Abstract
In this issue of Cell Stem Cell, Iglesias-Bartolome et al (2012) show that mTOR inhibition with rapamycin protects against mucositis in mice, suggesting potential treatment strategies against this harmful side effect of anti-cancer therapies. In normal tissues, rapamycin prevents epithelial stem cell senescence by reducing oxidative stress through increased MnSOD.
Surveys of patients undergoing aggressive treatment for cancer or who have undergone bone marrow transplants, often cite the development of painful mouth ulcers termed mucositis as their most dreaded and debilitating side effect (Sonis, 2011). These exquisitely tender lesions that develop on the mucosa of the mouth and oropharynx appear in most patients receiving combined chemotherapy and radiation and often leave the patient unable to eat or drink. Up to now, the management of this condition has been mostly supportive, with patients offered opioid-strength pain relief and hydration. That supportive but mostly ineffective strategy might however change if the findings from a paper in this issue of Cell Stem Cell can be extended from mice to humans (Iglesias-Bartolome et al., 2012). This new data suggests that pharmacological inhibition of the mechanistic target of rapamycin (mTOR) might eventually provide the basis for a molecular salve to protect patients from this prevalent and painful condition.
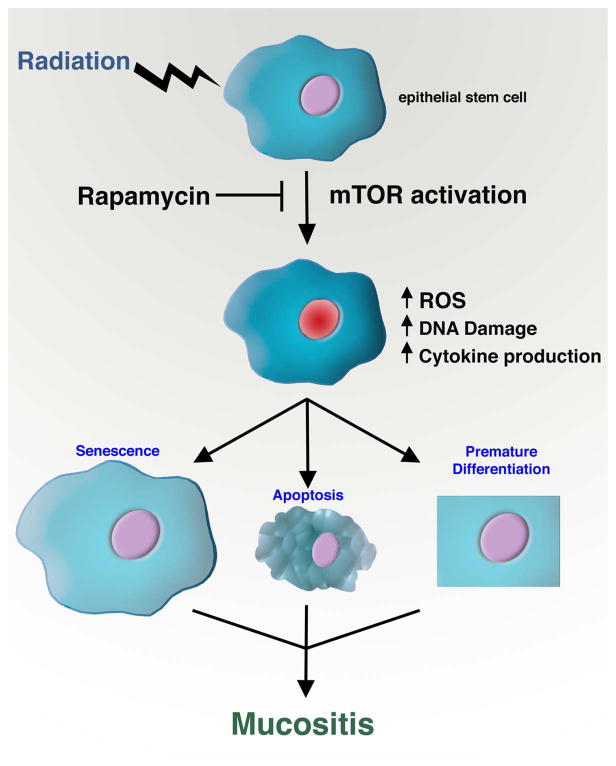
In this new study by Iglesias-Bartolome and colleagues, the authors began by asking whether the addition of the mTOR inhibitor rapamycin might sensitize head and neck cancer cells to radiation therapy. This made sense given that radiation is a major therapeutic approach for these tumors and mTOR is often activated in these malignacies. Like most good ideas, this notion of course turned out to be wrong. Yet as luck would have it, the authors realized that while mTOR inhibition did not appreciably alter their tumor cell lines, rapamycin treatment had a remarkable and completely unexpected effect on their control normal oral keratinocytes (NOK). These NOK cells can be isolated from biopsies obtained from the mouth of healthy human volunteers and the explanted cells can be demonstrated to possess long term self-renewal capacity, thus indicating the presence of epithelial stem cells with tissue regenerative capacity. The authors observed that rapamycin treatment had little effect on the proliferation of NOK but dramatically extended the replicative lifespan of these cells. Indeed, the authors calculate that in their culture system, a single epithelial stem cell that would normally give rise to 106 cells before undergoing senescence, would after rapamycin treatment, be able to give rise to nearly 1017 progeny before exhaustion. In addition, the secretion of various inflammatory cytokines that are normally robustly produced by senescent cells was dramatically reduced after rapamycin exposure. This might be particularly important as there is growing evidence that such pro-inflammatory cytokines might fuel mucositis (Sonis, 2011). The authors next showed that the premature senescence induced by exogenous gamma-irradiation was also inhibited in rapamycin treated NOK apparently because mTOR inhibition reduced the levels of reactive oxygen species (ROS). The basis for this reduced oxidative stress was traced to a heretofore unknown role for mTOR in regulating the expression of the mitochondrial antioxidant protein MnSOD (manganese superoxide dismutase). In rapamycin treated cells, lower levels of ROS produced lower levels of DNA damage, and therefore resulted in diminished expression of senescence-associated cell cycle inhibitors such as p16INK4A. These tantalizing observations were then extended to an in vivo model where mice received high dose fractionated radiation (6 Gy to the head and neck for five consecutive days). This regimen led to activation of mTOR in the irradiated tissues that could be suppressed by rapamycin treatment. Remarkably, intraperitoneal injection with rapamycin appeared to almost completely suppress the development of radiation-induced oral ulcerations. This finding suggests that as observed in vitro, mTOR inhibitors might lessen radiation-induced senescence and thus preserve the proliferative and intrinsic reparative properties of resident epithelial stem cells (Figure 1).
Figure 1.
The role of mTOR in radiation-induced mucositis. Irradiation of epithelial stem cells leads to mTOR activation. Inhibiting this activation with rapamycin treatment reduces the levels of ROS, inflammatory cytokine production and overall DNA damage and thereby prevents radiation-induced senescence. Rapamycin may also protect against the premature differentiation or apoptosis of stem and progenitor cells. These protective effects combine to prevent the appearance of clinically debilitating side-effects such as mucositis.
These observations add to a growing connection between mTOR activity and stem cell function. Previous work has suggested that constitutive activation of mTOR in hematopoietic stem cells (HSCs) led to an initial increase in the proliferation of HSCs, followed by a long-term decline in self-renewal (Chen et al., 2008). Again, this decline in self-renewal appeared to result from an increase in ROS levels within the mTOR hyperactivated stem cells. However, in this case, the rise in ROS levels was traced back to an increase in mitochondrial number and potentially ROS production (Chen et al., 2008), rather than as seen in the current manuscript, through a change in antioxidant defenses. Indeed, there appears to be an important and growing connection between mTOR activity, mitochondrial number and function and overall redox homeostasis. For instance, inhibiting mTOR genetically or pharmacologically can dramatically and sometimes rapidly alter mitochondrial metabolism in both cells and tissues (Laplante and Sabatini, 2012).
While the current study in Cell Stem Cell deals primarily with premature epithelial stem cell senescence induced by radiation, there is some evidence that the mechanisms described might be broadly applicable. Indeed, the authors of this study had previously shown that persistent Wnt signaling in the skin led to an initial hyperproliferative phase followed by epithelial stem cell exhaustion (Castilho et al., 2009). Surprisingly, these Wnt-induced stem cell effects were also mediated by increased mTOR signaling. In this model, as was seen with irradiation, Wnt-induced epidermal stem cell exhaustion was associated with increased DNA damage that was reduced by mTOR inhibition. This increase in DNA damage has also been seen in other models of constitutive Wnt signaling associated with accelerated aging models (Liu et al., 2007). Given the role of Wnt signaling in normal epithelial stem cell renewal, and the observation that an increase in DNA damage is a signature of aged stem cells (Rossi et al., 2007), these results suggest that the mTOR inhibition may be a general strategy to preserve and prolong the stem and progenitor population.
While the broader implication for stem cell biology need further study, the new results by Iglesias-Bartolome may have more immediate and practical implications for cancer patients suffering from radiation-induced mucositis. Rapamycin is already FDA approved for the treatment of graft rejection and coronary restenosis after angioplasty. Moreover, the compound is currently being tested for its efficacy in a wide range of diseases ranging from common malignancies to rare conditions such as the lung disease lymphangioleiomyomatosis. While systemic use of rapamycin is not without significant toxicities, the accessibility of the oral mucosa suggests other approaches are possible. For example, it might be possible to use a mouthwash or gel spiked with rapamycin, or even potentially newer, more potent mTOR inhibitors, to provide targeted relief from radiation-induced mucositis. This strategy would be conceptually similar to the currently approved and widely marketed use of topical cyclosporine (Restasis) for the treatment of dry eyes. Both cyclosporine and rapamycin are potent immunosuppressives when given orally, however, when given as eye drops or mouthwash, it is possible to achieve high local concentrations while avoiding the corresponding systemic toxicities. It should be noted that based on the current study, the beneficial effects of a theoretical rapamycin mouthwash (we will hereby christen it RapaWash) is not a result of its immunomodulatory effects but rather due to the role of mTOR in regulating the levels of ROS within the epithelial stem cell compartment. Interestingly, previous genetic studies have indicated that for patients undergoing stem cell transplant, polymorphisms within certain antioxidant genes are good predictors of which patients will develop mucositis (Hahn et al., 2010). Hopefully, it won’t be long before these patients, and countless others, can benefit from a simple, molecular-based strategy for this particularly vexing clinical problem.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Zhelnova E, Sucheston L, Demidova I, Savchenko V, Battiwalla M, Smiley SL, Ambrosone CB, McCarthy PL., Jr A deletion polymorphism in glutathione-S-transferase mu (GSTM1) and/or theta (GSTT1) is associated with an increased risk of toxicity after autologous blood and marrow transplantation. Biol Blood Marrow Transplant. 2010;16:801–808. doi: 10.1016/j.bbmt.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Iglesias-Bartolome, et al. this issue. [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Sonis ST. Oral mucositis. Anticancer Drugs. 2011;22:607–612. doi: 10.1097/CAD.0b013e3283462086. [DOI] [PubMed] [Google Scholar]