Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular disease. One contributory factor may be hemodynamic stress due to the negative intrathoracic pressure during each episode of apnea. Type B natriuretic peptide (BNP) is secreted by the cardiac ventricles in response to volume expansion and pressure load and the authors hypothesized that there would be an association between indices of OSA during the night and levels of BNP in the morning.
Setting:
Community-based in Uppsala, Sweden.
Participants:
There were 349 women who participated.
Measurements and Results:
Participants underwent full-night polysomnography and anthropometric measurements, and answered questionnaires about medical conditions and current medication. The morning after the polysomnography, blood samples were drawn for analysis of plasma BNP, C-reactive protein, creatinine, and hemoglobin. There was an increase in mean BNP as the severity of sleep apnea increased, increasing from a mean value of 8.5 ng/L among women with an apnea-hypopnea index (AHI) < 5 to 18.0 ng/L in women with an AHI ≥ 30. Elevated BNP levels (≥ 20 ng/L) were found in 29.8% of the women, whereas 70.2% had normal levels. The odds ratio was 2.2 for elevated BNP levels for women with an AHI of 5–14.9 in relation to women with an AHI < 5, 3.1 for women with an AHI of 15–29.9, and 4.6 for women with an AHI ≥ 30 after adjustment for age, body mass index, systolic blood pressure, antihypertensive drugs, and creatinine.
Conclusions:
There is a dose-response relationship in women between the severity of sleep apnea during the night and the levels of BNP in the morning.
Citation:
Ljunggren M; Lindahl B; Theorell-Haglöw J; Lindberg E. Association between obstructive sleep apnea and elevated levels of type B natriuretic peptide in a community-based sample of women. SLEEP 2012;35(11):1521-1527.
Keywords: Community-based, epidemiology, heart failure, polysomnography, sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by a recurrent partial or complete collapse of the upper airway during sleep. In combination with excessive daytime sleepiness, the condition is known as obstructive sleep apnea syndrome (OSAS), affecting 3%–7% of adult males and 2%–5% of adult women.1 OSAS is associated with an increased risk of cardiovascular disease and mortality2 but the underlying pathophysiologic mechanisms are not fully understood. One of the mechanisms that has been suggested as a contributing factor is the hemodynamic stress caused by the negative intrathoracic pressure during each apnea episode caused by attempts to breathe against an occluded airway.3
Type B natriuretic peptide (BNP), a diuretic and vasodilatory hormone, is secreted by the cardiac ventricles in response to volume expansion and pressure load.4 In everyday practice, BNP and N-terminal probrain natriuretic peptide (NT-pro-BNP) serve as diagnostic and prognostic markers of heart failure. In community-based studies, elevated levels of natriuretic peptides have also been reported to represent an increased risk of cardiovascular events and death.5,6
In 1998, Kita et al.7 reported increasing levels of BNP during sleep in patients with OSAS and reduced BNP levels with continuous positive airway pressure (CPAP) treatment. Subsequent studies have produced conflicting data, as a significant association between sleep apnea and natriuretic peptides has been reported by some8,9 but not by others.10–15 Most of these studies are small, based on symptomatic patients who are referred to sleep clinics, and generally include men or only few women. Little is known about the association between OSA and BNP in the general population.
The aim of this study was to analyze the possible association between OSA during the night and levels of BNP the following morning in a community-based sample of women.
METHODS
Population
In Phase I of the population-based study “Sleep and Health in Women” that started in the year 2000, a random sample of women age 20 years and older was sent a postal questionnaire with 109 questions on sleep disturbances and somatic disorders. The women were randomly selected from the population registry of the city of Uppsala, Sweden. The response rate was 71.6%, with a total of 7,051 women responding to the questionnaire. Based on their answer to the question “How often do you snore loudly and disturbingly?” the participants were categorized as nonsnorers (answering “never”, “seldom” or “sometimes”, n = 6,515) or snorers (answering “often” or “very often”, n = 518).
Of the total study population age 70 years and younger (n = 6,112), a random sample of 170 women from the entire study population and a random sample of 230 snorers participated in Phase II of the study. Women who were expected not to manage to perform the ambulatory recordings because of severe somatic or psychiatric disease were excluded.16 The current study is based on the Phase II cohort.
Study Protocol
All 400 participants underwent full-night polysomnography in their own home or at the patients' hotel at the hospital (see following paragraphs). In the evening before the polysomnography the participants arrived at the Sleep Laboratory at Uppsala University Hospital, where the polysomnography equipment was set up. They completed a questionnaire, which included specific questions about hypertension, myocardial infarction, chronic heart failure, angina pectoris, stroke, diabetes, and current medication. Daytime sleepiness was assessed by the Epworth Sleepiness Scale17 and a value of 10 or more was regarded as excessive daytime sleepiness. The women were also given vessels in which to collect their urine during the night of the polysomnography. The next morning they returned to the hospital while fasting, the polysomnography equipment was removed, and urine volume was measured. Blood samples were drawn and blood pressure was taken in the right arm after 15 min of rest in the supine position. The participants' height and weight were measured and body mass index (BMI) was calculated. Waist circumference was measured midway between the lower rib margin and the anterior superior iliac while standing and the sagittal abdominal diameter was measured while supine with the back against the underlying surface.18 An electrocardiogram was taken to detect atrial fibrillation.
The blood samples for C-reactive protein (CRP), creatinine, hemoglobin, and BNP, all taken between 07:00 and 09:00 the morning after the polysomnography, were analyzed at the Department of Clinical Chemistry and Pharmacology at Uppsala University. The plasma levels of BNP were analyzed using an immunoradiometric assay (Shionoria BNP, Shionogi, Japan). The lower detection limit was 4 ng/L and a value of ≥ 20 ng/L was regarded as elevated, in accordance with the reference value of the method.
Plasma BNP values are available for 350 of the 400 women. This is due to a change in the analysis method for BNP at the laboratory during the study period and problems obtaining blood from some participants. One woman did not have an acceptable polysomnography recording, leaving a total study population of 349 individuals.
Polysomnography
The polysomnography was conducted using the ambulatory EMBLA system (Flaga Inc., Iceland) with 16 channels: two electroencephalography leads (C3-A2, C4-A1), two electrooculography leads, three electromyography leads (submental, left and right anterior tibialis muscles), two airflow leads (oronasal thermistor and nasal flow pressure sensor), two respiratory effort leads from piezoelectric belts (thoracic and abdominal), two electrocardiography leads, one pharyngeal sound lead, one oximeter lead, and one body position lead. Data were downloaded to the Somnologica reviewing analysis software (Version 2.0; Flaga Inc.) and sleep was scored manually in 30-sec epochs according to the standard criteria of Rechtschaffen and Kales.19
An obstructive apnea was defined as the complete cessation of nasal and oral airflow lasting 10 sec or more with continuing abdominal and thoracic movements. An obstructive hypopnea was defined as a ≥ 50% reduction in both oronasal thermistor and nasal pressure for at least 10 sec, compared with baseline, accompanied by abdominal and thoracic movements, in combination with a desaturation of ≥ 3% or an arousal. The apnea-hypopnea index (AHI) was calculated as the mean number of obstructive apneas and hypopneas per hour of sleep. The oxygen desaturation index (ODI) was defined as the mean number of desaturations of ≥ 4% per hour of sleep. Central apneas were scored at the cessation of both oronasal thermistor and nasal pressure for 10 sec without respiratory movements, in combination with an oxygen desaturation of 3% or more. In addition, data on mean oxygen saturation, lowest oxygen saturation, time with saturation below 90%, 80%, and 70%, respectively, and AHI with different levels of accompanying hypoxia was obtained from the polysomnography recordings.
Statistical Methods
Statistical analyses were performed using Stata 10.0 (Stata Corporation, College Station, TX). Plasma BNP levels below the lower detection limit, 4 ng/L, were given a value of 2 ng/L. Comparisons of baseline data between groups were performed using the unpaired t-test for normally distributed variables, the Mann-Whitney U test for continuous variables that were not normally distributed, and the chi-square test for proportions. Association between plasma BNP and variables of OSA were analyzed with linear regression, first in a univariate model and then in two multivariate models: the first adjusting for age and BMI and the second also adjusting for systolic blood pressure, antihypertensive drugs, and P-creatinine. Before performing the calculations, the continuous variables that were not normally distributed (p-BNP, AHI, ODI, lowest oxygen saturation, and mean oxygen saturation) were log-transformed using the base-10 log. Censoring BNP values less than the lower limit of detection at a value of 2 ng/L may result in a left censored data set. To address this issue, Spearman rank correlation test and Spearman partial rank correlation tests were performed. Plasma BNP was divided into two categories, normal (< 20 ng/L) or elevated (≥ 20 ng/L) levels, whereas the AHI and ODI was divided into four categories based on commonly used clinical thresholds: zero to < 5, 5–14.9, 15–29.9, and ≥ 30 events/hr. The association between elevated levels of BNP and AHI groups and ODI groups, respectively, was analyzed using logistic regressions. To examine the association between sleep disordered breathing across desaturation thresholds, analyses were conducted with AHI across different oxyhemoglobin desaturation thresholds. Each multivariable model for a specific desaturation threshold also included a term that accounted and adjusted for sleep disordered breathing events with oxyhemoglobin desaturation above the threshold being examined. The null hypothesis was rejected at a level of P < 0.05.
The study was approved by the Ethics Committee at the Medical Faculty at Uppsala University (approval number 99486) and all the participants gave their informed consent.
RESULTS
Of the 349 participants, 121 (34.7%) had a normal AHI (defined as an AHI < 5), 113 (32.4%) had an AHI of 5–14.9, and 77 (22.1%) had an AHI of 15–29.9, whereas severe sleep apnea, with an AHI of ≥ 30, was found in 38 women (10.9%).
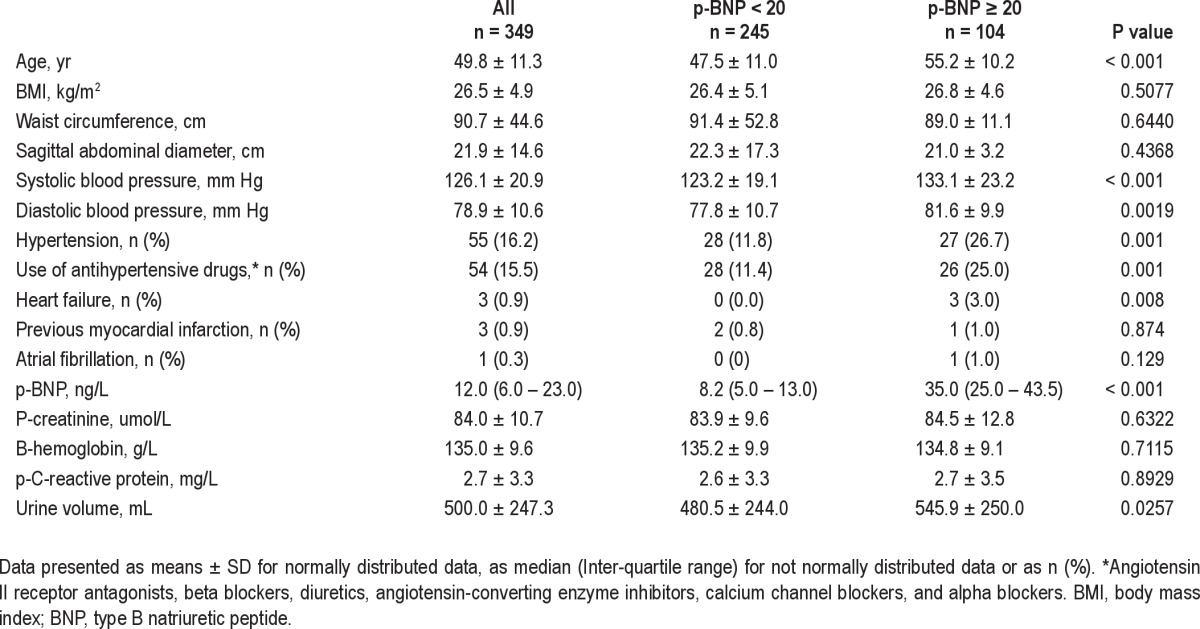
The plasma levels of BNP ranged from the lower detection level of < 4 ng/L to 113 ng/L, with a mean value of 11.4 ng/L. Elevated plasma BNP levels (≥ 20 ng/L) were found in 104 women (29.8% of the study population) whereas 245 participants (70.2%) had normal levels. The baseline characteristics are presented in Table 1. Participants with elevated plasma BNP levels were significantly older, had higher blood pressure, and were more frequently on antihypertensive medication (Table 1). There were no significant differences in BMI or central obesity or in the levels of hemoglobin, creatinine, or CRP between the two groups. A high level of plasma BNP was associated with somewhat higher urine volumes during the night.
Table 1.
Baseline characteristics
There was an increase in mean BNP as the severity of sleep apnea increased from a mean value of 8.5 ng/L among women with an AHI < 5 to 18.0 ng/L in women with severe sleep apnea with an AHI ≥ 30 (P for the trend < 0.001). A box plot showing the distribution of BNPs level in the AHI groups is presented in Figure 1.
Figure 1.
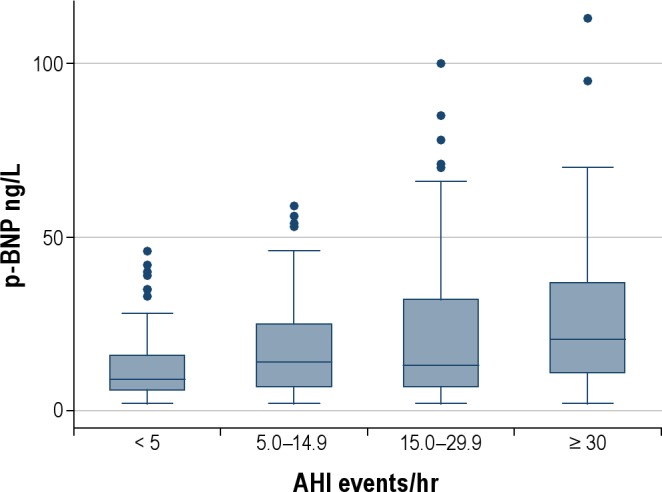
Distribution of BNP by AHI group. The box is the 25th – 75th percentile (including 50% of the participants), the line in the box is the median, the whiskers the 5th to 95th percentile, and the dots the outliers.
When the population was subdivided into normal or increased BNP, OSA was more common among women with increased BNP. On the other hand, the occurrence of daytime sleepiness did not differ significantly between the two groups (Table 2). The number of central apnea episodes was low in the entire study population.
Table 2.
Sleep disordered breathing and associations with p-BNP levels
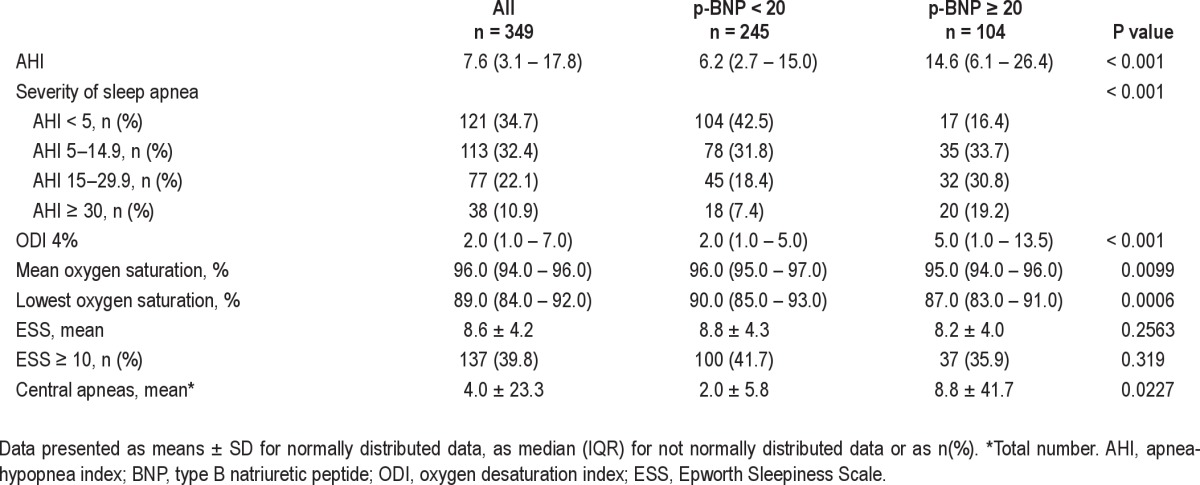
In the univariate regression model, there was a linear association between variables of sleep apnea and plasma BNP. For AHI and ODI, this association remained after adjustment for the confounders of age and BMI, as well as after adjustment for systolic blood pressure, antihypertensive drugs, and plasma creatinine (Table 3). Results from the Spearman rank correlation test and Spearman partial rank correlation tests (addressing the possible issue of a left censored data set) showed no difference compared with the results from the multiple linear regression models (data not shown).
Table 3.
Linear regression model with dependent variable p-BNP
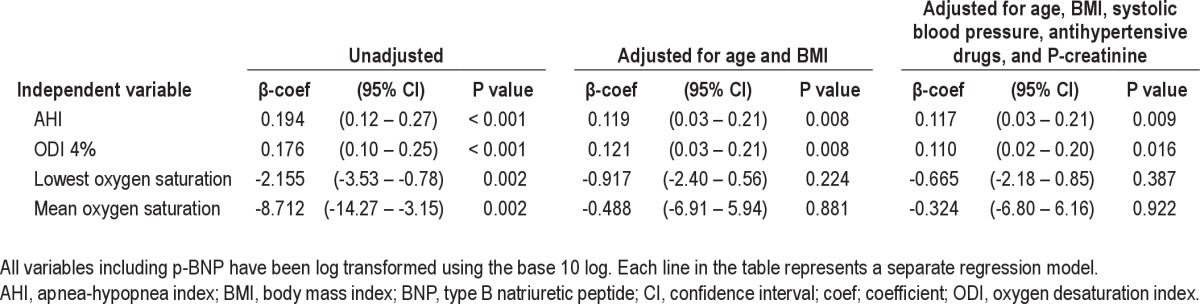
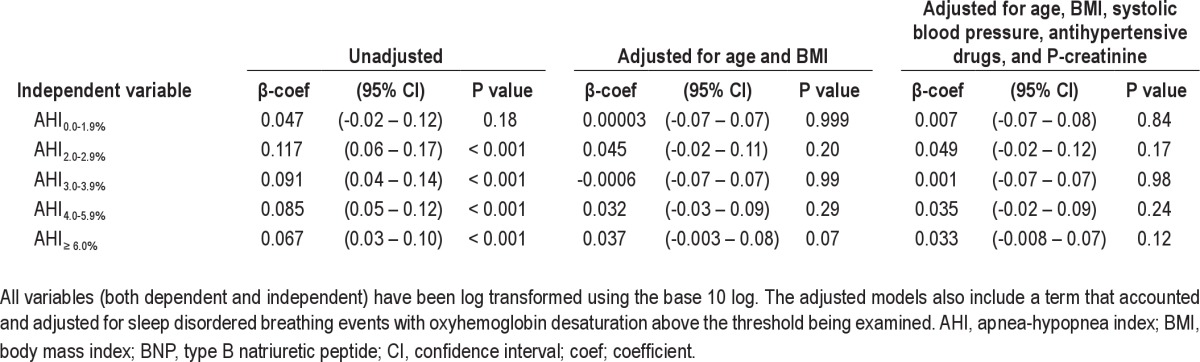
There was a dose-response relationship between increasing severity of sleep apnea and elevated BNP. This relationship remained after adjustment for confounders (Table 4). A similar dose-response relationship was seen between elevated BNP and ODI with adjusted odds ratios of 1.7 (0.9–3.2; 95% confidence interval [CI]), 3.0 (1.2–7.2) and 4.2 (1.2–14.8) for ODI 5–14.9, 15–29.9, and ≥ 30, respectively. To further analyze how different levels of hypoxia were associated with BNP, linear regression analyses were performed using AHI with different levels of accompanying hypoxia as independent variables. However, these analyses showed no associations with BNP after adjusting for confounders (Table 5).
Table 4.
Results of logistic regression analysis with dependent variable p-BNP > 20
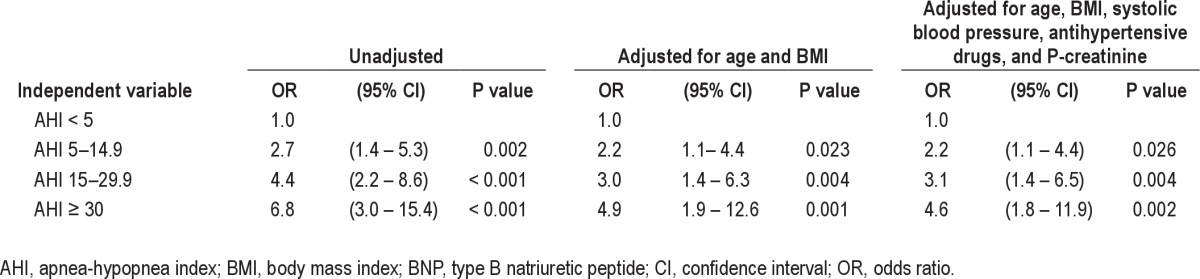
Table 5.
Linear regression model with dependent variable p-BNP
In the entire group, three persons had known heart failure and all of them had elevated levels of BNP (23 ng/L, 70 ng/L, and 100 ng/L). One of the participants with known heart failure had experienced a myocardial infarction. Another two women reported having had a myocardial infarction, but they had no known heart failure and normal levels of BNP. Atrial fibrillation was detected in one woman and she also had an elevated BNP level (25 ng/L). All significant associations reported in Tables 3 and 4 remained when these six women were excluded from the calculations.
DISCUSSION
The main finding in this study is that, in a community-based sample of women, there is a dose-response relationship between the severity of sleep apnea during the night and the levels of plasma BNP in the morning. This association could not be explained by known confounding variables such as age, BMI, blood pressure, or renal function.
Our findings are in accordance with some previous studies. Kita et al.7 found an increase in the plasma levels of BNP during the night in patients with severe OSAS, and treatment with nasal CPAP reduced the elevated levels of BNP during sleep. Their study population was limited to 12 male and 2 female patients. Moreover, in a Greek study in which children with habitual snoring were investigated with overnight polysomnography, Kaditis et al.9 found that an overnight increase in BNP levels was correlated with the severity of sleep apnea during sleep.
In the current study there was also a dose-response relationship between ODI levels and elevated BNP, although there was no significant association between lowest oxygen saturation, mean oxygen saturation, or AHI with different levels of hypoxia and the dependent variable BNP. The dose-response relationship between ODI levels and elevated BNP may indicate that intermittent hypoxia can play a role in the relationship between OSAS and BNP; however, there was no association between AHI with different levels of hypoxia and BNP. These results are both in agreement with and in discrepancy with results by Gottlieb et al.,20 who showed that frequency of sleep apnea and intermittent hypoxia was not related to elevated BNP whereas sustained hypoxia was. The fact that the study by Gottlieb et al. was performed in patients with heart failure as opposed to our population-based sample may explain our somewhat different findings.
There are also some studies in which no association between sleep disordered breathing and BNP has been found. Möller et al.,10 in a study comprising 24 patients (22 men and 2 women) with OSAS and 18 control participants, and Svatikova et al.,11 in a study comprising 10 men with moderate to severe OSAS, did not find any association between BNP and OSAS. These differences in results may be explained by differences in population groups and size. There is also one community-based study, where no association between OSA and the levels of BNP was found.12 In the cited study, however, the median length of time between the polysomnography and natriuretic peptide testing was 79 days, which may have affected the result, because both OSA and BNP levels may vary over time.
In everyday practice, BNP has often been replaced by the more stable NT-pro-BNP as a marker of cardiac failure. There are some studies of NT-proBNP and OSAS. Hübner et al.14 found no association between the levels of NT-pro-BNP and AHI in 60 patients with suspected OSAS. In addition, Vartany et al.13 found no relationship between AHI and overnight changes in NT-pro-BNP in patients with severe obesity (mean BMI 38.2) and OSAS. There are some differences between the physiologically active peptide BNP and its inactive split metabolite NT-pro-BNP, including a longer half-life of NT-pro-BNP.21 Whether these differences can explain the disparity in the association with sleep apnea remains unclear. The effect of male or female sex on the association between natriuretic peptides and sleep apnea is also unclear. The only other study, as far as we know, with a study population made up exclusively of women, found an association between NT-pro-BNP and sleep disordered breathing,8 in accordance with our findings. However, this study did not use polysomnography and sleep disordered breathing was defined by the Berlin Questionnaire.
BNP is produced and secreted from the myocytes in response to cardiac wall stretch. Hypoxia has also been reported to induce BNP secretion.22 In our study, there was a strong association between AHI and morning levels of plasma BNP, which might be explained by a change in intrathoracic pressure during the nightly apnea episodes. The negative intrathoracic pressure during each apnea episode increases ventricular transmural pressure and left ventricular afterload, as well as the end-systolic and end-diastolic volume, giving rise to both pressure load and volume expansion.23 Increased sympathetic nervous activity caused by repetitive apnea episodes with arousal and hypoxia,24 which leads to peripheral vasoconstriction and tachycardia, may also contribute. The increased left ventricular transmural pressure increases myocardial oxygen demand and reduces coronary blood flow and, in combination with the apnea-related hypoxia, may lead to myocardial ischemia and impaired cardiac contractility.23 Even in asymptomatic individuals without known cardiovascular disease, elevated levels of BNP are associated with an increased risk of cardiovascular events, heart failure, and death.25 OSAS is an independent risk factor for hypertension26 and is associated with an increased risk of ischemic heart disease, stroke, and heart failure.27–31 The cutoff value of BNP in our study is lower than the thresholds used clinically to diagnose heart failure. However, when used in community screening, BNP values above 20 ng/L are suggested to trigger additional evaluation32 and the increased risk of cardiovascular events and death has been demonstrated at these levels as well.5 Whether the elevated levels of BNP in our study are an indication of a future risk of heart failure and cardiovascular events, or whether OSA is a variable affecting BNP, such as sex and weight, remains to be investigated. However, considering the known association between OSAS and cardiovascular disease, it is tempting to suggest that BNP could serve as a risk marker to identify persons with OSA who run an increased risk of future cardiovascular events.
The strengths of this study include the large community-based sample, standardized polysomnographic data, adjustments for relevant confounding covariates, and the fact that all the blood samples were collected the morning after the polysomnography. The limitations of our study include the fact that, because it only included women, the results cannot be generalized to apply to men and that many of our participants were taking antihypertensive drugs. We made adjustments for the use of antihypertensive drugs, but we did not distinguish between different antihypertensive drug classes.
We conclude that, in a community-based sample of women, there is a dose-response relationship between the severity of sleep apnea during the night and the levels of plasma BNP in the morning that cannot be explained by known confounding factors.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Lindahl has received research grants from Roche Diagnostics and Radiometer A/S, lecture fees from Roche Diagnostics and Siemens Healthcare Diagnostics and is member of the scientific advisory boards of Siemens Healthcare Diagnostics, Philips Healthcare and Beckman Coulter Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported financially by the Swedish Heart Lung Foundation and the Uppsala County Association Against Heart and Lung Diseases. The authors thank Lars Berglund, MSc, PhD, UCR-Uppsala Clinical Research Center, for his statistical advice and Carin Sahlin, BSc, PhD, Department of Respiratory Medicine, Umeå University Hospital, for her assistance with the polysomnography scoring in this study. Work for this study was performed at Department of Medical Sciences, Respiratory Medicine and Allergology, Uppsala University, Sweden.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- BNP
type B natriuretic peptide
- CPAP
continuous positive airway pressure
- CRP
C-reactive protein
- EEG
electroencephalography
- EMG
electromyography
- EOG
electrooculography
- ESS
Epworth Sleepiness Scale
- NT-proBNP
N-terminal probrain natriuretic peptide
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- OSAS
obstructive sleep apnea syndrome
REFERENCES
- 1.Lindberg E. Epidemiology of OSA. Eur Respir Mon. 2010;50:51–68. [Google Scholar]
- 2.Parati G, Lombardi C, Narkiewicz K. Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1671–83. doi: 10.1152/ajpregu.00400.2007. [DOI] [PubMed] [Google Scholar]
- 3.Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429–37. doi: 10.1016/s0735-1097(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 4.Boerrigter G, Costello-Boerrigter L, Burnett JC., Jr Natriuretic peptides in the diagnosis and management of chronic heart failure. Heart Fail Clin. 2009;5:501–14. doi: 10.1016/j.hfc.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 6.Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 7.Kita H, Ohi M, Chin K, Noguchi T, Otsuka N, Tsuboi T, et al. The nocturnal secretion of cardiac natriuretic pepitdes during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res. 1998;7:199–207. doi: 10.1046/j.1365-2869.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- 8.Ybarra J, Planas F, Navarro-López F, Pujadas S, Pujadas J, Jurado J, et al. Association between sleep-disordered breathing, aminoterminal pro-brain natriuretic peptide (NT-proBNP) levels and insulin resistance in morbidly obese young women. Eur J Intern Med. 2009;20:174–81. doi: 10.1016/j.ejim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Kaditis AG, Alexopoulos EI, Hatzi F, Kostadima E, Kiaffas M, Zakynthinos E, et al. Overnight change in brain natriuretic peptide levels in children with sleep-disordered breathing. Chest. 2006;130:1377–84. doi: 10.1378/chest.130.5.1377. [DOI] [PubMed] [Google Scholar]
- 10.Möller DS, Lind P, Strunge B, Pedersen EB. Abnormal vaosactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274–80. doi: 10.1016/s0895-7061(02)03267-3. [DOI] [PubMed] [Google Scholar]
- 11.Svatikova A, Shamsuzzaman AS, Wolk R, Philips BG, Olson LJ, Somers VK. Plasma brain natriuretic peptide in obstructive sleep apnea. Am J Cardiol. 2004;94:529–32. doi: 10.1016/j.amjcard.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Patwardhan AA, Larson MG, Levy D, Benjamin EJ, Leip EP, Keyes MJ, et al. Obstructive sleep apnea and plasma natriuretic peptide levels in a community-based sample. Sleep. 2006;29:1301–6. doi: 10.1093/sleep/29.10.1301. [DOI] [PubMed] [Google Scholar]
- 13.Vartany E, Imevbore M, O'Malley M, Manfredi C, Pasquarella C, Scinto L, et al. N-terminal pro-brain natriuretic peptide for detection of cardiovascular stress in patients with obstructive sleep apnea syndrome. J Sleep Res. 2006;15:424–9. doi: 10.1111/j.1365-2869.2006.00551.x. [DOI] [PubMed] [Google Scholar]
- 14.Hübner RH, El Mokhtari NE, Freitag S, Rausche T, Göder R, Tiroke A, et al. NT-proBNP is not elevated in patients with obstructive sleep apnoea. Respir Med. 2008;102:134–42. doi: 10.1016/j.rmed.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Tasci S, Manka R, Scholtyssek S, Lentini S, Troatz C, Stoffel-Wagner B, et al. NT-pro-BNP in obstructive sleep apnea syndrome is decreased by nasal continuous positive airway pressure. Clin Res Cardiol. 2006;95:23–30. doi: 10.1007/s00392-006-0315-9. [DOI] [PubMed] [Google Scholar]
- 16.Theorell-Haglöw J, Berne C, Janson C, Lindberg E. Obstructive sleep apnoea is associated with decreased insuline sensitivity in females. Eur Respir J. 2008;31:1054–60. doi: 10.1183/09031936.00074907. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Riserus U, Arnlov J, Brismar K, Zethelius B, Berglund L, Vessby B. Sagittal abdominal diameter is a strong anthropometric marker of insulin resistance and hyperproinsulinemia in obese men. Diabetes Care. 2004;27:2041–6. doi: 10.2337/diacare.27.8.2041. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages in human subjects. Washington DC: US National Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 20.Gottlieb JD, Schwartz AR, Marshall J, Ouyang P, Kern L, Shetty V, et al. Hypoxia, not the frequency of sleep apnea, induces acute hemodynamic stress in patients with chronic heart failure. J Am Coll Cardiol. 2009;54:1706–12. doi: 10.1016/j.jacc.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner J, Guglin M. BNP or NTproBNP? A clinician's perspective. Int J Cardiol. 2008;129:5–14. doi: 10.1016/j.ijcard.2007.12.093. [DOI] [PubMed] [Google Scholar]
- 22.Woodard GE, Rosado JA. Natriuretic peptides in vascular physiology and pathology. Int Rev Cell Mol Biol. 2008;268:59–93. doi: 10.1016/S1937-6448(08)00803-4. [DOI] [PubMed] [Google Scholar]
- 23.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–27. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 24.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Angelantonio E, Chowdhury R, Sawar N, Ray KK, Gobin R, Saleheen D, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–87. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 26.Butt M, Dwivedi G, Khair O, Lip GY. Obstructive sleep apnea and cardiovascular disease. Int J Cardiol. 2010;139:7–16. doi: 10.1016/j.ijcard.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–4. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 28.Peker Y, Kraiczi H, Hedner J, Löth S, Johansson Å, Bende M. An independent association between obstructive sleep apoea and coronary artery disease. Eur Respir J. 1999;13:179–84. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 29.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 30.Munoz R, Duran-Cantolla J, Martínez-Vila E, Gallego J, Rubio R, Aizpuru F, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 31.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Naresh M, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough PA, Omland T, Maisel AS. B-type natriuretic peptides: a diagnostic breakthrough for clinicans. Rev Cardiovasc Med. 2003;2:72–80. [PubMed] [Google Scholar]


