Abstract
The combination of the mTOR inhibitor everolimus with the aromatase inhibitor exemestane was evaluated in the randomized Phase III BOLERO-2 trial. Research has indicated that aberrant signaling through the mTOR pathway is associated with resistance to endocrine therapies. The BOLERO-2 trial examined the effects on progression-free survival of the addition of everolimus to exemestane in a patient population of postmenopausal, hormone receptor-positive, advanced breast cancer. At the interim analysis, the median progression-free survival assessed by local investigators was 6.9 months for everolimus plus exemestane versus 2.8 months for placebo plus exemestane (hazard ratio: 0.43; p < 0.001), and by central assessment was 10.6 versus 4.1 months, respectively (hazard ratio: 0.36; p < 0.001). The everolimus plus exemestane arm showed greater number of grade 3 and 4 adverse events. This study suggests that the addition of everolimus to exemestane is a potential viable treatment option for this patient population.
Keywords: advanced breast cancer, BOLERO-2, endocrine resistance, everolimus, exemestane, hormone receptor-positive, mTOR inhibitor, postmenopausal
Background
Approximately 60–70% of breast cancers express estrogen receptor (ER)-α. For patients with these tumors, endocrine therapy is the foundation of treatment [1]. In postmenopausal patients with advanced breast cancer, treatment guidelines recommend that the initial endocrine therapy consists of an aromatase inhibitor (AI), either nonsteroidal (anastrozole or letrozole) or steroidal (exemestane) [2–6]. Unfortunately, in up to 50% of breast cancer patients who relapse with distant metastasis, this hormonal therapy is no longer effective (de novo resistance), or if it is initially effective, resistance will still develop (acquired resistance) [1]. Subsequent options after resistance include changing the class of AI or changing to an ER antagonist such as fulvestrant or the selective ER modulator tamoxifen [7, 8]. The PI3K–Akt–mTOR signaling pathway modulates growth through signaling communicated by ER and the EGF receptor family of receptor tyrosine kinases [9–11]. Activation of the PI3K–Akt–mTOR pathway has been implicated in resistance to endocrine therapy in breast cancer [12–14]. Given the major clinical issue of endocrine resistance, extensive preclinical research has been performed examining the molecular basis of endocrine resistance and using inhibitors of this pathway to attempt to combat this resistance [15–19]. Specifically, some researchers have focused their efforts on developing mTOR inhibitors derived from rapamycin (the prototypical mTOR inhibitor).
Introduction to the trial
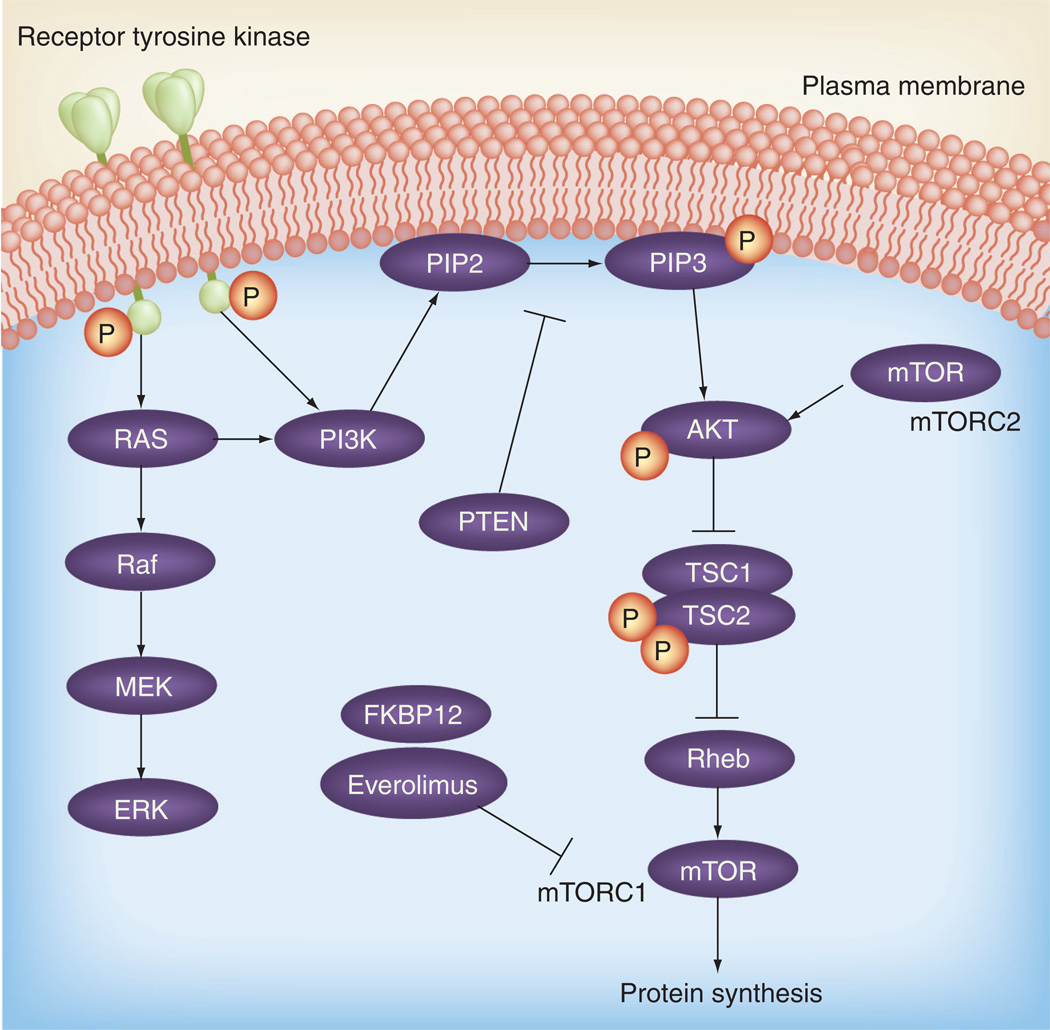
Everolimus (Af initor®, Novartis, Basel, Switzerland) is one such rapamycin analogthat is orally active and has a more favorable pharmacological prof ile than its predecessors [20–22]. Specifically, everolimus is an allosteric mTOR antagonist that acts through binding to FKBP12, which then binds directly to the FKBP12–rapamycin binding domain of mTOR. mTOR itself is the catalytic component of two distinct multimeric complexes, mTORC1 and mTORC2, and both play key roles in the phosphorylation of downstream proteins that mediate signaling pathways important for proliferation and cell growth. Importantly, rapamycin and its analogs allosterically inhibit mTORC1 only (Figure 1). Previous studies demonstrated that inhibition of mTORC1 blocks the phosphorylation of the activation domain 1 of ER [17, 23]. Everolimus is a US FDA-approved drug for the treatment of renal cell cancer [24]. In clinical studies in breast cancer, single-agent mTOR inhibitors such as temsirolimus have shown approximately a 10% response rate [25]. In addition, preclinical research has shown that, in combination, the addition of endocrine therapy to everolimus results in an antiproliferative effect [17]. These positive results of everolimus in combination with an antiestrogen have been recapitulated in clinical studies. A recent randomized Phase II study examining neoadjuvant everolimus plus letrozole showed a higher response rate and decreased cancer cell growth compared with letrozole alone [26]. In addition, unpublished data from the TAMRAD trial, a randomized, Phase II study involving postmenopausal, ER-positive, advanced breast cancer patients treated with everolimus and tamoxifen, has so far demonstrated an improved progressionfree survival compared with tamoxifen alone, as well as improved overall survival [27]. Owing to this potential for increased efficacy, the BOLERO-2 trial attempted to define both the efficacy and safety of the addition of a mTOR inhibitor to an AI [28]. This study compared a combination arm consisting of everolimus (at 10 mg/day orally) plus exemestane (at 25 mg/day orally) with a control arm of exemestane plus placebo in postmenopausal, hormone-refractory advanced breast cancer patients [28].
Figure 1. PI3K–AKT–mTOR pathway.
mTORC: mTOR complex.
Study design
Patient characteristics
The BOLERO-2 trial is an ongoing, randomized, international, double-blinded Phase III placebo-controlled study in postmenopausal women with ER-positive, HER2-nonamplified advanced breast cancer whose disease was deemed refractory to nonsteroidal AIs (letrozole or anastrozole). This endocrine resistance was defined as either disease recurrence during adjuvant treatment or within 12 months of adjuvant therapy completion, progression during the treatment of advanced disease or progression within 1 month of treatment completion. One prior chemotherapy regimen for advanced disease was allowed, as was other hormonal therapy (excluding exemestane). Patients were excluded if they had received prior exemestane or mTOR inhibitors, or had an Eastern Cooperative Oncology Group (ECOG) performance status of three or greater. Patients were required to have measurable disease; although, in the absence of such, mainly lytic bone lesions were also allowed. The interim analysis occurred after 359 progression-free survival events. A total of 724 women were randomized in a 2:1 ratio to receive either exemestane (25 mg/day) plus everolimus (10 mg/day; n = 485) or exemestane at the same dose plus placebo (n = 239). Patients receiving everolimus or matched placebo could have two possible dose reductions to 5 mg/day and subsequently 5 mg every other day and still remain on the trial.
End points
The primary end point was investigator-determined progression-free survival as determined by radiographic studies. The secondary end points consisted of overall survival, clinical benefit rate, overall response rate, time to deterioration of ECOG performance status, safety and quality of life (as assessed by standard questionnaires). Subgroup analysis of 80 patients evaluated blood levels of everolimus, and levels of exemestane and estradiol.
Baseline characteristics
Patient and tumor characteristics between the two arms of the study were well matched with no significant differences, including age (median: 62 years), race, visceral involvement (56%), bone metastasis (76%) and ECOG performance status. In addition, prior therapies were well balanced between the arms with all having received letrozole or anastrozole, 48% having received tamoxifen, 16% fulvestrant and 68% chemotherapy. Prior sensitivity to endocrine therapy (defined as ≥24 months of endocrine treatment prior to disease recurrence in the adjuvant setting or response/stabilization of advanced disease for ≥24 weeks of endocrine treatment) accounted for the majority of the patients (84%).
Data analysis
The efficacy analysis regarding the primary end point of progression-free survival was stratified with respect to the presence of visceral metastasis and prior sensitivity to endocrine therapy. An interim analysis was performed after the study reached approximately 60% of the progression-free survival events (n = 359). The trial will run until the final analysis of 528 progression-free survival events. Progression was assessed every 6 weeks by imaging studies (CT scan, MRI or bone scan). Adverse events were assessed based on grade according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events [29]. There was no crossover between arms.
Results
Efficacy
At the time of the interim analysis, 227 patients (47%) were still receiving exemestane and everolimus, and 69 patients (29%) were still receiving placebo and exemestane. The trial met its primary end point of progression-free survival. According to local investigators, the median progression-free survival was 6.9 months for the combination everolimus plus exemestane arm versus 2.8 months for the placebo plus exemestane arm (hazard ratio for progression or death: 0.43; 95% CI: 0.35–0.54; p < 0.001). When the same data were examined by a central assessment, the median progression-free survival was 10.6 months for the combination arm and 4.1 months for the placebo arm (hazard ratio: 0.36; 95% CI: 0.27–0.47; p < 0.001). It is speculated that the difference between local and central assessment is secondary to subjectivity in interpretation of patients’ radiographic scans. Taking all subgroups into account (including age, race, ECOG status, progesterone receptor positivity, prior treatment type, sensitivity to prior treatments and number of prior treatments) these progression-free survival data remained consistent. Plasma concentrations of estradiol were found not to be statistically different between the study arms. When comparing objective response rates between local and central assessments, the two were similar at 9.5 and 7%, respectively, in the combination arm and 0.4% in the placebo arm. At the time of analysis and publication, overall survival results were immature.
Safety/toxicity
The median treatment exposure of everolimus was 14.6 weeks compared with 12 weeks of placebo, and median exposure to exemestane was 17.4 weeks in the combination arm compared with 12 weeks in the placebo arm. In the combination arm, 23% of patients had serious adverse events, with approximately half of these events (11%) thought to be related to the study treatment. There were seven deaths (two deaths from sepsis and one death from each of the following: pneumonia, tumor hemorrhage, cerebrovascular incident, renal failure and suicide). In the placebo arm, 12% of patients had serious adverse events, although only a small fraction (1% of the overall patients) were attributed to treatment, and one death occurred (from pneumonia). There was also an increased withdrawal of consent (5 vs 2%) in the combination arm and a higher proportion of patients who discontinued everolimus compared with placebo (19 vs 4%). In addition, a higher number of patients discontinued exemestane from the combination arm compared with discontinuation of exemestane in the placebo arm (7 vs 3%). The most common adverse events (grade 3 or 4) in the combination arm as compared with the placebo arm were stomatitis (8 vs 1%), anemia (6 vs <1%), fatigue (4 vs 1%) and pneumonitis (3 vs 0%). However, according to quality-of-life end points and ECOG status, there was found to be no statistically significant difference between the study groups.
Conclusion
The BOLERO-2 trial met its primary end point of progression-free survival at the interim analysis. Results showed that the combination of everolimus with exemestane had increased efficacy compared with exemestane plus placebo with respect to progression-free survival in the range of 4–6 months in a patient population of postmenopausal, hormone receptor-positive, advanced breast cancer patients. Taken with other clinical data indicating activity of everolimus combined with other hormonal therapies, and the clinical difficulty of hormone therapy resistance, the addition of everolimus to exemestane appears to be a viable option [26, 27]. The progression-free survival benefit seen in the BOLERO-2 trial is similar to or better than that of other approved hormonal therapies and chemotherapies given after initial hormone resistance [8, 30]. However, given the increased toxicity profile of the combination arm of exemestane and everolimus, the FDA and the prescribing oncologist will need to consider the benefit of the combination against the added toxicity it brings. Moreover, the omission of an everolimus-only arm precludes confidence that everolimus is truly overcoming endocrine therapeutic resistance per se; rather, it is possible that mTOR inhibition by itself may also be a potential effective therapy for hormone-refractory disease in this patient population.
Future perspective
As previously hormone-sensitive breast cancer patients develop endocrine resistance, decisions need to be weighed regarding switching within the endocrine therapies or moving toward chemotherapy. The findings of the BOLERO-2 trial would indicate that the addition of everolimus to exemestane in postmenopausal patients who have developed endocrine resistance would be able to extend their chemotherapy-free period by a maximum of 10 months. For ease of oral dosing, doctor visits and flexibility of schedule, one might imagine that everolimus would be much favored over conventional chemotherapy. The toxicity profile of everolimus–exemestane will need to be weighed against the benefit of delaying chemotherapy. The response rate seen in this trial, of approximately 10% for everolimus/exemestane, is similar to that seen for single-agent temsirolimus in a similar patient population, as well as some single-agent multitargeted tyrosine kinase inhibitors [25, 31]. Therefore, an unanswered question is what the response rate and progression-free survival of everolimus alone might have been, had the trial incorporated such an arm. In addition, since the majority of patients in the BOLERO-2 trial had acquired hormonal resistance, it is unclear if the results will be applicable to patients with de novo resistance. Regardless, there is great anticipation as the results of BOLERO-2 mature, specifically analysis of the overall survival data. If there is indeed a significant improvement in overall survival seen with the combination arm, this would only strengthen the case for making everolimus combined with exemestane a standard of care in this patient population.
There are a number of anticipated or ongoing clinical trials that will try to establish additional options for the treatment of advanced endocrine-resistant breast cancer. Specifically, ongoing studies in both the adjuvant and metastatic setting are evaluating the efficacy of metformin, an approved drug for diabetes whose mechanism of action involves indirect mTOR inhibition, which may show promise in this regard. In a similar vein, the ENCORE study is evaluating the addition of etinostat, a benzamide histone deacetylase inhibitor, to exemestane. In addition, an adjuvant everolimus study has been proposed by the Southwest Oncology Group (SWOG) in hormone receptor-positive breast cancer patients, based on their cancers’ recurrence scores by the Oncotype® DX assay (Genomic Health, Inc., CA, USA), randomized to hormonal therapy with or without 1 year of everolimus.
It is clear that more work needs to be done in understanding the complex pathways that lead toward the development of endocrine resistance. Combined inhibition of ER signaling with PI3K–AKT–mTOR pathway inhibitors is logical from our current knowledge of these pathways, which is further substantiated by emerging clinical observations that ER-positive breast cancers that are PIK3CA mutation positive and/or HER2 positive do not respond as favorably to hormone therapy. However, there is a great opportunity to advance our knowledge of the molecular mechanisms leading to endocrine resistance and therefore improve upon clinical outcomes. Therefore, it will be critical in the coming years to design additional therapeutic strategies to combine key signal transduction inhibitors as well as improve on currently available inhibitors. For example, while everolimus is an allosteric inhibitor of mTORC1, it does not affect mTORC2 (which independently activates AKT) and, in addition, has a documented effect of paradoxically increasing PI3K (and MAPK) pathway activation proximally, owing to inhibition of a negative feedback loop [32–34]. Currently, there is much work being performed to develop dual mTORC1/2 kinase inhibitors that also target PI3K [35].
In the coming decade, as we strive to improve outcomes, it will also be critical to develop biomarkers that predict the response to mTOR inhibition and other therapies for breast cancer. These biomarkers will probably come from preclinical data that lead to a better understanding of the complex signaling pathways and crosstalk involved in endocrine resistance, as well as better-designed clinical trials with more in-depth subgroup analyses. Examples of such work in relation to everolimus and mTOR inhibition have shown that increased Ki67 or HER2 positivity can predict the response [36]. Also, existing research has analyzed the response outcomes to everolimus in patients with PIK3CA mutations; however, results have been somewhat conflicting and further study is required [22, 26]. In the Phase II TAMRAD study, clinical benefit seemed to be improved for the subgroup of patients in whom estrogen resistance was acquired [27]. Specific biomarkers such as pS6K and 4EBP1 were analyzed in this study, and the combination arm of everolimus– tamoxifen showed a correlation between these biomarkers of mTOR activation and efficacy in preliminary data. It will be interesting to see additional in-depth biomarker analyses in large randomized trials to define a particular patient population in which the response to therapies is robust. In addition to the discovery of additional targets that modulate hormone resistance, future preclinical and clinical studies will be able to better define appropriate patient subpopulations that will respond to given therapies, thereby drastically improving on current predictors of ‘good’ response.
Executive summary.
Background
-
▪
Given the clinical issue of endocrine resistance in breast cancer, researchers have examined the pathways that lead to endocrine resistance in the hope of identifying targets for drug development.
-
▪
Aberrant signaling through the mTOR pathway is implicated in endocrine resistance.
-
▪
Everolimus is a mTOR inhibitor that has shown efficacy in a Phase II trial in combination with letrozole, as well as in the ongoing Phase II TAMRAD trial where it is combined with tamoxifen.
Methods
-
▪
The BOLERO-2 trial is an ongoing randomized, international, Phase III trial examining the addition of daily oral 10 mg of everolimus to daily oral 25 mg of exemestane compared with 25 mg of exemestane plus placebo.
-
▪
Hormone receptor-positive, postmenopausal, advanced breast cancer patients were stratified according to visceral metastasis and prior response to endocrine therapy.
-
▪
The primary end point was progression-free survival, with secondary end points of response rate, clinical benefit rate, time to deterioration of Eastern Cooperative Oncology Group (ECOG) status, safety, quality of life and overall survival.
Results
-
▪
At the interim analysis the trial met its primary end point of progression-free survival.
-
▪
According to local investigators, the median progression-free survival was 6.9 months for the combination everolimus–exemestane arm versus 2.8 months for the placebo–exemestane arm (hazard ratio for progression or death: 0.43; 95% CI: 0.35–0.54; p < 0.001).
-
▪
According to the central assessment, the median progression-free survival was 10.6 months for the combination everolimus–exemestane arm and 4.1 months for the placebo–exemestane arm (hazard ratio: 0.36; 95% CI: 0.27–0.47; p < 0.001).
-
▪
Overall response rates were calculated as being more similar between local and central assessment with 9.5 and 7% response rate, respectively, in the everolimus–exemestane arm and 0.4% for both assessments in the placebo–exemestane arm.
-
▪
Serious adverse events were more common in the everolimus–exemestane arm (23%) compared with the placebo–exemestane arm (12%).
-
▪
Adverse events leading to discontinuation of drug were more common in the everolimus–exemestane arm (19%) versus the placebo–exemestane arm (4%).
-
▪
The most common adverse events (grade 3 or 4) in the everolimus–exemestane arm compared with the placebo–exemestane arm were stomatitis (8 vs 1%), anemia (6 vs <1%), fatigue (4 vs 1%) and pneumonitis (3 vs 0%).
Conclusion
-
▪
The combination of exemestane and everolimus has increased efficacy compared with exemestane alone with respect to progression-free survival in a patient population of postmenopausal, hormone receptor-positive, advanced breast cancer patients.
-
▪
Everolimus has significantly greater toxicity compared with placebo.
-
▪
The role for combination therapy in hormone-refractory breast cancer is evolving and may include everolimus.
Acknowledgments
BH Park and JA Beaver have received funding from the NIH (P50 CA88843, R01 CA109274 and T32CA009071).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer. 2002;2(2):101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 2.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N. Engl. J. Med. 2003;348(24):2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 3.Mouridsen H, Sun Y, Gershanovich M, et al. Superiority of letrozole to tamoxifen in the first-line treatment of advanced breast cancer: evidence from metastatic subgroups and a test of functional ability. Oncologist. 2004;9(5):489–496. doi: 10.1634/theoncologist.9-5-489. [DOI] [PubMed] [Google Scholar]
- 4.Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J. Clin. Oncol. 2000;18(22):3758–3767. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 5.Bonneterre J, Thurlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J. Clin. Oncol. 2000;18(22):3748–3757. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 6.Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J. Natl Cancer Inst. 2006;98(18):1285–1291. doi: 10.1093/jnci/djj357. [DOI] [PubMed] [Google Scholar]
- 7.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J. Clin. Oncol. 2008;26(10):1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 8.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM Phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J. Clin. Oncol. 28(30):4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 9.Kurokawa H, Arteaga CL. ErbB (HER) receptors can abrogate antiestrogen action in human breast cancer by multiple signaling mechanisms. Clin. Cancer Res. 2003;9(1 Pt 2):S511–S515. [PubMed] [Google Scholar]
- 10.Stoica GE, Franke TF, Wellstein A, et al. Estradiol rapidly activates Akt via the ErbB2 signaling pathway. Mol. Endocrinol. 2003;17(5):818–830. doi: 10.1210/me.2002-0330. [DOI] [PubMed] [Google Scholar]
- 11.Stoica GE, Franke TF, Wellstein A, et al. Heregulin-beta1 regulates the estrogen receptor-alpha gene expression and activity via the ErbB2/PI 3-K/Akt pathway. Oncogene. 2003;22(14):2073–2087. doi: 10.1038/sj.onc.1206311. [DOI] [PubMed] [Google Scholar]
- 12.Burstein HJ. Novel agents and future directions for refractory breast cancer. Semin. Oncol. 2011;38(Suppl. 2):S17–S24. doi: 10.1053/j.seminoncol.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Johnston SR. Clinical efforts to combine endocrine agents with targeted therapies against epidermal growth factor receptor/human epidermal growth factor receptor 2 and mammalian target of rapamycin in breast cancer. Clin. Cancer Res. 2006;12(3 Pt 2):S1061–S1068. doi: 10.1158/1078-0432.CCR-05-2125. [DOI] [PubMed] [Google Scholar]
- 14.Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin. Cancer Res. 2004;10(1 Pt 2):S331–S336. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 15.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584(1):124–128. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J. Biol. Chem. 2009;284(10):6361–6369. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 17.Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin. Cancer Res. 2005;11(14):5319–5328. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- 18.Beeram M, Tan QT, Tekmal RR, Russell D, Middleton A, Degraffenried LA. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann. Oncol. 2007;18(8):1323–1328. doi: 10.1093/annonc/mdm170. [DOI] [PubMed] [Google Scholar]
- 19.Degraffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin. Cancer Res. 2004;10(23):8059–8067. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 20.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120(6):747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 21.Johnston SR. Are we missing the mTOR target in breast cancer? Breast Cancer Res. Treat. 2011;128(3):607–611. doi: 10.1007/s10549-010-1207-2. [DOI] [PubMed] [Google Scholar]
- 22.Macaskill EJ, Bartlett JM, Sabine VS, et al. The mammalian target of rapamycin inhibitor everolimus (RAD001) in early breast cancer: results of a pre-operative study. Breast Cancer Res. Treat. 2011;128(3):725–734. doi: 10.1007/s10549-010-0967-z. [DOI] [PubMed] [Google Scholar]
- 23.Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr. Opin Cell Biol. 2010;22(2):169–176. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 25.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2005;23(23):5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 26. Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. 2009;27(16):2630–2637. doi: 10.1200/JCO.2008.18.8391. ▪ Demonstrated the initial efficacy and safety of using neoadjuvant everolimus combined with the aromatase inhibitor letrozole in patients with early-stage estrogen receptor-positive breast cancer in a Phase II trial.
- 27. Bachelot T, Bourgier C, Cropet CEA. TAMRAD: a GINECO randomized Phase II trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients with hormone-receptor positive, HER2 negative metastatic breast cancer with prior exposure to aromatase inhibitors. Presented at: 33rd Annual San Antonio Breast Cancer Symposium; 8–20 December, 2010; San Antonio TX, USA. ▪ Evaluated the efficacy and safety of everolimus combined with the selective estrogen receptor modulator tamoxifen in patients with hormone receptor-positive, HER2-negative metastatic breast cancer. All patients had received prior endocrine therapy with aromatase inhibitors.
- 28. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormonereceptor- positive advanced breast cancer. N. Engl. J. Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. ▪▪ Demonstrated the efficacy and safety of everolimus in combination with the aromatase inhibitor exemestane in hormone receptor-positive advanced breast cancer previously treated with nonsteroidal aromatase inhibitors. This is the primary research article on which this commentary is based.
- 29.Common Terminology Criteria for Adverse Events V3.0 V. MD, USA: National Cancer Institute; 2006. [Google Scholar]
- 30.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, Phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J. Clin. Oncol. 2011;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 31.Burstein HJ, Elias AD, Rugo HS, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 2008;26(11):1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 32.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65(16):7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 33.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004;6(11):1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 35.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68(19):8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 36.Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J. Natl Cancer Inst. 2007;99(2):167–170. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]