Abstract
Continuous exposure to oxygen is essential for nearly all vertebrates. We found that embryos of the zebrafish Danio rerio can survive for 24 h in the absence of oxygen (anoxia, 0% O2). In anoxia, zebrafish entered a state of suspended animation where all microscopically observable movement ceased, including cell division, developmental progression, and motility. Animals that had developed a heartbeat before anoxic exposure showed no evidence of a heartbeat until return to terrestrial atmosphere (normoxia, 20.8% O2). In analyzing cell-cycle changes of rapidly dividing blastomeres exposed to anoxia, we found that no cells arrested in mitosis. This is in sharp contrast to similarly staged normoxic embryos that consistently contain more than 15% of cells in mitosis. Flow cytometry analysis revealed that blastomeres arrested during the S and G2 phases of the cell cycle. This work indicates that survival of oxygen deprivation in vertebrates involves the reduction of diverse processes, such as cardiac function and cell-cycle progression, thus allowing energy supply to be matched by energy demands.
Most animals are very sensitive to reduced levels of oxygen. Known vertebrate responses to low oxygen concentrations (hypoxia) include changes in carbohydrate metabolism, an increase in nitric oxide, and stimulation of red blood cell and hemoglobin production (1). Hypoxia can also induce the expression of a select set of genes, which includes glycolytic enzymes, glycoprotein hormone erythropoeitin, and the inducible nitric oxide synthase (1, 2). Hypoxia-inducing factor has been shown to play a central role in this transcriptional response (3, 4). Extreme hypoxia is central to the pathology of several diseases involving cardiac and pulmonary dysfunction (5). Additionally, it is known that in solid tumors, cancerous cells that are hypoxic are often more resistant to radiation and chemotherapy (6). Identification of the response that organisms have to low oxygen tension may facilitate the development of treatment for rescue or prevention of damaged ischemic tissue or for the destruction of tumor cells with low oxygen tensions (7).
Given the central role of oxygen in physiology, several animal model systems have been developed to understand the response that organisms have to reduced oxygen levels. It has been shown that some invertebrates, such as Caenorhabditis elegans, Artemia franciscana, and Drosophila melanogaster, have the ability to survive in the absence of molecular oxygen (anoxia) (8–11). The brine shrimp, A. franciscana, has been shown to survive 4 years of continuous anoxia, exhibiting an arrest of development, a decrease in intracellular pH, a reduction in protein synthesis, and an accumulation of heat shock proteins (10, 12). It has been shown that both C. elegans and D. melanogaster can survive at least 1 day of anoxia exposure by arresting development until oxygen supply is reestablished (9, 11). The survival of anoxia likely depends on the organism's ability to curb energy usage by shutting down nonessential cellular functions, maintaining stable and low permeability of membranes, and synthesizing ATP by glycolytic processes (13, 14). Recent studies in D. melanogaster and mammalian tissue have demonstrated that the nitric oxide/cGMP signaling pathway is involved in the response to oxygen deprivation (15–17).
To better understand the response of organisms to oxygen deprivation, we tested the possibility that zebrafish would be capable of surviving anoxia. Here, we present evidence that zebrafish embryos survive anoxia by entering into a state of suspended animation, where all microscopically observable movement ceases. We suggest this is a natural response that increases the fitness of this fish.
Materials and Methods
Maintenance of Zebrafish.
Embryos were obtained by mating three females and two males. Embryos were carefully staged as described (18) and kept separate to create populations of synchronized embryos. For all experiments, embryos were incubated in Petri dishes with ≈15 ml of fish water at 28.5°C, unless otherwise stated.
Oxygen Deprivation Environments.
For all studies, we used the anaerobic bio-bag type A environmental chamber according to the manufacturer's instructions (Becton Dickinson). This method contains a resazurin indicator that allows one to determine when the anoxic environment is established. We used a second method to verify suspended animation results. This method involved use of a chamber perfused with 100% N2 gas (Airgas or Byrne Gas, Seattle) and monitored for oxygen by using a fyrite O2 gas analyzer (Bacharach, Pittsburgh) and resazurin indicator (Becton Dickinson). By using these anoxia-producing methods, it took approximately 2 h for the oxygen concentration to reach zero. Development of embryos could continue for another 1–2.5 h, depending on the amount of water present. This suggests that a small amount of oxygen remained in the water during the first few hours of the experiment. We used a third method to determine whether embryos could be quickly arrested. Fish water (25 ml) was preconditioned in anoxia for 24 h by using an anaerobic bio-bag type A. Embryos at the 8- to 16-cell stage were collected and quickly transferred into the preconditioned anoxic water by using a nitex filter (TEKTO, Elmsford, NY) to eliminate transfer of oxygenated water. The time of exposure of the preconditioned anoxic water to room air during embryo transfer was less than 1 min. Given that opening the chamber results in some oxygen entry, it took ≈20 min for the resazurin indicator inside the chamber to indicate an anoxic environment. These embryos remained in this environment for 24 h (26°C) and were then quickly removed and fixed; the nuclei were then stained (see below).
Viability Assays.
Embryos were synchronized and collected during specific stages of embryogenesis and subjected to anoxia for 24 h. Embryo viability was scored, on return to a normoxic environment, by having the ability to develop to the larval stage with swim bladders. To control for the small number of embryos that die during embryogenesis independent of oxygen availability, the viability of control embryos in normoxia was used as a standard to compare with embryos exposed to anoxia. At least 50 embryos, at the two-cell stage or shield stage, which were subjected to 24 h of anoxia, were allowed to recover in air and were then raised to sexually mature adults. Fish from these populations were mated and determined to have the capacity to produce offspring.
Staining of Nuclei.
Young (16-cell) embryos were placed in a Petri dish with just enough water to keep them hydrated. This reduced the time period until developmental arrest and enabled better imaging of embryo nuclei. Control embryos (normoxia) were collected and fixed when the experimental embryos arrested development, ≈2.5 h after introduction into the anoxic producing chamber, at approximately the sphere stage of embryogenesis. Experimental embryos remained in the anoxic environment for 24 h and were either immediately fixed (anoxia) or allowed to recover in air for 2 h before fixation (postanoxia). The method used to stain nuclei is similar to that previously described (19). Embryos were fixed in 4% formaldehyde in PBS for 3 h, followed by a wash with, and incubation in, PBS. Embryos were dechorionated and deyolked carefully with forceps. The mass of embryonic cell caps was incubated with the DNA-binding dye 4′,6-diamidino-2-phenylindole (DAPI) for ≈20 min and washed once with block buffer (3% BSA/0.1% Tween 20/2 mM MgCl2 in PBS). Microscopy was done on a Zeiss axioscope. Images were collected and analyzed by using ADOBE PHOTOSHOP 5.5. Determination of the number of interphase or mitotic blastomeres present in control and anoxia-exposed embryos was done by examining more than 1,000 nuclei from each of several anoxic and control embryos.
Flow Cytometric DNA Content Analysis.
Four-cell embryos were collected and exposed to either a normoxic or an anoxic environment. Embryos exposed to anoxia arrested development at the shield stage of embryogenesis, ≈4.5 h after initiation of producing the anoxic environment. Embryos exposed to anoxia for 24 h were either immediately analyzed (anoxia) or recovered in air for 2 h before analysis (postanoxia). Control embryos were analyzed at the shield stage of embryogenesis. The method used to analyze zebrafish DNA content by flow cytometry analysis was previously described (20), with the exception that DNA was stained with DAPI. The nuclear suspensions were analyzed by the LSR flow cytometer (Becton Dickinson), and the DNA histograms were analyzed by CELL QUEST and MODFIT LT (Ver. 2, Verity Software House, Topsham, ME).
Results and Discussion
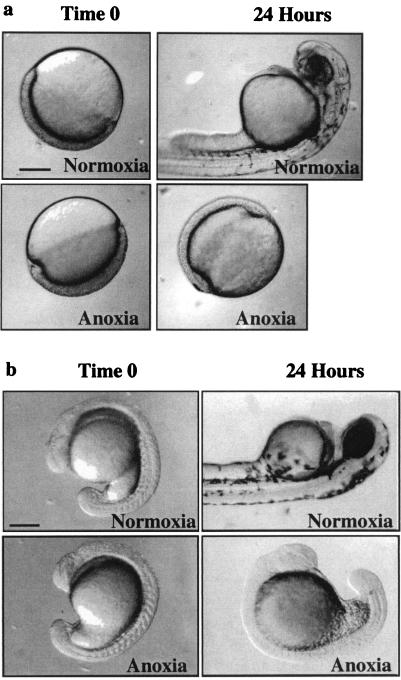
The ability to survive anoxia (0% O2) has been observed in small invertebrate organisms that lack a circulatory system and are therefore able to rapidly adapt to changes in oxygen levels (11, 21). Fully developed vertebrates that rely on circulatory systems instead of diffusion may be unable to adjust rapidly enough to survive anoxia. Here, we show that zebrafish embryos exposed to an anoxic environment, in normal culture conditions and temperature, enter a state of suspended animation that can be maintained for 24 h without deleterious effect (see Materials and Methods). In the anoxic environment, development stopped (Fig. 1). On reexposure to a normoxic (20.8% O2) environment, embryos continued with development. To determine whether exposure to anoxia caused any long-term effects, we raised 100 embryos, which were exposed to anoxia, to sexual maturity. We found that they were able to produce offspring and were indistinguishable from fish raised under normal conditions.
Figure 1.
Zebrafish embryos arrest when exposed to anoxia. (a) Four-cell embryos were collected and exposed to either a normoxic environment (air, 20.8% O2) or an anoxic (0% O2) environment. (b) Five-somite stage embryos were collected and exposed to either a normoxic or an anoxic environment for 24 h. Time 0 is the time when embryos began to arrest development, ≈4.5 h after initiation of producing an anoxic environment. The second time point is 24 h after time 0. Images were collected and analyzed by using ADOBE PHOTOSHOP 5.5. (Bar, ≈200 μm.)
Several invertebrates that survive anoxia are only able to do so at specific times in development (11, 12). To define developmental stages when zebrafish embryos can survive anoxia, embryos at the periods of cleavage, blastula, gastrula, segmentation, straightening, and hatching were collected and subjected to anoxia for 24 h (22). Zebrafish embryos 25 h postfertilization (h.p.f.) and younger were capable of surviving 24 h of anoxia (Table 1). As embryos progress through development to the period of straightening (30 h.p.f.), the length of time that they could survive anoxia was reduced. Animals older than 48 h.p.f. were quite sensitive to anoxia (Table 1). This transition to anoxia sensitivity indicates that zebrafish are useful for studying the developmental acquisition of anoxia sensitivity.
Table 1.
Zebrafish viability in anoxia
| Period | Hours after fertilization* | Percent alive after 24 h of anoxia (N) |
|---|---|---|
| Cleavage | 2 | 83.1 (89) |
| Blastula | 4 | 83.2 (85) |
| Gastrula | 6 | 97.7 (90) |
| Segmentation | 13 | 98.8 (85) |
| Straightening | ||
| Early | 25 | 64.0 (100) |
| Middle | 30 | 4.4 (91) |
| Hatching | 50 | 0 (130) |
N is total number of embryos.
h.p.f. when placed in the anoxic environment.
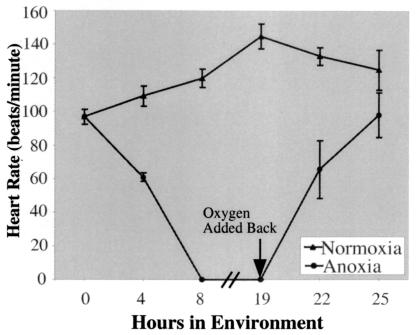
Zebrafish embryos exposed to anoxia had a great reduction in motility such as whole body movement and heartbeat. For example, 29 h.p.f. embryos exposed to anoxia displayed stopping of the heartbeat at 28.5°C, which normally beats at ≈100 beats/min (23) (Fig. 2). If these embryos were exposed to the anoxic environment for less than 8 h, the heartbeat could return within several minutes on exposure to oxygen. However, if the embryos were exposed to anoxia for 19 h, it took ≈6 h of exposure to air for the heart rate to return to normal (Fig. 2). There are rare situations in nature when heartbeat can cease for long periods of time without detrimental effects to the organism. For example, the freeze-tolerant frogs (Rana sylvatica and Hyla veriscolor) and turtle (Chrysemys picta) display a stopping of heartbeat and blood flow at cold temperatures. Heartbeat is reestablished in these species on thawing (24, 25). It will be of interest to determine the genetic mechanisms allowing a vertebrate species to survive periods of time without functional heartbeat and circulation.
Figure 2.
Zebrafish embryos arrest heartbeat when exposed to anoxia. Twenty-nine h.p.f. embryos were collected and subjected to either a normoxic or an anoxic environment. Embryos were exposed to anoxia for the times indicated and then removed. Heart rate was immediately determined by visual inspection on at least five embryos per data point. Embryos exposed to anoxia for 19 h were allowed to recover in air, and heart rate of these recovering embryos was determined.
To determine whether zebrafish embryos in a state of recoverable suspended animation arrest at a specific point in the cell cycle, we compared embryos exposed to anoxia with untreated embryos. Untreated embryos contained blastomeres with both mitotic and interphase nuclei (Fig. 3) (19). In contrast, blastomeres of anoxic embryos arrested in interphase but not mitosis (Fig. 3). We also saw no mitotic cells when embryos from other stages of development (25 and 30 h.p.f.) were placed into anoxia (data not shown). In addition, we found that the chromosomal DNA in anoxia-treated embryos is not uniformly distributed throughout the nucleus, as it is in normal embryos (Fig. 3). Similar results were obtained when embryos were rapidly transferred to anoxic conditions (see Materials and Methods). These results may indicate that anoxia exposure reduces transcription and DNA synthesis that is known to cause redistribution of the DNA in the nucleus. When arrested embryos were allowed to recover, they progressed in development with a frequency of mitotic cells comparable to untreated embryos (Fig. 3). That zebrafish embryos arrest in interphase and not mitosis contrasts with studies of Drosophila, where embryos exposed to oxygen deprivation arrest during interphase, prophase, metaphase, and telophase (11, 26, 27).
Figure 3.
Cell biology analysis of anoxia-exposed zebrafish embryos. Embryos were collected and exposed to either a normoxic or an anoxic environment. Control embryos (normoxia) were collected and fixed when the experimental embryos arrested development. Experimental embryos remained in the anoxic environment for 24 h and were either immediately fixed (anoxia) or allowed to recover in air for 2 h before fixation (postanoxia). White arrowheads point to examples of blastomeres in mitosis, and white arrows point to examples of blastomeres in interphase. [Bar, ≈50 μm (Upper) and 10 μm for enlarged image of interphase nuclei (Lower).]
To identify where in interphase arrest occurs, we analyzed DNA content by flow cytometry analysis. The control embryos showed a characteristic cell-cycle pattern for zebrafish embryos past the midblastula stage (Fig. 4) (20). The majority of cells from embryos exposed to anoxia arrested throughout S phase. G0/G1 cells appeared to be absent, and there were more blastomeres in the G2 phase compared with untreated embryos at the same stage of development (Fig. 4). As expected, cells with G1 DNA content were detected in the anoxia-exposed embryos after they were allowed to recover in normoxia for 2 h (data not shown). Together, the absence of mitosis observed by DAPI staining and flow cytometry analysis show that anoxia exposure causes cells to arrest in the S and G2 phases of the cell cycle.
Figure 4.
Flow cytometry analysis of anoxia-exposed zebrafish embryos. Embryos were collected and exposed to either a normoxic or an anoxic environment. Control embryos (normoxia) were at the shield stage of embryogenesis. Experimental embryos, arrested at the shield stage of embryogenesis, remained in the anoxic environment for 24 h and were immediately processed for flow cytometry analysis (anoxia). Regions 1 (R1) and 2 (R2) were defined to exclude debris and clumps of nuclei. DNA content histograms were determined by the fluorescent height histograms of gated events.
The amount of DNA in the arrested S-phase nuclei was highly variable, indicating that there are many different points in S phase that arrest can occur. This result contrasts with mammalian cell work that shows the majority of cells arrest in G0/G1 (28–31). There are at least two possible explanations for the S and G2 phase arrest we see in zebrafish. The first is that a checkpoint could be activated, in S and G2, when oxygen levels are reduced. Alternatively, the establishment of an anoxic environment can take 4.5 h, and the average length of the cell cycle is relatively short (≈80 min) at this time in development (32). If S and G2 phases have a higher requirement for oxygen than other points in the cell cycle, it could account for the observed results.
The work presented here indicates that vertebrate defense strategies for survival of oxygen deprivation are pervasive and involve many independent cellular processes. The finding that in zebrafish a state of suspended animation is established in response to oxygen deprivation is consistent with previous work of others by using D. melanogaster, C. elegans, and A. franciscana (9–11, 15). Unlike D. melanogaster blastomeres, zebrafish blastomeres do not arrest in mitosis, suggesting that there are vertebrate-specific responses to oxygen deprivation. One general hypothesis to account for the capacity to survive anoxia is that low oxygen levels reduce oxidative phosphorylation that, in turn, results in a reduction of ATP and cellular processes (14). If this hypothesis is correct, it will be of interest to determine how the reduction in ATP is matched with reduction of activity, and how these processes are reversed.
The capacity of vertebrates to respond to environmental changes by arresting development has been shown previously. In nature, almost 100 mammalian species have the capacity to arrest embryo development (33). This phenomenon has been referred to as diapause or delayed implantation (33, 34). The ecological result of diapause is an enhancement of reproductive fitness by increasing the gestation period, thus allowing young to be born at optimal times of the year (33, 34). It is possible that the ability of an organism to arrest development in response to anoxia also enhances reproductive fitness. It is therefore intriguing to speculate that the suspended animation resulting from oxygen deprivation may be related to embryonic diapause.
Acknowledgments
We thank Cecelia Moens, Landon Moore, Sue Biggins, Brian Buchwitz, Debbie Frank, Todd Nystul, Jeff Stear, Meng-Chao Yao, and Laurie Roth for discussions and comments regarding work in this article. We thank Andrew Berger and Michelle Black for help with the flow cytometry analysis. This work was supported in part by National Institutes of Health Grant GM48435 (to M.B.R.) and by a National Science Foundation postdoctoral research fellowship (to P.A.P.).
Abbreviations
- h.p.f.
hours postfertilization
- DAPI
4′,6-diamidino-2-phenylindole
References
- 1.Guillemin K, Krasnow M A. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 2.Iyer N V, Kotch L E, Agani F, Leung S W, Laughner E, Wenger R H, Gassmann M, Gearhart J D, Lawler A M, Yu A Y, Semenza G L. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza G L. Cell. 1999;98:281–284. doi: 10.1016/s0092-8674(00)81957-1. [DOI] [PubMed] [Google Scholar]
- 4.Semenza G L. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 5.Semenza G L. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 6.Brown J M. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- 7.Lipton P. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 8.Anderson G L. Can J Zool. 1978;56:1786–1791. [Google Scholar]
- 9.Van Voorhies W A, Ward S. J Exp Biol. 2000;203:2467–2478. doi: 10.1242/jeb.203.16.2467. [DOI] [PubMed] [Google Scholar]
- 10.Hand S C. In: Surviving Hypoxia Mechanisms of Control and Adaptation. Hochachka P W, Lutz P L, Sick T, Rosenthal M, van den Thillart G, editors. Boca Raton, FL: CRC; 1993. pp. 171–185. [Google Scholar]
- 11.Foe V E, Alberts B M. J Cell Biol. 1985;100:1623–1636. doi: 10.1083/jcb.100.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clegg J. J Exp Biol. 1997;200:467–475. doi: 10.1242/jeb.200.3.467. [DOI] [PubMed] [Google Scholar]
- 13.Hochachka P W. Science. 1986;231:234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- 14.Hochachka P W, Buck L T, Doll C J, Land S C. Proc Natl Acad Sci USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wingrove J A, O'Farrell P H. Cell. 1999;98:105–114. doi: 10.1016/S0092-8674(00)80610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clementi E, Brown G C, Foxwell N, Moncada S. Proc Natl Acad Sci USA. 1999;96:1559–1562. doi: 10.1073/pnas.96.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giulivi C. Biochem J. 1998;332:673–679. doi: 10.1042/bj3320673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westerfield M. The Zebrafish Book: Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene, OR: Univ. of Oregon Press; 1995. [Google Scholar]
- 19.Yager T D, Ikegami R, Rivera-Bennetts A K, Zhao C, Brooker D. Biochem Cell Biol. 1997;75:535–550. [PubMed] [Google Scholar]
- 20.Zamir E, Kam Z, Yarden A. Mol Cell Biol. 1997;17:529–536. doi: 10.1128/mcb.17.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochachka P W, Lutz P L, Sick T, Rosenthal M, van den Thillart G E. Surviving Hypoxia Mechanisms of Control and Adaptation. Boca Raton, FL: CRC; 1993. [Google Scholar]
- 22.Kimmel C B, Ballard W W, Kimmel S R, Ullmann B, Schilling T F. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 23.Baker K, Warren K S, Yellen G, Fishman M C. Proc Natl Acad Sci USA. 1997;94:4554–4559. doi: 10.1073/pnas.94.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storey K B. Am J Physiol. 1990;258:R559–R568. doi: 10.1152/ajpregu.1990.258.3.R559. [DOI] [PubMed] [Google Scholar]
- 25.Storey K B. Comp Biochem Physiol A Physiol. 1997;117:319–326. doi: 10.1016/s0300-9629(96)00270-8. [DOI] [PubMed] [Google Scholar]
- 26.DiGregorio P J, Ubersax J A, O'Farrell P H. J Biol Chem. 2001;276:1930–1937. doi: 10.1074/jbc.M003911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas R M, Xu T, Haddad G G. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1555–R1563. doi: 10.1152/ajpregu.2001.280.5.R1555. [DOI] [PubMed] [Google Scholar]
- 28.Krtolica A, Krucher N A, Ludlow J W. Oncogene. 1998;17:2295–2304. doi: 10.1038/sj.onc.1202159. [DOI] [PubMed] [Google Scholar]
- 29.Green S L, Freiberg R A, Giaccia A J. Mol Cell Biol. 2001;21:1196–1206. doi: 10.1128/MCB.21.4.1196-1206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner L B, Li Q, Park M S, Flanagan W M, Semenza G L, Dang C V. J Biol Chem. 2000;276:7919–7926. doi: 10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
- 31.Amellem O, Pettersen E O. Cell Prolif. 1991;24:127–141. doi: 10.1111/j.1365-2184.1991.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 32.Kane D A. In: Methods in Cell Biology. Detrich H W III, Westerfield M, Zon L I, editors. Vol. 59. New York: Academic; 1999. pp. 11–26. [DOI] [PubMed] [Google Scholar]
- 33.Renfree M B, Shaw G. Annu Rev Physiol. 2000;62:353–375. doi: 10.1146/annurev.physiol.62.1.353. [DOI] [PubMed] [Google Scholar]
- 34.Mead R A. J Exp Zool. 1993;266:629–641. doi: 10.1002/jez.1402660611. [DOI] [PubMed] [Google Scholar]