renal disease. However, hypoxia has a crucial role in the tubule interstitium before structural microvasculature damage in the corresponding region, emphasizing the pathogenic role of this condition from an early stage of kidney disease [57]. Several mechanisms may contribute to decreased tissue oxygenation, including anaemia, increased vasoconstriction driven by an increase in vasoconstrictors and/or loss of vasodilators, increased metabolic demand as a result of hyperfiltration and hypertrophy of uninjured nephrons, and increased oxygen diffusion distances as the extracellular matrix accumulates between blood vessels and adjacent cells.
HIF-1 is the transcription factor that activates an adaptive response to a low concentration of oxygen [58]. HIF-1 positively regulates the expression of the gene encoding erythropoietin, and angiogenic and antioxidative enzymes, and it protects against damaging reactive oxidative molecules from mitochondria in response to hypoxia [58]. Lim et al. [49] reported that SIRT1 may inhibit HIF-1α-dependent transcription by deacetylation at Lys674. These findings imply that HIF-1α is involved in the pathogenesis of renal injury, as activation of HIF responses could modulate disease activity at an early stage, when tissue oxygenation is not yet impaired. Several signalling molecules, some with key roles in the pathogenesis of CKD, induce stabilization of HIF-1α under normoxia. These include NO, ROS, TNF-α, interleukin-1, AngII (angiotensin II), TGF-β, hyperglycaemia and growth factors such as epidermal growth factor, insulin and IGFs; these either inhibit HIF prolyl-hydroxylation directly or indirectly increase HIF-1α levels [59]. Given the wide range of HIF-regulated biological functions, normoxic stabilization of HIF-1α is likely to modulate disease activity at an early stage before the occurrence of significant hypoxia. For example, increased renal HIF-1α is observed in the early phases of diabetic nephropathy in db/db mice [60] or ZDF (Zucker diabetic fatty) rats [61]. However, it is not known whether acetylated HIF-1α is increased in the diabetic kidney.
On the other hand, SIRT1 may activate HIF-2α via deacetylation during hypoxia [50], leading to overexpression of erythropoietin or Mn-SOD. HIF-2α, but not HIF-1α, is highly expressed in the renal cortical interstitia of aged rodents [62]. Therefore a reduction in SIRT1 could lead to the impairment of the response to hypoxia by inactivation of HIF-2α, resulting in chronic renal injury. Although it seems likely that SIRT1 regulates HIF-1α and HIF-2α in an opposite manner in response to hypoxia, the inhibition of HIF-1α and the activation of HIF-2α by SIRT1 may be a beneficial response to the cellular stress caused by hypoxia or growth factors in the kidney.
Further studies are needed to determine the relationship between HIF-1α and HIF-2α under tissue hypoxia or relative hypoxia in various renal injuries.
Interstitial fibrosis, tubular cell apoptosis and sirtuins in the kidney (Table 2)
Tubulointerstitial fibrosis is considered a central event in the progression of CKD, independent of aetiology. Even in glomerulopathies, tubulointerstitial fibrosis correlates better than glomerular injury with the evolution and prognosis of the disease [63]. Renal tubular cell apoptosis is implicated in the progression of renal injuries.
He et al. [64] found that SIRT1 is abundantly expressed in mouse medullary interstitial cells, where it increases cell resistance to oxidative stress. In Sirt1+/− mice, the decreased SIRT1 concentration is associated with increased apoptosis and fibrosis after UUO (unilateral ureteral obstruction), whereas its activation by treatment with resveratrol or SRT2183 in wild-type mice reduced apoptotic and fibrotic changes in the UUO. In addition, SIRT1 deficiency decreases the COX2 (cyclo-oxygenase 2) induction in medullary interstitial cells under oxidative stress, whereas exogenous PGE2 (prostaglandin E2) reduces apoptosis in oxidatively stressed SIRT1-deficient cells. These findings not only indicate the protective function of SIRT1, but also identify COX2 as one of its targets. It remains to be established whether this effect of SIRT1 is due to its deacetylation of COX2 or is mediated via FOXO or other established targets of SIRT1.
The TGF-β1/Smad3 signalling pathway plays a central role in the pathogenesis of tissue fibrosis in the kidney. Li et al. [65] reported that SIRT1 activation by resveratrol inhibits the acetylation of Smad3, resulting in reduction of TGF-β1-induced collagen IV and fibronectin expression in a UUO animal model and in cultured cells (rat fibroblasts, NRK49F; rat proximal tubular cells, NRK52E).
SIRT1 also protects against renal tubular cell apoptosis. Hasegawa et al. [66] found that SIRT1 protects against oxidative- stress-induced apoptosis by inducing catalase via deacetylation of FOXO3 in cultured proximal tubular cells. Moreover, Hasegawa et al. [67] also reported that renal proximal tubular cell-specific SIRT1 transgenic mice showed resistance to cisplatin-induced renal tubular cell injuries, such as apoptosis, by maintaining peroxisome number and function, concomitant up-regulation of catalase and elimination of renal ROS. In addition, SIRT1 activation by resveratrol reduces cisplatin-induced proximal tubular cell apoptosis through deacetylation of p53 [68].
Inflammation and sirtuins in the kidney (Table 2)
The inflammatory process is one of the pivotal mechanisms for the initiation and progression of age-related diseases, such as diabetes, cardiovascular diseases, neurodegenerative disorders, pulmonary disease and kidney diseases including diabetic nephropathy [69,70]. Therefore the control of the inflammatory process might be a potential therapeutic target for attenuating the progression of such age-related diseases.
The NF-κB signalling pathway plays a central role in the regulation of the expression of several inflammation-related proteins, such as MCP-1 (monocyte chemotactic protein-1), ICAM-1 (intercellular adhesion molecule 1) and VCAM-1 (vascular cell adhesion protein 1) [71]. The observation that several lysine residues of the NF-κB p65 subunit can be acetylated has highlighted the potential regulatory role of lysine acetylation in NF-κB function [72]. Among these acetylated lysine residues in NF-κB p65, acetylation at Lys310 might promote superior transcriptional activity while also being a substrate for SIRT1 [51]. Therefore SIRT1 may act as a negative regulator of NF-κB activity by deacetylating Lys310 of p65. Reduced levels of SIRT1 result in the up-regulation of acetylated NF-κB, leading to an increase in the inflammatory response in adipocytes [73], monocytes/macrophages [74,75], myeloid cells [76], endothelial cells [77] and microglia [78] of several experimental animal or cell models. In those studies, SIRT1 up-regulation using chemical activators of SIRT1 or overexpression of a SIRT1 gene improved the inflammation through deacetylation of NF-κB.
We found that SIRT1 protein expression is significantly decreased and that acetylated NF-κB p65 and inflammation-related gene expression levels (ICAM-1, VCAM-1 and MCP-1) are clearly increased in the kidneys of WFRs (Wistar fatty diabetic rats) compared with WLRs (Wistar lean non-diabetic rats) [79]. The increased levels of acetylated NF-κB and inflammation-related genes in WFRs were decreased by CR, consistent with the restoration of SIRT1 protein expression in the kidney. Therefore renal inflammation is induced by increased levels of acetylated NF-κB p65 owing to reduced SIRT1 protein expression, whereas CR exerts anti-inflammatory effects by restoring SIRT1 expression in the kidneys of WFRs [79].
SIRT6 is also a negative regulator of NF-κB signalling. SIRT6 attenuates NF-κB signalling by functioning at the chromatin level. Although it directly binds to p65 as SIRT1 does, SIRT6 deacetylates H3K9 in the promoters of NF-κB target genes to decrease promoter occupancy by p65, rather than directly modulating p65 [80]. So far, however, there are no reports about SIRT6 in renal diseases.
Role of sirtuins in glomerular cells (Table 2)
Apoptosis is a distinct form of cell death that is observed under various physiological and pathological conditions [81]. Glomerular cells, including mesangial cells and podocytes, exhibit up-regulated apoptosis in human and experimental kidney diseases, such as diabetic nephropathy, hypertensive nephrosclerosis and glomerulonephritis. This apoptosis is considered to be involved in the progression of these diseases. Therefore preventing glomerular cell apoptosis may help prevent various kidney diseases.
Interestingly, SIRT1 diminishes mesangial cell apoptosis induced by oxidative stress by reducing p53 activity [47], and attenuates TGF-β-induced apoptotic signalling mediated by the effector molecule Smad7 [82]. SIRT1-dependent deacetylation of Smad7 at Lys60 and Lys70 also enhances the ubiquitin-dependent proteasomal degradation of this effector by Smurf1 (Smad ubiquitination regulatory factor 1). Glomerular mesangial cells are thus protected from not only oxidative stress, but also TGF-β-dependent apoptosis. Both oxidative stress and TGF-β accelerate and contribute to renal diseases, implying that SIRT1 could be a therapeutic target for preventing glomerular diseases, including diabetic nephropathy.
Chuang et al. [83] found that AGE-BSA (AGE-modified BSA) increased FOXO4 acetylation and suppressed the expression of the SIRT1 protein in glomerular podocytes of db/db diabetic mice and diabetic patients. Acetylated FOXO4 promotes the expression of the pro-apoptosis gene Bcl2l11 (also known as Bim) and leads to podocyte apoptosis.
NO is a protective factor in vascular tissues, including the kidneys. eNOS (endothelial NO synthase) deficiency due to endothelial cell dysfunction plays an important role in the pathophysiologies of cardiovascular diseases (hypertension and atherosclerosis) and renal injuries, including diabetic nephropathy [84]. SIRT1 promotes vasodilation and protects vascular tissues through increased NO production by deacetylating eNOS in endothelial cells [85].
Autophagy and sirtuins in renal diseases (Table 2)
Autophagy is a lysosomal degradation pathway that plays a crucial role in removing protein aggregates and damaged or excess organelles, such as mitochondria, leading to the maintenance of intracellular homoeostasis and promoting cellular health under various stress conditions, including hypoxia, ER (endoplasmic reticulum) stress or oxidative stress [86,87]. Autophagy plays a crucial role in several organs, especially metabolic organs, and its alteration is involved in the pathogenesis of metabolic and age-related diseases, including renal diseases [88]. According to experiments in renal injury models, the autophagy system is important in renal tubular cells and podocytes. Furthermore, autophagy is regulated by nutrition-sensing signals such as SIRT1, mTOR (mammalian target of rapamycin) and AMPK. Results that demonstrate the role of SIRT1 in autophagy are still sparse compared with those for mTOR and AMPK, but they have been accumulating. SIRT1 can deacetylate essential autophagic factors, such as Atg5, Atg7 and LC3, leading to the induction of autophagy. Furthermore, SIRT1 deacetylates the transcription factor FOXO3a, which leads to enhanced expression of proautophagic Bnip3 (Bcl-2/adenovirus E1V 19-kDa interacting protein 3).
Hypoxia can cause renal tubular damage in kidney diseases, as described below, and it also stimulates autophagy [87]. Hypoxia-induced autophagy largely depends on HIF-1α, which activates the transcription of Bnip3 and Bnip3L (Bnip3-like), which in turn induce autophagy. Normally, Beclin 1 interacts with Bcl-2 proteins. Bnip3 can disrupt this interaction, liberating Beclin 1 from Bcl-2 and leading to autophagy [89]. Thus HIF-1α-induced Bnip3 overexpression promotes autophagy. We have shown that hypoxia-induced autophagy activity declines with age in mice, which leads to accumulations of damaged mitochondria and mitochondrial ROS in the kidney [46]. Interestingly, long-term CR restores autophagy activity, even in aged kidneys. When we investigated the mechanism of this effect, we found that SIRT1-mediated autophagy is essential in the CR-mediated protection of aged kidneys [46]. Bnip3 expression is essential for inducing autophagy under hypoxic conditions and is positively regulated by FOXO3a rather than HIF-1α. This regulation is altered in aged kidneys. However, CR-mediated SIRT1 activation deacetylates and activates FOXO3a transcriptional activity and subsequent Bnip3-mediated autophagy, even in aged kidneys. Furthermore, the kidneys of Sirt1+/− mice exhibit lower autophagy activity and decreased Bnip3 expression; thus they are resistant to CR-mediated anti-aging effects. These findings suggest that SIRT1 is essential for CR-mediated renoprotection. Chronic hypoxia causes damage to the mitochondria and promotes the intracellular accumulation of ROS. Removing the damaged mitochondria under hypoxic conditions is also an important role of Bnip3-mediated autophagy. Thus restoring autophagic activity even under diabetic conditions should be valuable for protecting the kidney from hypoxia.
In addition, we observed that mitochondrial morphological damages in the proximal tubular cells and p62/Sqstm1 accumulation in kidneys of WFRs, suggesting that an impairment of the autophagy system can induce mitochondrial damage. Because SIRT1 is a positive regulator of autophagy, the decrease in SIRT1 expression observed in the kidneys of WFRs may lead to the dysregulation of autophagy. CR improves the function of the autophagy system, which resulted in normalization of mitochondrial morphological changes and p62/Sqstm1 accumulation, which was accompanied by the restoration of SIRT1 expression [79].
On the other hand, Hartleben et al. [90] have demonstrated an important role for the autophagy system in podocytes under basal conditions and under several renal injury conditions using GFP (green fluorescent protein)–LC3 transgenic mice and podocyte-specific Atg5-knockout mice. They [90] showed that podocytes exhibit an unusually high level of constitutive autophagy and that podocyte-specific deletion of Atg5 leads to glomerulopathy in aging mice that is accompanied by an accumulation of oxidized and ubiquitinated proteins, ER stress and proteinuria. These changes ultimately result in podocyte loss and histological glomerulosclerosis. Moreover, autophagy is substantially increased in glomeruli from mice with protein-overload-induced renal injury and in glomeruli from patients with glomerular diseases, such as focal glomerulosclerosis or membranoproliferative glomerulonephritis. Furthermore, mice lacking Atg5 in podocytes exhibit strongly increased susceptibility to drug-induced glomerular injuries (i.e. induced by puromycin aminonucleoside and adriamycin). These findings highlight the importance of induced autophagy as a key homoeostatic mechanism for maintaining podocyte integrity.
Thus it is evident that autophagy deficiency is associated with podocyte and renal tubular cell injuries. These findings indicate that autophagy deficiency should contribute to the pathogenesis of renal diseases such as diabetic nephropathy. Therefore dysregulation of SIRT1 in the kidney could result in the accumulation of mitochondrial ROS via suppression of autophagy, which may be associated with the initiation of the early stages of diabetic nephropathy. Both hypoxia- and proteinuria-induced ER stress may also contribute to proximal tubular cell damage in the progressive stages of diabetic nephropathy. Autophagy deficiency in kidneys from diabetic animals may make tubular cells fragile under hypoxic and ER stress and may lead to progression of diabetic nephropathy. Therefore activation of autophagy may be a therapeutic option for the advanced stages of diabetic nephropathy [88].
Role of sirtuins in Na+ handling and regulation of blood pressure (Table 2)
The development and rate of renal deterioration are most closely related to the patient's blood pressure. AngII plays important roles in the pathogenesis of not only hypertension, but also chronic renal diseases, including diabetic nephropathy. AngII is a mediator of glomerular haemodynamic adaptation and injury. It also has pro-inflammatory actions that lead to the up-regulation of chemokines, adhesion molecules and other fibrogenic growth factors, culminating in a decline in renal function. Most of the traditional cardiovascular effects of angII, such as vasoconstriction, water retention and Na+ retention, are mediated by the AT1R (angiotensin type 1 receptor), a protein that is implicated in the pathogenesis of hypertension. SIRT1 may negatively regulate AT1R expression. Miyazaki et al. [91] reported that overexpression of SIRT1 or treatment with resveratrol, an activator of SIRT1, suppressed AT1R expression by reducing Sp1 (specificity protein 1) binding to the AGTR1 promoter in cultured smooth muscular cells. Moreover, they found that resveratrol suppresses AT1R expression in the aorta and improves AngII-induced hypertension in mice. Interestingly, the lifespans of Agtr1−/− mice are extended compared with wild-type mice, and the expression of SIRT3, but not SIRT1, was significantly increased in the kidneys of Agtr1−/− mice [92].
Aldosterone increases renal tubular Na+ absorption largely by increasing the transcription of α-ENaC (epithelial Na+ channel α-subunit) in the apical membranes of collecting duct principal cells. This increase contributes to the pathogenesis of hypertension [93]. SIRT1 represses the expression of α-ENaC in mIMCD3 cells independent of its deacetylase activity [94]. SIRT1 interacts with Dot (disruptor of telomeric silencing)-1, a histone H3K79 methyltransferase, resulting in histone H3K79 hypermethylation in chromatin along the α-ENaC promoter, leading to repression of α-ENaC transcription. In addition, treatment with aldosterone decreases SIRT1 mRNA expression, whereas SIRT1 inhibits aldosterone-induced α-ENaC promoter activity independent of mineralocorticoid receptor signalling [94].
Lipid metabolism and sirtuins in the kidney
Clinical and animal model observations have suggested that abnormal lipid metabolism contributes to the pathogenesis and progression of renal diseases, including diabetic nephropathy [95,96]. Lipid metabolites can induce cellular dysfunction, a process known as lipotoxicity, to release pro-fibrotic and pro-inflammatory cytokines and chemokines, increase the generation of ROS, and promote the expression of extracellular matrix proteins, contributing to oxidative stress, inflammation and fibrosis in diabetic nephropathy. The increased expression or activity of SREBP and the decreased expression or activity of PPAR-α, FXR and LXR are observed in the kidneys of animal models of diabetes or diet-induced obesity [97–99]. Treatment with PPAR-α agonists or FXR agonists can improve lipid metabolism abnormalities and renal injuries [100–104].
SIRT1 is associated with lipid metabolism through its regulation of PPAR-α [43], LXR, FXR and SREBP [44]. SIRT1 is a positive regulator of LXR proteins, which are nuclear receptors that function as cholesterol sensors and regulate whole-body cholesterol and lipid homoeostasis [105]. SIRT1 also positively regulates FXR [106] and negatively regulates SREBP. Therefore SIRT1 can modulate liver and renal lipid metabolism and can prevent the progression of renal diseases. However, further investigations are required to determine the significance of SIRT1-regulated lipid metabolism in renal diseases.
NEFA (non-esterified fatty acid)-mediated renal lipotoxicity is associated with the progression of tubulointerstitial inflammation in proteinuric renal disease [107,108]. SIRT3 exerts antioxidant and anti-inflammatory effects under conditions of palmitate-induced lipotoxicity by promoting the enhancement of mitochondrial oxidative capacity and antioxidant defences in proximal tubular cells [109]. Therefore the activation of SIRT3 could be useful in the design of new therapies to prevent proteinuric renal diseases, including advanced diabetic nephropathy.
SNPs (SINGLE NUCLEOTIDE POLYMORPHISMS) AND SIRTUINS IN DIABETIC NEPHROPATHY
Four SNPs within SIRT1 have been nominally associated with susceptibility to diabetic nephropathy [110]. The haplotype, consisting of the 11 SNPs in SIRT1, had a stronger association with diabetic nephropathy than any single SNP in four independent Japanese case-control studies. SNPs in other sirtuin genes have not demonstrated any associations with diabetic nephropathy. Thus SIRT1 may be a good pharmaceutical target for diabetic nephropathy, although the association should be evaluated further in independent studies [110].
CONCLUSIONS AND PROSPECTS
In recent decades, numerous investigators have made efforts to identify the molecular mechanisms involved in the initiation and progression of diabetic nephropathy to develop new therapeutic strategies. However, end-stage renal failure due to diabetic nephropathy continues to increase worldwide. There is an urgent need to identify new therapeutic targets to prevent diabetic nephropathy.
Over the last decade, the understanding of sirtuins has expanded from the original description of a single NAD+-dependent class III histone deacetylase that can control the lifespan of yeast. SIRT1 deacetylates not only histones, but also many transcriptional regulators, thereby modulating diverse biological processes. SIRT1 exerts renoprotective effects by conferring resistance to cellular stress such as hypoxia, reducing fibrosis, inhibiting apoptosis and inflammation, inducing autophagy, and regulating blood pressure (Figure 4). Therefore SIRT1 may be a novel therapeutic target for renal diseases including diabetic nephropathy.
Although other sirtuins, including the mitochondrial sirtuins SIRT3, SIRT4 and SIRT5 and the nuclear sirtuins SIRT6 and SIRT7, may have important roles in cytoprotective functions, their molecular targets and biological functions, and possible roles in renoprotection, are largely unknown. Further investigation into the targets and functions of other sirtuins will help develop new strategies for protection against renal diseases.
FUNDING
This work was supported by Novo Nordisk Pharma, a Grant-in-Aid for Scientific Research (C) [number 24591218] and a Grant for Promoted Research from Kanazawa Medical University [number S2012-4] (to M.K.), Grants for Collaborative Research [number C2012-1] and Specially Promoted Research from Kanazawa Medical University [number SR2012-06] and the Japanese Society of Anti-Aging Medicine [4th Annual Research Award Grant (to D.K.)].
References
- 1.Jun M., Perkovic V., Cass A. Intensive glycemic control and renal outcome. Contrib. Nephrol. 2011;170:196–208. doi: 10.1159/000325664. [DOI] [PubMed] [Google Scholar]
- 2.Calcutt N. A., Cooper M. E., Kern T. S., Schmidt A. M. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat. Rev. Drug Discovery. 2009;8:417–429. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitada M., Zhang Z., Mima A., King G. L. Molecular mechanisms of diabetic vascular complications. J. Diabetes Invest. 2010;1:77–89. doi: 10.1111/j.2040-1124.2010.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galle J. Reduction of proteinuria with angiotensin receptor blockers. Nat. Clin. Pract. Cardiovasc. Med. 2008;5(Suppl. 1):S36–S43. doi: 10.1038/ncpcardio0806. [DOI] [PubMed] [Google Scholar]
- 5.Barlovic D. P., Cooper M. E. Diabetes: RAS inhibition: probably not a one-size-fits-all approach. Nat. Rev. Nephrol. 2009;5:669–670. doi: 10.1038/nrneph.2009.189. [DOI] [PubMed] [Google Scholar]
- 6.Coresh J., Astor B. C., Greene T., Eknoyan G., Levey A. S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 7.Rule A. D., Amer H., Cornell L. D., Taler S. J., Cosio F. G., Kremers W. K., Textor S. C., Stegall M. D. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann. Intern. Med. 2010;152:561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu C. Y., Iribarren C., McCulloch C. E., Darbinian J., Go A. S. Risk factors for end-stage renal disease: 25-year follow-up, Arch. Intern. Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana L., Partridge L., Longo V. D. Extending healthy life span–from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouque D., Pelletier S., Mafra D., Chauveau P. Nutrition and chronic kidney disease. Kidney Int. 2011;80:348–357. doi: 10.1038/ki.2011.118. [DOI] [PubMed] [Google Scholar]
- 11.Guarente L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N. Engl. J. Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 12.Someya S., Yu W., Hallows W. C., Xu J., Vann J. M., Leeuwenburgh C., Tanokura M., Denu J. M., Prolla T. A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCay C. M., Crowell M. F., Maynard L. A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 14.Colman R. J., Anderson R. M., Johnson S. C., Kastman E. K., Kosmatka K. J., Beasley T. M., Allison D. B., Cruzen C., Simmons H. A., Kemnitz J. W., Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana L., Meyer T. E., Klein S., Holloszy J. O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer T. E., Kovacs S. J., Ehsani A. A., Klein S., Holloszy J. O., Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Coll. Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 17.Imai S., Armstrong C. M., Kaeberlein M., Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 18.Liang F., Kume S., Koya D. SIRT1 and insulin resistance. Nat. Rev. Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 19.Cohen H. Y., Miller C., Bitterman K. J., Wall N. R., Hekking B., Kessler B., Howitz K. T., Gorospe M., de Cabo R., Sinclair D. A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 20.Bordone L., Cohen D., Robinson A., Motta M. C., van Veen E., Czopik A., Steele A. D., Crowe H., Marmor S., Luo J., et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 21.Boily G., Seifert E. L., Bevilacqua L., He X. H., Sabourin G., Estey C., Moffat C., Crawford S., Saliba S., Jardine K., et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanfi Y., Naiman S., Amir G., Peshti V., Zinman G., Nahum L., Bar-Joseph Z., Cohen H. Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 23.Mostoslavsky R., Chua K. F., Lombard D. B., Pang W. W., Fischer M. R., Gellon L., Liu P., Mostoslavsky G., Franco S., Murphy M. M., et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 24.Kanfi Y., Shalman R., Peshti V., Pilosof S. N., Gozlan Y. M., Pearson K. J., Lerrer B., Moazed D., Marine J. C., de Cabo R., Cohen H. Y. Regulation of SIRT6 protein levels by nutrient availability. FEBS Lett. 2008;582:543–548. doi: 10.1016/j.febslet.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanfi Y., Peshti V., Gil R., Naiman S., Nahum L., Levin E., Kronfeld-Schor N., Cohen H. Y. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell. 2010;9:162–173. doi: 10.1111/j.1474-9726.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 26.Michan S., Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frye R. A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 28.Tanno M., Sakamoto J., Miura T., Shimamoto K., Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 29.North B. J., Verdin E. Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS ONE. 2007;2:e784. doi: 10.1371/journal.pone.0000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundaresan N. R., Samant S. A., Pillai V. B., Rajamohan S. B., Gupta M. P. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol. Cell. Biol. 2008;28:6384–6401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michishita E., Park J. Y., Burneskis J. M., Barrett J. C., Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vakhrusheva O., Smolka C., Gajawada P., Kostin S., Boettger T., Kubin T., Braun T., Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 33.Vaquero A., Scher M., Lee D., Erdjument-Bromage H., Tempst P., Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol. Cell. 2004;16:93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Vaquero A., Scher M., Erdjument-Bromage H., Tempst P., Serrano L., Reinberg D. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007;450:440–444. doi: 10.1038/nature06268. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 36.Rodgers J. T., Lerin C., Gerhart-Hines Z., Puigserver P. Metabolic adaptations through the PGC-1 α and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun C., Zhang F., Ge X., Yan T., Chen X., Shi X., Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J. Biol. Chem. 2007;282:34356–34364. doi: 10.1074/jbc.M706644200. [DOI] [PubMed] [Google Scholar]
- 39.Moynihan K. A., Grimm A. A., Plueger M. M., Bernal-Mizrachi E., Ford E., Cras-Meneur C., Permutt M. A., Imai S. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Machado De Oliveira R., Leid M., McBurney M. W., Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakahata Y., Kaluzova M., Grimaldi B., Sahar S., Hirayama J., Chen D., Guarente L. P., Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asher G., Gatfield D., Stratmann M., Reinke H., Dibner C., Kreppel F., Mostoslavsky R., Alt F. W., Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 43.Purushotham A., Schug T. T., Xu Q., Surapureddi S., Guo X., Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker A. K., Yang F., Jiang K., Ji J. Y., Watts J. L., Purushotham A., Boss O., Hirsch M. L., Ribich S., Smith J. J., et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 46.Kume S., Uzu T., Horiike K., Chin-Kanasaki M., Isshiki K., Araki S., Sugimoto T., Haneda M., Kashiwagi A., Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kume S., Haneda M., Kanasaki K., Sugimoto T., Araki S., Isono M., Isshiki K., Uzu T., Kashiwagi A., Koya D. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radical Biol. Med. 2006;40:2175–2182. doi: 10.1016/j.freeradbiomed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Rajamohan S. B., Pillai V. B., Gupta M., Sundaresan N. R., Birukov K. G., Samant S., Hottiger M. O., Gupta M. P. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol. Cell. Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim J. H., Lee Y. M., Chun Y. S., Chen J., Kim J. E., Park J. W. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1α. Mol. Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 50.Dioum E. M., Chen R., Alexander M. S., Zhang Q., Hogg R. T., Gerard R. D., Garcia J. A. Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 51.Yeung F., Hoberg J. E., Ramsey C. S., Keller M. D., Jones D. R., Frye R. A., Mayo M. W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee I. H., Cao L., Mostoslavsky R., Lombard D. B., Liu J., Bruns N. E., Tsokos M., Alt F. W., Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price N. L., Gomes A. P., Ling A. J., Duarte F. V., Martin-Montalvo A., North B. J., Agarwal B., Ye L., Ramadori G., Teodoro J. S., et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choudhury D., Levi M. Kidney aging – inevitable or preventable? Nat. Rev. Nephrol. 2011;7:706–717. doi: 10.1038/nrneph.2011.104. [DOI] [PubMed] [Google Scholar]
- 55.Imai S. Dissecting systemic control of metabolism and aging in the NAD world: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett. 2011;585:1657–1662. doi: 10.1016/j.febslet.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braidy N., Guillemin G. J., Mansour H., Chan-Ling T., Poljak A., Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in Wistar rats. PLoS ONE. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 58.Haase V. H. Hypoxia-inducible factors in the kidney. Am. J. Physiol. Renal Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haase V. H. The sweet side of HIF. Kidney Int. 2010;78:10–13. doi: 10.1038/ki.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makino H., Miyamoto Y., Sawai K., Mori K., Mukoyama M., Nakao K., Yoshimasa Y., Suga S. Altered gene expression related to glomerulogenesis and podocyte structure in early diabetic nephropathy of db/db mice and its restoration by pioglitazone. Diabetes. 2006;55:2747–2756. doi: 10.2337/db05-1683. [DOI] [PubMed] [Google Scholar]
- 61.Takiyama Y., Harumi T., Watanabe J., Fujita Y., Honjo J., Shimizu N., Makino Y., Haneda M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1α expression and oxygen metabolism. Diabetes. 2011;60:981–992. doi: 10.2337/db10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka T., Kato H., Kojima I., Ohse T., Son D., Tawakami T., Yatagawa T., Inagi R., Fujita T., Nangaku M. Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:795–805. doi: 10.1093/gerona/61.8.795. [DOI] [PubMed] [Google Scholar]
- 63.Nath K. A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 64.He W., Wang Y., Zhang M. Z., You L., Davis L. S., Fan H., Yang H. C., Fogo A. B., Zent R., Harris R. C., et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Invest. 2010;120:1056–1068. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J., Qu X., Ricardo S. D., Bertram J. F., Nikolic-Paterson D. J. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am. J. Pathol. 2010;177:1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hasegawa K., Wakino S., Yoshioka K., Tatematsu S., Hara Y., Minakuchi H., Washida N., Tokuyama H., Hayashi K., Itoh H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem. Biophys. Res. Commun. 2008;372:51–56. doi: 10.1016/j.bbrc.2008.04.176. [DOI] [PubMed] [Google Scholar]
- 67.Hasegawa K., Wakino S., Yoshioka K., Tatematsu S., Hara Y., Minakuchi H., Sueyasu K., Washida N., Tokuyama H., Tzukerman M., et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 2010;285:13045–13056. doi: 10.1074/jbc.M109.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim D. H., Jung Y. J., Lee J. E., Lee A. S., Kang K. P., Lee S., Park S. K., Han M. K., Lee S. Y., Ramkumar K. M., et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am. J. Physiol. Renal Physiol. 2011;301:F427–F435. doi: 10.1152/ajprenal.00258.2010. [DOI] [PubMed] [Google Scholar]
- 69.Chung H. Y., Sung B., Jung K. J., Zou Y., Yu B. P. The molecular inflammatory process in aging. Antioxid. Redox Signaling. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 70.Navarro-Gonzalez J. F., Mora-Fernandez C., Muros de Fuentes M., Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 71.Hayden M. S., Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 72.Kiernan R., Bres V., Ng R. W., Coudart M. P., El Messaoudi S., Sardet C., Jin D. Y., Emiliani S., Benkirane M. Post-activation turn-off of NF-κB-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 2003;278:2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- 73.Yoshizaki T., Milne J. C., Imamura T., Schenk S., Sonoda N., Babendure J. L., Lu J. C., Smith J. J., Jirousek M. R., Olefsky J. M. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol. Cell. Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoshizaki T., Schenk S., Imamura T., Babendure J. L., Sonoda N., Bae E. J., Oh D. Y., Lu M., Milne J. C., Westphal C., et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010;298:E419–E428. doi: 10.1152/ajpendo.00417.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajendrasozhan S., Yang S. R., Kinnula V. L., Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schug T. T., Xu Q., Gao H., Peres-da-Silva A., Draper D. W., Fessler M. B., Purushotham A., Li X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell. Biol. 2010;30:4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stein S., Schafer N., Breitenstein A., Besler C., Winnik S., Lohmann C., Heinrich K., Brokopp C. E., Handschin C., Landmesser U., et al. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging. 2010;2:353–360. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J., Zhou Y., Mueller-Steiner S., Chen L. F., Kwon H., Yi S., Mucke L., Gan L. SIRT1 protects against microglia-dependent amyloid-β toxicity through inhibiting NF-κB signaling. J. Biol. Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 79.Kitada M., Takeda A., Nagai T., Ito H., Kanasaki K., Koya D. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp. Diabetes Res. 2011;2011:908185. doi: 10.1155/2011/908185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawahara T. L., Michishita E., Adler A. S., Damian M., Berber E., Lin M., McCord R. A., Ongaigui K. C., Boxer L. D., Chang H. Y., Chua K. F. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hattori T., Shindo S., Kawamura H. Apoptosis and expression of Bax protein and Fas antigen in glomeruli of a remnant-kidney model. Nephron. 1998;79:186–191. doi: 10.1159/000045023. [DOI] [PubMed] [Google Scholar]
- 82.Kume S., Haneda M., Kanasaki K., Sugimoto T., Araki S., Isshiki K., Isono M., Uzu T., Guarente L., Kashiwagi A., Koya D. SIRT1 inhibits transforming growth factor β-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J. Biol. Chem. 2007;282:151–158. doi: 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- 83.Chuang P. Y., Dai Y., Liu R., He H., Kretzler M., Jim B., Cohen C. D., He J. C. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS ONE. 2011;6:e23566. doi: 10.1371/journal.pone.0023566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakagawa T., Tanabe K., Croker B. P., Johnson R. J., Grant M. B., Kosugi T., Li Q. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat. Rev. Nephrol. 2011;7:36–44. doi: 10.1038/nrneph.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mattagajasingh I., Kim C. S., Naqvi A., Yamamori T., Hoffman T. A., Jung S. B., DeRicco J., Kasuno K., Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kroemer G., Marino G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka Y., Kume S., Kitada M., Kanasaki K., Uzu T., Maegawa H., Koya D. Autophagy as a therapeutic target in diabetic nephropathy. Exp. Diabetes Res. 2012;2012:628978. doi: 10.1155/2012/628978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bellot G., Garcia-Medina R., Gounon P., Chiche J., Roux D., Pouyssegur J., Mazure N. M. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartleben B., Godel M., Meyer-Schwesinger C., Liu S., Ulrich T., Kobler S., Wiech T., Grahammer F., Arnold S. J., Lindenmeyer M. T., et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyazaki R., Ichiki T., Hashimoto T., Inanaga K., Imayama I., Sadoshima J., Sunagawa K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 92.Benigni A., Corna D., Zoja C., Sonzogni A., Latini R., Salio M., Conti S., Rottoli D., Longaretti L., Cassis P., et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rossier B. C., Pradervand S., Schild L., Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu. Rev. Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 94.Zhang D., Li S., Cruz P., Kone B. C. Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate α-ENaC transcription in collecting duct. J. Biol. Chem. 2009;284:20917–20926. doi: 10.1074/jbc.M109.020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moorhead J. F., Chan M. K., El-Nahas M., Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;ii:1309–1311. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 96.Ruan X. Z., Varghese Z., Moorhead J. F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 2009;5:713–721. doi: 10.1038/nrneph.2009.184. [DOI] [PubMed] [Google Scholar]
- 97.Sun L., Halaihel N., Zhang W., Rogers T., Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J. Biol. Chem. 2002;277:18919–18927. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 98.Proctor G., Jiang T., Iwahashi M., Wang Z., Li J., Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502–2509. doi: 10.2337/db05-0603. [DOI] [PubMed] [Google Scholar]
- 99.Wang Z., Jiang T., Li J., Proctor G., McManaman J. L., Lucia S., Chua S., Levi M. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328–2335. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 100.Wang X. X., Jiang T., Shen Y., Caldas Y., Miyazaki-Anzai S., Santamaria H., Urbanek C., Solis N., Scherzer P., Lewis L., et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59:2916–2927. doi: 10.2337/db10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang T., Wang X. X., Scherzer P., Wilson P., Tallman J., Takahashi H., Li J., Iwahashi M., Sutherland E., Arend L., Levi M. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56:2485–2493. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- 102.Wang X. X., Jiang T., Shen Y., Adorini L., Pruzanski M., Gonzalez F. J., Scherzer P., Lewis L., Miyazaki-Anzai S., Levi M. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am. J. Physiol. Renal Physiol. 2009;297:F1587–F1596. doi: 10.1152/ajprenal.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X. X., Jiang T., Levi M. Nuclear hormone receptors in diabetic nephropathy. Nat. Rev. Nephrol. 2010;6:342–351. doi: 10.1038/nrneph.2010.56. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka Y., Kume S., Araki S., Isshiki K., Chin-Kanasaki M., Sakaguchi M., Sugimoto T., Koya D., Haneda M., Kashiwagi A., et al. Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int. 2011;79:871–882. doi: 10.1038/ki.2010.530. [DOI] [PubMed] [Google Scholar]
- 105.Li X., Zhang S., Blander G., Tse J. G., Krieger M., Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 106.Kemper J. K., Xiao Z., Ponugoti B., Miao J., Fang S., Kanamaluru D., Tsang S., Wu S. Y., Chiang C. M., Veenstra T. D. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burton C., Harris K. P. The role of proteinuria in the progression of chronic renal failure. Am. J. Kidney Dis. 1996;27:765–775. doi: 10.1016/s0272-6386(96)90512-0. [DOI] [PubMed] [Google Scholar]
- 108.Thomas M. E., Schreiner G. F. Contribution of proteinuria to progressive renal injury: consequences of tubular uptake of fatty acid bearing albumin. Am. J. Nephrol. 1993;13:385–398. doi: 10.1159/000168653. [DOI] [PubMed] [Google Scholar]
- 109.Koyama T., Kume S., Koya D., Araki S., Isshiki K., Chin-Kanasaki M., Sugimoto T., Haneda M., Sugaya T., Kashiwagi A., et al. SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radical Biol. Med. 2011;51:1258–1267. doi: 10.1016/j.freeradbiomed.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 110.Maeda S., Koya D., Araki S., Babazono T., Umezono T., Toyoda M., Kawai K., Imanishi M., Uzu T., Suzuki D., et al. Association between single nucleotide polymorphisms within genes encoding sirtuin families and diabetic nephropathy in Japanese subjects with type 2 diabetes. Clin. Exp. Nephrol. 2011;15:381–390. doi: 10.1007/s10157-011-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]