Abstract
Study Objectives:
The objectives of this study were to characterize sleep patterns and determine factors, including sex, age, season, and environmental pressures, that influence sleep in the endangered black rhinoceros (rhino; Diceros bicornis bicornis).
Design:
To noninvasively observe sleep behavior of wild rhinos, digital infrared cameras were erected on poles at two bedding sites from September 2009 to March 2010.
Setting:
The study site was located in South Africa's Addo Elephant National Park (AENP) in the Main Camp (Addo) and Nyathi sections.
Participants:
A total of 2,417 photos captured rhino sleep behavior on eight individual rhinos during 40 separate sleeping bouts (Addo, n = 15; Nyathi, n = 25).
Inventions:
N/A.
Results:
Results demonstrated that age and season did not affect rhino sleep behavior (P > 0.05); however, sex did influence the length of sleep bouts with males (n = 27; mean, 105.6 ± 11.3 min; range, 14.0–202.0 min) sleeping longer (F1,48 = 6.93, P = 0.01) than females (n = 13; mean, 58.6 ± 10.4 min; range, 11.0–132.0 min). Park section did not influence the length of sleep episodes, but did affect (rw40 = 0.88; P < 0.025) the time at which rhinos slept (Addo, 20:00–24:00; Nyathi, 20:00–04:00).
Conclusions:
This is the first study to characterize sleep behavior in wild black rhinos. This study resulted in a greater understanding of the biologic factors that affect sleep in wild rhinos and can provide information to assist their management and conservation.
Citation:
Santymire R; Meyer J; Freeman EW. Characterizing sleep behavior of the wild black rhinoceros (Diceros bicornis bicornis). SLEEP 2012;35(11):1569-1574.
Keywords: Black rhinoceros, in situ population, predation risks, sex, sleep characterization
INTRODUCTION
The fundamental theory of sleep is constantly debated and considered one of the most important unsolved mysteries of science.1 However, all animals studied to date engage in some form of sleep, making it one of the most prevalent animal behaviors.2 Definitions of sleep in vertebrates are typically based on behavioral and electrophysiologic criteria.3 Behaviorally, sleep is distinguished by (1) a distinctive sleeping site; (2) a species-specific body posture; (3) physical quiescence2–4 characterized by reduced motor activity5; (4) an elevated arousal threshold,2–4 which suggests reduced motor and sensory brain activity5; (5) a rapid ability to reverse the sleep/wake state2–4; and (6) rebound sleep, the homeostatic regulation of sleep.2,3,6 These behavioral factors correlate strongly with electroencephalographic (EEG) changes.3,4 Sleep can be separated into two states based on electroencephalogram (EEG) activity: rapid eye movement (REM) and nonrapid eye movement (NREM).3
Although the function of sleep may not be fundamentally different among mammalian species,3 sleep patterns vary across species and within an individual's life span.7 The length of sleep bouts ranges widely among mammals from approximately 6.5 min in laboratory rats (Rattus norvegicus) and up to 3.3 hrs lemurs (Lemur macaco fulvus).4 Total sleep time (TST) also varies across mammals; from 15–20 h/day in bats (Eptesicus fuscus, Myotis lucifugus, Cynopterus sphinx, Eonycteris spelaea) and opossums (Didelphis marsupialis) to 3 to 4 h/day in giraffe (Giraffa camelopardalis reticulata) and elephants (Elephas maximus; Loxodonta africana).4,6,8 Additionally, time of sleep relative to the 24-h light-dark (LD) cycle varies among species, with patterns being monophasic, biphasic, or polyphasic.2,3
Sleep parameters are dynamic and adaptively respond to differences in trophic levels, sex, and environmental and physiologic variation.1,2 For instance, sleep time is correlated with diet.6 Herbivores demonstrate the shortest recorded TST over a 24-h period, with omnivores having a higher TST and carnivores the highest.6 For most mammals, TST over a 24-h period correlates positively with basal metabolic rate and negatively with brain weight and body weight.3,6 Few studies in animals have directly examined the influence of sex on sleep,8 and those that have, observed differences in sleep patterns.9–12 The different energy requirements of males and females, such as parental investment and care,13 may affect the amount of awake time for each sex.9–12 Varying sleep patterns between the sexes also may be controlled by reproductive hormones14 and environmental influences and stressors.11,14,15
Most systematic studies of mammalian sleep have been conducted on a handful of domesticated species, including rats, mice, cats, dogs, and monkeys.7,16 However, there are some studies of sleep in large herbivores that have occurred ex situ. For instance, in a zoo study of 14 southern white rhinos, most sleep bouts lasted less than 2 hours, with 75% of resting occurring at 22:00–02:00.17 In another zoo study three adult and one juvenile elephants were documented to participate in standing sleep 110–150 min/day and recumbent sleep 200–260 min/day.3 Additionally, 12 female Asian elephants (including one juvenile) from circuses (n = 7) and zoos (n = 5)18 had sleep bouts that included 13.8–130.9 min of standing sleep and an average duration of 72 min of recumbent sleep between 21:00 and 04:00 hours. Finally, six adult zoo giraffe slept approximately 40 min standing and approximately 230 min recumbently.3
Although sleep studies of large mammals have been conducted in zoos, sleep patterns in mammals under naturalistic conditions are lacking.7 Studying sleep behavior of animals in their natural environment is difficult, especially when the animals are diurnal or crepuscular. However, we cannot assume that laboratory and/or zoo-based results reflect true sleep patterns19 because the constant food and reduced stress of no predators or environmental changes in captivity may alter sleep patterns. Thus, it is critical that more sleep studies are conducted in the field, recording animals in the natural environments in which they evolved and providing insight into the evolutionary factors that influence when, where, and how species sleep.16 In addition, it would be interesting to study large herbivores, which appear to sleep relatively little, because their vulnerability to predators and other environmental stressors make it maladaptive for them to experience long periods of the reduced sensory responsiveness that defines sleep.7
The current study was aimed at collecting normative sleep data from wild black rhinoceros (Diceros bicornis bicornis) in Addo Elephant National Park (AENP), South Africa. Black rhinos are currently listed as critically endangered by the International Union for Conservation of Nature (IUCN) Red List, with only 4,200 rhinos surviving in the wild.20 Due to small populations and the secretive nature of rhinos, many aspects of their ecology and behavior remain poorly understood. Black rhinos are crepuscular in their movements and have bedding sites within their home ranges, which they visit during the daytime to rest when temperatures are high.21,22 However, there are no measurable data on the sleeping behavior of black rhinos nor any documentation of black rhinos sleeping during the night in either captive or wild populations. Variability in sleep patterns does occur within species; therefore, we investigated the influence of sex, age, and the season (wet versus dry) on sleep patterns and duration. Additionally, sleep patterns were compared across two sections of AENP, which differ greatly in biotic and abiotic factors and thus could create differences in sleep behavior between the subpopulations. Understanding the biologic factors that affect sleep patterns in a wild population of black rhinos will not only enhance the world of sleep science, but also the management of this highly endangered species.
METHODS
Study Area
The study site was located in the Eastern Cape of South Africa at the AENP. The AENP was originally founded in 1931 to protect the remaining 11 elephants in the area.23 The black rhinoceros subspecies, of the arid-adapted western ecotype D bicornis bicornis, was extirpated from the Eastern Cape when the last individual was shot in 1858.24 The first black rhino was reintroduced to Addo in 1961 and of the East African ecotype Diceros bicornis michaeli.25 In 1995, black rhinoceros of the indigenous ecotype D bicornis bicornis were reintroduced into the AENP and the D bicornis michaeli rhino were slowly removed. The AENP has now expanded to multiple sections, with three containing black rhinos. Our study focused on two sections, Addo's Main Camp and the Nyathi.
The Addo Main Camp (Addo) section of AENP currently consists of 11,500 ha of habitat that ranges from xeric subtropical succulent thicket to open, grassy plains23,26 and receives on average 445 mm of rain per year.27 Water within is pumped from a reservoir, ensuring a continuous water supply to seven main waterholes. There also are natural water pans throughout Addo that are filled by seasonal rainfall. Addo has a higher density of elephants, 3.58 elephants/km2 (J. Meyer, personal observation, AENP, December 2010), and predators, including lion (Panthera leo) and spotted hyena (Crocuta crocuta), than the Nyathi section and also receives the greatest number of tourists.
Nyathi is located 1 km north of the Addo section and the two are separated by fences and a highway. Nyathi is composed of 14,000 ha of grassland and thicket that receives an annual rainfall of 445–600 mm.27 The Coerney River, an ephemeral river that flows for a few months after heavy rains, provides the main water source; additionally, multiple dams and pans are dispersed throughout Nyathi. The density of elephants in Nyathi is low (0.71 elephants/km2; J. Meyer, personal observation, AENP, December 2010) relative to the Addo section. Like Addo, Nyathi is rich in ungulates, but there are no large predators (such as lion or hyena). Additionally, Nyathi is not open to the general public; only visitors of two concessionaires are allowed to traverse this section of the park.
Rhino Population
There were 44 black rhinos managed in the two sections of AENP, Addo: n = 18 (nine females, eight males, one unsexed) and Nyathi: n = 26 (11 females, 13 males, two unsexed). Each rhino is darted approximately 2 to 4 yr of age, and given a name and a specific pattern of ear notches that can be used to identify individuals. Prior to notching, rhinos can be positively identified by other anatomic features such as their size, horn, and bodily scars.
Characterizing Sleep Patterns
In each section, there was one known bedding site that was frequently used by black rhinos. The bedding site in Addo was in a 2 × 4 m open sand pit surrounded by broken bush (Figure 1A). In Nyathi, an old burn site provided a 2 × 3 m ash pit surrounded by broken bush where the rhino frequently slept (Figure 1B). Both sites were similar to previously described black rhino bedding sites, which included a bare patch of earth enclosed by a canopy of trees or bushes.28,29 Digital camera traps (ScoutGuard 550V, HCO, Norcross, GA, USA) were erected on poles at these bedding sites and camouflaged with bushes. Due to the remoteness of the landscape, photographs were chosen over video to ensure that batteries would remain charged between visits to the camera. A high-quality digital photograph was quietly taken with an infrared flash when the passive motion detector on the camera was activated. The camera was set to take three photographs when it was triggered and reset itself every 15 sec. Each photograph was labeled with the date and time. Photographs were recovered from the camera and batteries checked every other day.
Figure 1.

Examples of camera trap images of black rhinoceros sleeping in the (A) Addo and (B) Nyathi sections of Addo Elephant National Park, South Africa.
It has been difficult to define sleep in large herbivores.3 In elephants (E maximus),18 giraffe (Giraffa camelopardalis),30 and white rhinos (Ceratotherium simum),17 sleep has been defined as recumbent sleep and standing sleep. In this study, we only observed rhinos in recumbent sleep, with legs straight out (Figure 1B) or on their hunches (Figure 1A), similar to the preferred positions of the elephant and horse (Equus caballus).3 Although it was not possible to confirm an animal was in paradoxical sleep from the photographs, sleeping bouts could be assumed based on the rhino's appearance and recumbent position across successive images. Therefore, sleeping bouts were calculated from the time on the photograph when the rhino first lay down until the time when the rhino stood up to leave the bedding site. Small movements in the ears or legs of sleeping animals would cause the camera to trigger and take up to 300 photographs during a sleeping bout, thus visually documenting the duration of the recumbent sleep session.
Data Collection
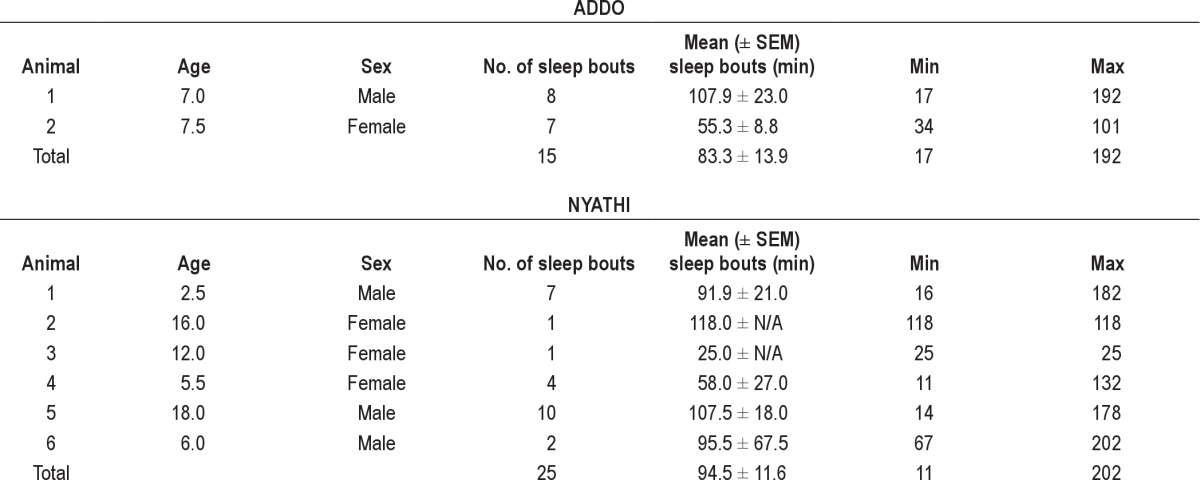
Data were collected from September 2009 to March 2010 from eight rhinos (Table 1) that slept in front of camera traps placed at the two bedding sites. A total of 2,417 photographs (Addo, n = 1,626; Nyathi, n = 914) captured rhino behavior during 40 separate sleeping bouts (Addo, n = 15; Nyathi, n = 25). The average age of the rhinos involved in the study was 10.3 ± 2.4 yr for females and 8.4 ± 3.4 yr for males. Images were collected of two calves that slept with their mothers, one in each section of AENP, but they were not used in our analyses.
Table 1.
Description of individual black rhinos and sleeping bouts measured in two sections (Addo, Nyathi) of Addo Elephant National Park, South Africa
Data Analysis
Data were analyzed using SigmaStat (v. 11.0 2008, Systat Software, San Jose, CA). A Kolmogorov-Smirnov test was used for normality assumption testing and the Levene median test for equal variance assumption testing. For nonnormal data, a nonparametric test was used. A t-test was used unless data were not normal; otherwise, a Mann-Whitney rank-sum test was used. When these data permitted, analysis of variance (ANOVA) was used. Comparisons of means among treatments were performed using Tukey test. If the normality failed, a Kruskal-Wallis ANOVA on ranks was used and a Dunn test was used to compare ranks. For all analyses, P < 0.05 was considered significant and data were reported as mean ± standard error of the mean (SEM).
Black rhinos were categorized into three age classes, calf (0–3 yr), subadult (3–7 yr), and adult (7+ yr). Calves are individuals that are still with their mothers and suckling until age 3 yr; after this time they cease nursing and become independent. At approximately 7 yr of age, rhinos become reproductively active and are considered adults; all animals between weaning and reproductive maturity are considered subadults. Of the eight rhinos in this study, there were five adults (three females, two males), two subadults (one female, one male), and one male calf without his mother. A one-way ANOVA was used to compare among age categories. Precipitation data were provided by the South African Weather Station in Port Elizabeth. The months of data recorded spanned over the wet (November-April) and dry (May-October) seasons. To determine if there was a seasonal effect on sleep patterns, ANOVA was used to look for differences between seasons within rhinos.
To determine whether male and female rhinos had distinct sleep patterns, we took the hour for each sleeping bout and converted them into an angular direction on a circular scale. The uniform distribution of each male's and female's sleep bout around the circle was tested using the Rayleigh test.31,32 Then, the mean time of male and female was compared using the Watson-Williams test.31 Determination of distinct sleeping patterns between the park sections also was conducted using these methods.
RESULTS
Black rhinos in the AENP were recorded in a recumbent sleep position for 90.3 ± 9.0 min (n = 40; range, 11.0 to 202.0 min; Table 1) on average. Overall, sleeping bouts did not vary (F5,32 = 1.77, P = 0.15) among individuals; therefore, each sleeping bout recorded was considered an individual bout (Table 1). Sex did influence the length of sleep with males (n = 27; mean, 105.6 ± 11.3 min; range, 14.0–202.0 min) sleeping longer (t-test, t38 = −2.63 = 6.93, P = 0.012) than female rhinos (n = 13; mean, 58.6 ± 10.4 min; range, 11.0–132.0 min). There was no difference (Kruskal-Wallis ANOVA on ranks, H2 = 0.318, P = 0.853) in the length of sleep bouts among the age categories: calf, 91.9 ± 21.0 min (n = 7; range, 16–182 min); subadult, 83.5 ± 29.2 min (n = 6; range, 11–202 min); and adult 91.4 ± 10.7 min (n = 27; range, 14–192 min; Table 1). The section had no effect (Kruskal-Wallis ANOVA on ranks, H1 = 0.34, P = 0.56) on the sleeping duration of the rhino: Addo (n = 15; mean, 83.3 ± 13.9 min; range, 17–192 min) and Nyathi (n = 25; mean, 94.5 ± 11.6 min; range, 11.0–202.0 min). Additionally, wet/dry seasons did not affect (F1,36 = 0.08, P = 0.77) the duration of the sleep bout with no interaction (F1,36 = 2.68, P = 0.11) between sex and season. Males (n = 21; 114.0 ± 12.3 min; range, 16.0–202.0 min) had a sleep duration (F1,36 = 2.516, P = 0.12) similar to females (n = 9; 50.4 ± 11.6 minutes; range, 11.0–132.0 min) in the wet compared with the dry season (males, n = 6: 76.0 ± 25.4 min, range, 14.0–18.0 min; females, n = 4, 77.0 ± 20.5 min, range, 26.0–118.0 min).
Overall, rhinos slept between 20:00–04:00. Sex did not affect the time of day that the rhinos slept (rw40 = 0.87; P > 0.10). However, within female black rhinos, sleep patterns at our bedding sites were distinct (r13 = 0.94; P < 0.001), with sleep occurring between 20:00 and 24:00. Similar results were observed in males (r27 = 0.83; P < 0.001) with sleep at our sites occurring between 20:00 and 04:00. However, the time at which the rhinos slept varied (rw40 = 0.88; P < 0.025) between the sections, with the Addo rhinos sleeping between 20:00 and 24:00 and Nyathi between 20:00 and 04:00 (Figure 2).
Figure 2.
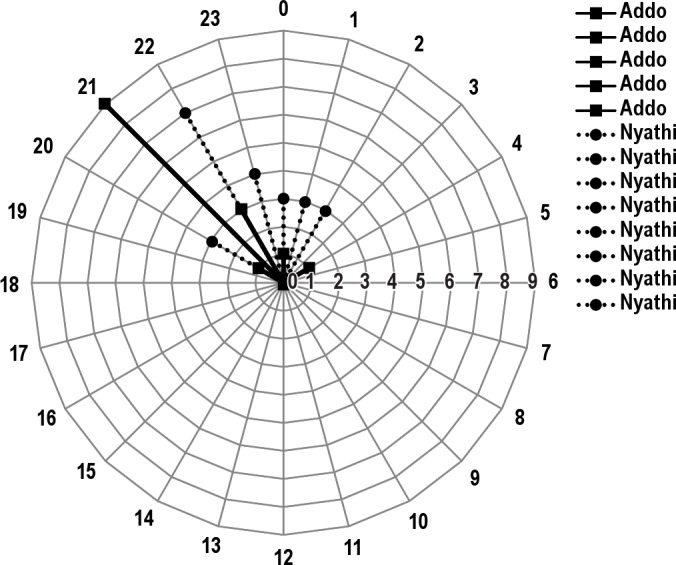
The light/dark (LD) cycle pattern for black rhinos that slept in bedding sites in Addo Elephant National Park, South Africa. Circular statistics determined that LD varied (rw40 = 0.88; P < 0.025) between the two sections monitored, Addo (n = 15) and Nyathi (n = 25).
DISCUSSION
This is the first study to characterize sleep behavior in wild black rhinos. Interestingly, age and season did not affect sleep behavior; however, sex did influence the length of sleep bouts, but not the sleep pattern. The park sections (Addo versus Nyathi) did not influence sleep episodes, but did affect the LD cycle in which the black rhinos slept.
Large herbivores, such as the black rhino, may have evolved reduced sleep because the low caloric density of their diet means they need to spend more time awake foraging.6,16 Given that sleep cycles are negatively correlated with maximum life span, brain, and body mass,30 black rhinos, which weigh between 800–1,400 kg33 with a brain weight approximately 400–600 g, are predicted to sleep for approximately 3.5 to 4.5 h/day. Similar to other large herbivores, such as white rhinos, cattle (Bos taurus) and elephants, black rhinos are most likely polyphasic in their sleep patterns, and with an average sleeping bout of 90 min, this would equate to approximately three to four sleep cycles for adult rhino each day. Although black rhinos were only recorded sleeping at night in the current study, they are known to sleep throughout the day, during times of high heat.20,22,28 Therefore, the results of the current study should not be considered TST for the black rhino, but rather the length of a nighttime sleeping bout. Because the black rhinos in the study were never recorded sleeping twice on the same day, we expected that other bedding sites were likely located throughout their home range that they might be using at other times of the day and/or night.
Sleeping animals are generally unresponsive and unaware of changes in their environment, which makes it a dangerous behavior.2 Thus, risk of predation strongly influences sleep duration in mammals; studies suggest that predators provide selective pressure that favors the evolution of short sleeping time in prey species.19 As such, large herbivores may have evolved reduced sleep because they are more vulnerable to predators and because of the risks associated with their sleeping site.6,16 Large mammals must lie down during REM sleep due to the loss of muscle tone, yet this supine position makes them more vulnerable to predators.2 Black rhino sleep LD pattern varied between the sections of AENP in which the individuals resided. In Addo, rhinos slept between 20:00 and 24:00 whereas those in the Nyathi section slept until 04:00. The rhinos in the Addo section were exposed to lions and hyenas and were perhaps influenced by the risk of predation between 01:00 and 04:00. Hayward and Hayward34 documented lion activity throughout the day in AENP, with highest levels observed around sunrise and sunset. Hyenas were active mostly at night from 22:00 to 03:00.34 Although reports about predation of adult black rhinos are rare, young rhinos are vulnerable to predation by spotted hyenas and lions and rates of predation may be underreported because they are rarely witnessed.35 Therefore, presence of these predators could contribute to differences in sleep times documented between the two sections of AENP.
Although it is generally regarded that sex of the individuals affects the sleep-wake cycle of a wide range of species, very few studies have examined its direct influence on sleep.9 Male black rhinos slept approximately 47 min longer per bout than females. In contrast, the study of captive white rhinos found no differences in the proportion of time adult males and females spent resting.17 However, in a wild population of African elephants, bulls were recorded in recombinant sleep for 120 min, compared with only 40.3 min in cows.12 Hunger is a natural stressor in wild populations and affects the amount of sleep in laboratory rats, which declines with days of food deprivation.14 Under laboratory baseline conditions, female mice9,11 and rats10 spend less time asleep than males; however, gonadectomy in both species eliminates the sex difference. These sex differences may also be a result of differing energy requirements, such as reproduction and caring for offspring. In the current study we did not observe a difference in sleep duration between female rhinos with and without calves (approximately 63 and 51 min, respectively).
Age is known to affect the pattern of sleep stages and TST in humans.36 Interestingly, we did not observe a difference in sleep bouts among the age classes of wild black rhinos. Similarly, no differences among age classes were found in captive white rhinos, except for two female calves (younger than 1 yr) that spent more time lying down than the adults and subadults.17 In captive Asian elephants, one infant (younger than 1 yr) and two juveniles (3 yr old) had longer TST than adults.28 However, the increased length of TST was not attributed to longer sleep bouts. In fact, the infant exhibited more frequent and shorter sleep bouts (39.3 min) than adults (approximately 60.7–77.2 min).18 Here, only one black rhino calf (2 yr old) was observed. Although this rhino's sleep bouts were in the same range as the adults, it may be sleeping more frequently throughout the day and night. Additional calves are needed to validate these data and potentially analyzing sleep in yearlings may help with determining the effect of age on sleep patterns in the black rhino.
Seasonality of sleep behavior has received little attention due to the difficulty of investigating the same animals across seasons. When ambient temperatures fall outside of the thermoneutral zone, REM sleep is suppressed more strongly than NREM sleep.6 In the Asian elephant, seasonal differences influenced the TST and the distribution of sleep within the night with more sleep occurring in the winter.18 Females in general need more nutrients due to energetic costs of gestation and lactation; therefore, females should spend more time foraging and less time sleeping. The time of year can affect nutritional quality and quantity of diets, which can modify sleep durations and patterns. However, no differences in black rhino sleeping bout duration between the wet and dry seasons were documented in the current study. Although there are distinct wet and dry season within AENP, animals within the park do have access to drought-resistant vegetation,37 artificial water sources, and rainfall year-round.38 Black rhinos in AENP show very little difference in their body condition between the seasons based on photos from camera traps, which may explain why no differences in sleeping bouts were documented between the wet and dry seasons. Populations of black rhinos that live in areas with more extreme differences in food availability and rainfall throughout the year may exhibit more pronounced sleeping patterns with respect to season.
CONCLUSION
When studying a wild population of black rhino, it is difficult to directly observe them and impossible to observe them at night. Although we were not able to investigate REM versus NREM sleep in wild black rhinos because we did not use the video mode on the camera traps, we were taking the first step in characterizing wild black rhino sleep behavior. Studies of animals in the wild frequently rely on behavior data alone; however, it is difficult to distinguish quiet waking states from true sleep when physiologic data are not collected.7 Given that we were not able to confine the wild rhinos to an enclosure; our study only recorded the average sleep bouts at two sleep sites, not TST. However, our data were comparable to a captive African elephant study when the observer recorded when the elephant was in the recumbent position and assumed they were sleeping when lying down.12 Additionally, the relationship between sleep and hormones is very complex, with the physiologic linkage poorly understood, but both endogenous and exogenous steroid hormones have an effect on sleep.14 Therefore, it would be interesting to analyze fecal steroid hormones to determine if gonadal or adrenal hormones affected the rhino sleep patterns. Preliminary analysis has demonstrated that fecal glucocorticoid metabolites (stress hormones) are significantly higher in Addo rhino versus Nyathi.39 Perhaps the elevated stress in Addo explains why a difference in the sleep pattern was observed between the two sections. Further investigation is warranted. Overall, these data provide a greater understanding of the biologic factors that can influence the success of this highly endangered species. Now, park managers can use this knowledge to improve the conservation of the black rhino.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Savage VM, West GB. A quantitative, theoretical framework for understanding mammalian sleep. Proc Nat Acad Sci. 2007;104:1051–6. doi: 10.1073/pnas.0610080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima SL, Rattenborg NC, Lesku JA, Amlaner CJ. Sleeping under the risk of predation. Anim Behav. 2005;70:723–36. [Google Scholar]
- 3.Tobler I. Is sleep fundamentally different between mammalian species? Behav Brain Res. 1995;69:35–41. doi: 10.1016/0166-4328(95)00025-o. [DOI] [PubMed] [Google Scholar]
- 4.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav R. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- 5.Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr Biol. 2008;18:R670–R9. doi: 10.1016/j.cub.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–71. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel JM. Do all animals sleep? Trends Neurosci. 2008;31:208–13. doi: 10.1016/j.tins.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao X, Sun H, Zhanhui T, Flanders J, Zhang S, Ma Y. Characterization of the sleep architecture in two species of fruit bats. Behav Brain Res. 2010;208:497–501. doi: 10.1016/j.bbr.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Paul K, Dugovic C, Turek FW, Laposky AD. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29:1211–23. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- 10.Fang J, Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734:275–85. [PubMed] [Google Scholar]
- 11.Koehl M, Battle S, Meerlo P. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep. 2006;29:1224–31. doi: 10.1093/sleep/29.9.1224. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt JR, Eltringham SK. The daily activity of the elephant in the Rwenzori National Park, Uganda. Afr J Ecol. 1974;12:273–89. [Google Scholar]
- 13.Trivers RL. Parental investment and sexual selection. In: Campbell BG, editor. Sexual selection and the descent of man: the Darwinian pivot. Chicago: Aldine Press; 1972. pp. 136–75. [Google Scholar]
- 14.Manber R, Armitage R. Sex, steroids and sleep: a review. Sleep. 1999;22:540–55. [PubMed] [Google Scholar]
- 15.Jacobs BL, McGinty DJ. Effects of food deprivation on sleep and wakefulness in the rat. Exp Neurol. 1971;30:212–22. doi: 10.1016/s0014-4886(71)80002-x. [DOI] [PubMed] [Google Scholar]
- 16.Lesku JA, Roth TC, II, Amlaner CJ, Lima SL. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. Am Nat. 2006;168:441–53. doi: 10.1086/506973. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor SM. Activity cycles of the Southern white rhinoceros (Ceratotherium s. simum) in captivity: implications for management. Int Zoo Yb. 1986;24/25:297–303. [Google Scholar]
- 18.Tobler I. Behavioral sleep in the Asian elephant in captivity. Sleep. 1992;15:1–12. [PubMed] [Google Scholar]
- 19.Lesku JA, Roth TC, 2nd, Rattenborg NC, Amlaner CJ, Lima SL. History and future of comparative analyses in sleep research. Neurosci Biobehav Rev. 2009;33:1024–36. doi: 10.1016/j.neubiorev.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Emslie R. Diceros bicornis ssp. bicornis. IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1. 2011. < www.iucnredlist.org >. Downloaded on 01 August 2012.
- 21.Joubert E, Eloff F. Notes on the ecology and behaviour of the black rhinoceros Diceros bicornis Linn. 1758 in South West Africa. Madoqua. 1971;1:5–53. [Google Scholar]
- 22.Shenkel R, Shenkel-Hulliger L. Ecology and behaviour of the black rhinoceros (Diceros bicornis): a field study. Hamburg: Mammalia Depicta; 1969. [Google Scholar]
- 23.Whitehouse AM, Hall-Martin AJ. Elephants in Addo Elephant National Park, South Africa: reconstruction of the population's history. Oryx. 2000;34:46–55. [Google Scholar]
- 24.Skead CJ. Cape Town: Department of Nature and Environmental Conservation of the Provincial Administration of the Cape of Good Hope; 1987. Historical mammal incidence in the Cape Province: The eastern half of the Cape Province, including the Ciskei, Transkei and East Griqualand. Volume 2. [Google Scholar]
- 25.Hall-Martin AJ, Penzhorn BL. Behaviour and recruitment of translocated black rhinoceros Diceros bicornis. Koedoe. 1977;20:147–62. [Google Scholar]
- 26.Whitehouse AM, Hall-Martin AJ, Knight MH. A comparison of methods used to count the elephant population of the Addo Elephant National Park, South Africa. Afr J Ecol. 2001;39:140–5. [Google Scholar]
- 27.SANParks. Addo Elephant National Park. 2009. Available from: http://www.sanparks.org/parks/addo/default.php. Downloaded on March 1, 2009.
- 28.Rice MB, Jones M. Characteristics of black rhinoceros (Diceros bicornis) bedding sites. Afr J Ecol. 2006;44:452–7. [Google Scholar]
- 29.Price EE, Stoinski TS. Group size: determinants in the wild and implications for the captive housing of wild mammals in zoos. Appl Anim Behav Sci. 2006;103:255–264. [Google Scholar]
- 30.Tobler I, Schwierin B. Behavioural sleep in the giraffe (Giraffa camelopardalis) in a zoological garden. J Sleep Res. 1996;5:21–32. doi: 10.1046/j.1365-2869.1996.00010.x. [DOI] [PubMed] [Google Scholar]
- 31.Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River: Prentice-Hall, Inc; 1999. [Google Scholar]
- 32.Di Bitetti MS, Janson CH. Reproductive socioecology of tufted capuchins (Cebus apellanigritus) in northeastern Argentina. Int J Primatol. 2001;22:127–42. [Google Scholar]
- 33.Estes RD. The behavior guide to African mammals. Los Angeles: University of California Press; 1991. [Google Scholar]
- 34.Hayward MW, Hayward GJ. Activity patterns of reintroduced lion Panthera leo and spotted hyaena Crocuta crocuta in the Addo Elephant National Park, South Africa. Afr J Ecol. 2007;45:135–141. [Google Scholar]
- 35.Plotz RD, Linklater WL. Black rhinoceros (Diceros bicornis) calf succumbs after lion predation attempt: implications for conservation management. Afr Zool. 2009;44:283–7. [Google Scholar]
- 36.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 37.Stuart-Hill G, Aucamp A. Carrying capacity of the succulent valley bushveld of the Eastern Cape. Afr J Range Forage Sci. 1993;10:1–10. [Google Scholar]
- 38.Gough KF, Kerley GIH. Demography and population dynamics in the elephants Loxodonta africana of Addo Elephant National Park, South Africa: is there evidence of density dependent regulation? Oryx. 2006;40:434–41. [Google Scholar]
- 39.Santymire RM, Meyer J, Bird J, Schulte BA, Freeman E. Using fecal hormonal analysis to determine the factors affecting the success of the black rhinoceros in Addo Elephant National Park, South Africa. Proceedings of the International Elephant and Rhino Conservation and Research Symposium; 2011 Oct 10-14; Rotterdam, Netherlands. International Elephant Foundation; 2011. [Google Scholar]


