Abstract
Objective
C-type natriuretic peptide (CNP) is a paracrine regulatory factor of the growth plate and plays a key role in endochondral growth. Its amino-terminal propeptide (NTproCNP) is an equimolar product of CNP biosynthesis and is easily measured in plasma. Preliminary studies suggest that NTproCNP levels correlate with height velocity in sheep and children. The objectives of the study were to correlate NTproCNP levels with height velocity and to define the reference range for plasma CNP and NTproCNP across childhood.
Design
This was a prospective, cross-sectional, observational study of healthy children.
Patients
Participants were 258 healthy children between 2 months and 20 years of age.
Measurements
Anthropometrics were obtained and CNP and NTproCNP levels were determined by radioimmunoassay.
Results
For both sexes, CNP and NTproCNP levels were high in infancy, lower in early childhood, rising during puberty, then falling to low adult levels. Levels of NTproCNP peaked at 14.1 years in boys and 11.9 years in girls, coincident with the age of peak height velocity. Levels of NTproCNP varied with pubertal status, peaking at genital Tanner stage IV in boys and III in girls. There was a highly significant correlation between NTproCNP and height velocity.
Conclusions
C-type natriuretic peptide plays an integral role in endochondral growth. We show here that CNP synthesis (as measured by NTproCNP levels in plasma) is closely related to linear growth in healthy children at all ages. We propose NTproCNP as a biomarker of linear growth.
Key terms: C-type natriuretic peptide, height velocity, biomarker, reference range, radioimmunoassay
Introduction
C-type natriuretic peptide (CNP) was first isolated in 1990, based on its structural similarity to atrial and B-type natriuretic peptide (ANP and BNP). All three natriuretic peptides are small, single-chain peptides that contain a 17-amino acid ring structure. The functions of CNP are still being elucidated. Because of its association with ANP and BNP, many studies have focused on a role for CNP in the cardiovascular system. However, genetically altered mice experiments and genetic studies in humans have shown that the most obvious role of the CNP system is the regulation of growth and bone metabolism.
Knock-out mice for CNP 1 or its cognate receptor natriuretic peptide receptor B (NPR-B) 2 are dwarfed, with the tail, legs, and paws being disproportionately affected. The CNP transgeneic mouse is overgrown, with disproportionately long tails, legs, and paws 3. In humans, homozygous loss of function mutations in the NPR-B gene cause acromesomelic dysplasia, Maroteaux type (OMIM #602875), a severe form of short-limbed dwarfism 4. Conversely, three patients have now been described with translocations involving chromosome 2q37 (the location of the CNP gene) that result in overexpression of CNP and elevated blood levels of CNP 5,6. These patients had overgrowth with a Marfanoid body habitus, including disproportionately long limbs and arachnodactyly. A similar pattern of abnormal growth has been recently described in a family with an activated mutation in NPR-B 7.
In vitro studies using rat growth plate chondrocytes and mouse ATDC5 cells have shown that CNP is expressed and found in conditioned medium 8. The addition of exogenous CNP to these cells inhibits cell proliferation and enhances the hypertrophic chondrocyte phenotype. The expression of both CNP and NPR-B in the growth plate demonstrates that this peptide can act through a paracrine mechanism.
During CNP biosynthesis, an amino-terminal propeptide (NTproCNP) is released from cells at an equimolar ratio with CNP 9. The biologically active forms of CNP are found in plasma in low concentrations, due to several clearance mechanisms. The amino-terminal propeptide, however, is not subject to these clearance pathways and it is found in the circulation at 20- to 50-fold higher concentrations. This readily detectable level hence provides a window into the study of CNP biosynthesis in vivo. Plasma NTproCNP concentrations are stable in healthy adults and show no diurnal variation, nor change in response to eating 10. Because of its key role in growth regulation, we hypothesized that levels would be increased in growing children. This was first demonstrated in lambs and in a small heterogeneous group of children attending hospital outpatient clinics 10. In that study, significant correlation was noted between NTproCNP level and growth velocity, raising the possibility that NTproCNP may be a biomarker of linear growth.
The purpose of this study was to prospectively study the relationship between NTproCNP levels and height velocity throughout childhood as well as to describe the reference range of CNP and NTproCNP in a healthy population.
Material and Methods
Subjects
Subjects were healthy children older than 2 months and less than 20 years of age and were recruited through fliers, newspaper, and internet advertisements. Sibling enrollment was encouraged. Subjects with chronic diseases or medications that might affect growth or bone metabolism were excluded. Chronic diseases specifically not excluded were well-controlled asthma and well-controlled hypothyroidism. For children with hypothyroidism, if the TSH level drawn at the time of the study visit was abnormal, study samples were discarded. Children with attention deficit disorder not treated with amphetamine derivatives were enrolled. Subjects on chronic antibiotics were excluded, except those on antibiotics for acne. Use of vitamins, asthma medications, antihistamines, levothyroxine, and oral contraceptives were allowed. Subjects with minor acute illnesses (upper respiratory infections, streptococcal pharyngitis, gastroenteritis, urinary tract infection, etc.) had to be free of symptoms and off antibiotics for at least one month prior to the study. Subjects with significant acute illnesses (pneumonia, meningitis, pyelonephritis, any illness requiring hospitalization, etc.), surgery for any reason, or who had a fracture had to be free of symptoms for six months prior to the study. This study was approved by the Nemours Florida Institutional Review Board. All subjects had written consent or parental permission obtained. Written child assent was obtained for children between 7 and 18 years of age.
Results from 23 boys participating as healthy controls in a previous study 11 were also included in this dataset.
Study procedures
Children were seen in the Pediatric Endocrinology clinic at Nemours Children’s Clinic, Jacksonville, FL. Screening family and medical histories were obtained. Anthropometrics were done, including length by recumbent stadiometer (for subjects less than two years) or height by Harpendon stadiometer, seated height by Harpendon stadiometer, arm span, standing lower segment measurement, and weight by electronic scale. A physical exam was performed by one of two investigators (R.C.O or J.W.P.) and included Tanner staging. Blood was drawn. A second visit was requested about six months after the first visit. An interval medical history, anthropometrics and Tanner staging were repeated at this time. A second blood draw was optional at this visit.
Assays
Blood was drawn into EDTA tubes and stored at 4 C until processed (generally less than one hour). Blood was centrifuged at 4 C and plasma aliquoted and frozen at −80 C until assayed.
The radioimmunoassay used for CNP was as previously described 12, using a commercial anti-CNP-22 antiserum (Phoenix Pharmaceuticals, Belmont, CA). Limit-of-detection for this assay is 1.0 pM (0.2 pM after sample concentration). Within- and between-assay coefficients of variation of the assay are 4.9% and 8.9% respectively, at 2.1 pM. Cross reactivity with atrial natriuretic peptide (ANP) in this assay is <0.004%. Cross reactivity with human B-type natriuretic peptide (BNP)(at 100 pM) is approximately 4%.
The radioimmunoassay used for NTproCNP was as previously described 13,14 with the following alterations: 100 µl of standard or sample extract was preincubated with 50 µL primary rabbit antiserum (J39) raised against synthetic human proCNP1–15 (diluted to 1:6,000) and incubated for 22–24 hours. The antiserum J39 epitope on proCNP spans amino acid residues 3–15. Fifty microliters of radiolabeled tracer (1,500 cpm) was then added. The detection limit of this assay is 1.2 pM (0.3 pM after sample concentration). Within- and between-assay coefficients of variation are 6.8% and 8.4% respectively, at 14 pM. Cross reactivity with ANP propeptide in this assay is <0.07% and with human BNP propeptide is <0.4%.
A subset of samples was also assayed for NTproCNP using a commercially available sandwich enzyme-linked immunosorbent assay (Biomedica Medizinprodukte GmbH & Co KG, Vienna, Austria, distributed in the US by ALPCO Diagnostics, Salem, NH). Manufacturer’s instructions were followed. Limit of detection for this assay was 0.55 pM.
Statistical analysis
Height, weight, and BMI standard deviation scores (SDS) were calculated using Center for Disease Control 2000 growth charts 15. Height velocity SDS were calculated using data from the World Health Organization (children less than 2 years)16 or from Tanner and Davies 17. Upper-to-lower segment ratio SDS were calculated from data from McKusick 18, as reprinted by Hall, et al. 19. Reference range curves for CNP and NTproCNP were estimated by the LMS procedure 20, using LmsChartMaker software (version 2.4, Harlow Printing Limited, South Shields, UK). These reference range estimates were then used to calculate CNP and NTproCNP SDS.
No assumptions of normal distribution of the data were made. Data are summarized as full range or median and interquartile range (25th – 75th percentiles). Comparisons of NTproCNP based on sex and Tanner stage were done using Kruskal-Wallis Tests with Dunn’s Test post-hoc pairwise comparisons. Correlations between measurements were done by fitting a line by least squares and performing linear regression analysis. Pearson product-moment correlation coefficients (r) are reported. The above analyses were done using Primer of Biostatistics software (version 5.0; McGraw Hill Professional, New York, NY). Significance was assumed for p values less than 0.05.
Results
Subjects
The characteristics of the subject population are summarized in Table 1. The racial/ethnicity makeup of the study population reflects that of the general population in Northeast Florida. As a whole, the subjects were somewhat taller than the general population (median height SDS of 0.3, p<0.005), and somewhat heavier (median BMI SDS of 0.2, p<0.05). Height velocity data were available from 139 subjects and height velocity did not differ from the general population (median height velocity SDS of 0.1), although the range was wider than expected, likely due to short interval between height measurements.
Table 1.
Subject Characteristics
| Number | 258 |
| Gender (M:F) | 155:103 |
| Race/ethnicity (white:black:Hispanic:Asian) | 181:57:14:3 |
| Age at baseline, years (range) | 0.2 – 19.8 |
| Height SDS [median (interquartile range)] | 0.3 (−0.5 – 0.8)* |
| BMI SDS [median (interquartile range)] | 0.2 (−0.4 – 0.8)** |
| Number that returned for second visit | 139 |
| Time between visits, months (range) | 2.4 – 11.9 |
| Height velocity, cm/y (range) | 0 – 26.9 |
| Height velocity SDS [median (interquartile range)] | 0.1 (−1.6 – 1.4) |
| Number that had second blood sample | 94 |
| Total number of blood samples | 352 |
differs from general US population, p<0.005
differs from general US population, p<0.05
CNP and NTproCNP levels by age
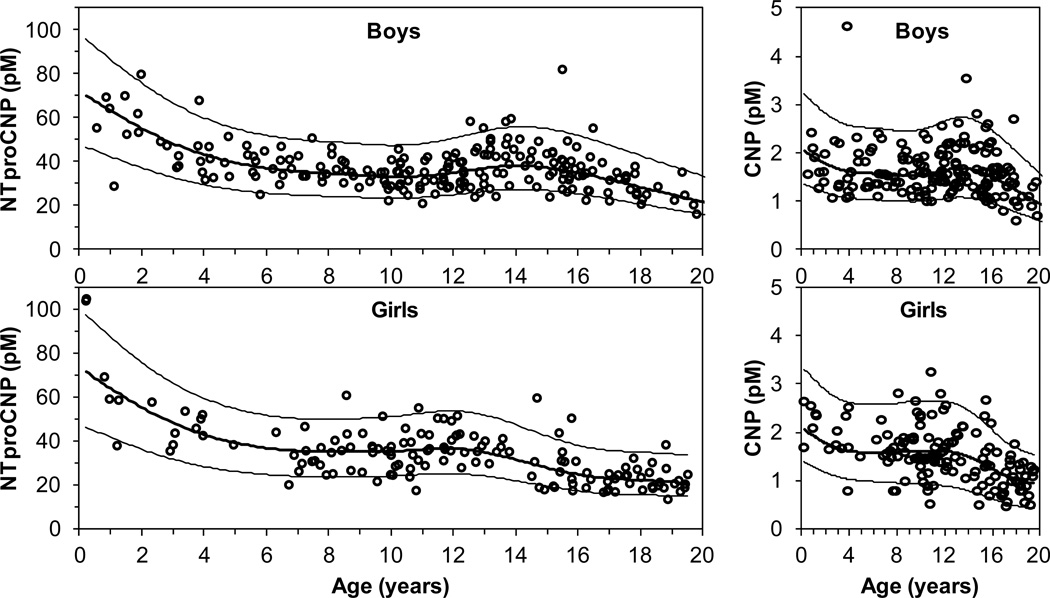
Plasma levels for CNP and NTproCNP measured by RIA varied by sex and age (Figure 1). For both analytes, levels were high in infants and declined with age until puberty. Levels were higher in pubertal subjects, then declined again to adult levels. For children less than nine years of age, there was no difference between boys and girls (data not shown). For all ages, there was no difference between Caucasian, African-American, or Hispanic subjects (data not shown). During puberty, median NTproCNP levels peaked at 14.1 years in boys and at 11.9 years in girls.
Figure 1.
CNP and NTproCNP levels vary by age. Plasma levels of CNP and NTproCNP were determined by RIA for healthy children between the age of 2 months and 19.8 years of age. Open circles, individual data points; heavy line, median; light lines, 5th and 95th percentiles. Top left panel, NTproCNP levels for boys (n = 209), top right panel, CNP levels for boys (n = 178). Bottom left panel, NTproCNP levels for girls (n = 143), bottom right panel, CNP levels for girls (n = 140).
CNP and NTproCNP levels by Tanner stage
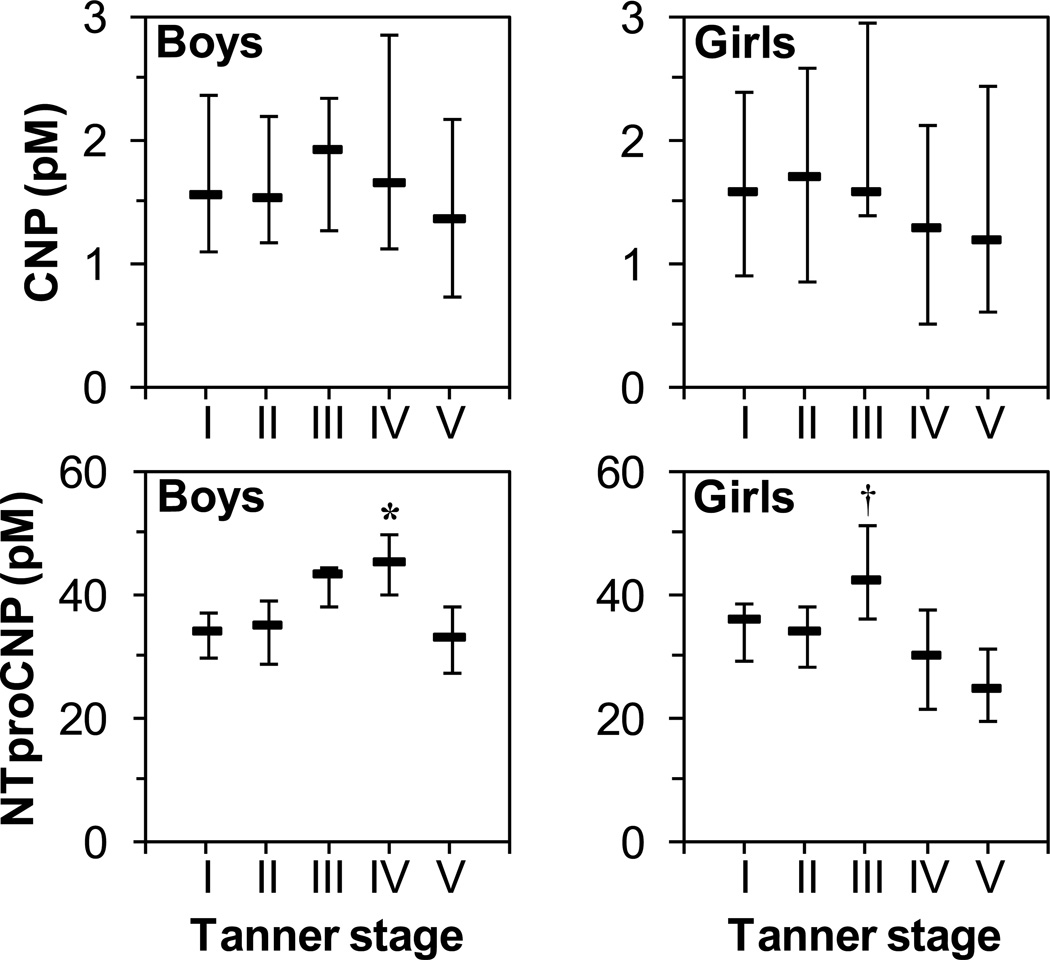
Levels of CNP differed in both boys and girls depending on genitalia Tanner stage (p<0.005 for both), although post-hoc pair-wise comparison reached significance only between boys of Tanner stage III and IV (p<0.05)(Figure 2, upper panels). Differences were more pronounced for levels of NTproCNP (Figure 2, lower panels). For both sexes, NTproCNP differed by Tanner stage group (p<0.0005). For boys, levels were highest in boys at genitalia Tanner stage IV (for IV vs. I, IV vs. II, and IV vs. V, p<0.05; for IV vs. III, p was not significant). For girls, NTproCNP levels were highest for girls at breast Tanner stage III (for III vs. IV, III vs. V, p<0.05; for III vs. I and III vs. II, p was not significant).
Figure 2.
CNP and NTproCNP levels vary by Tanner stage. Bar, median; error bars, 25th and 75th percentiles. For boys, penile Tanner stage was used, for girls, breast Tanner stage. Children of Tanner stage I were older than 6 years of age; Children of Tanner stage V were less than 20 years of age. Top left panel, CNP levels for boys, top right panel, CNP levels for girls. Bottom left panel, NTproCNP levels for boys, bottom right panel, NTproCNP levels for girls. For all four panels, levels differed as group by Kruskal-Wallis Test (p<0.005 for all). *, p<0.05 compared to boys of Tanner stage I, II, and V by Dunn’s Test post hoc pair-wise comparison. †, p<0.05 compared to girls of Tanner stage IV and V.
Correlations with NTproCNP levels
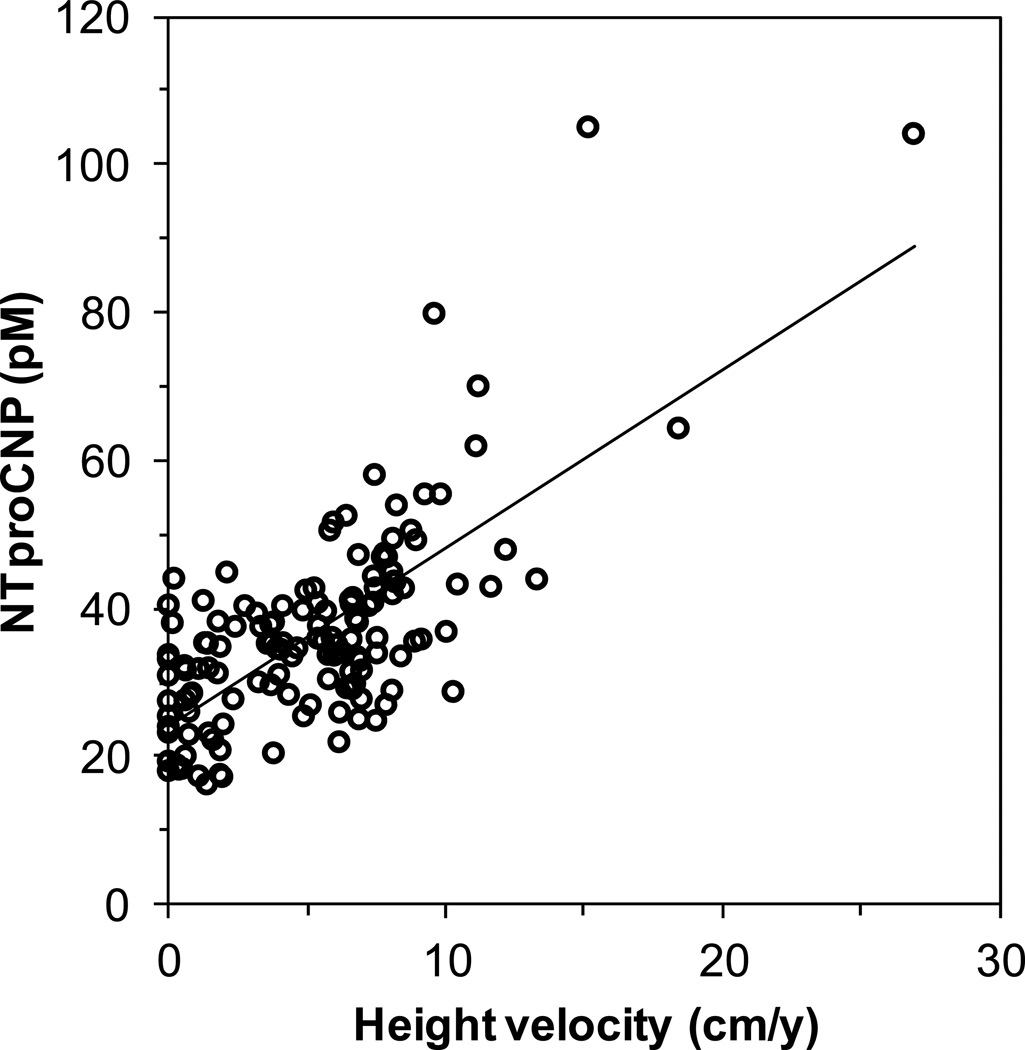
For all subjects, plasma concentrations of NTproCNP were strongly correlated with CNP in the same sample (r=0.566, p<0.0005)(Table 2). There was a linear correlation between CNP level and annualized height velocity (n=138, r=0.314, p<0.0005). There was an even stronger linear correlation between NTproCNP and height velocity (n=139, r=0.711, p<0.0005)(Table 2 and Figure 3). For both CNP and NTproCNP, there were no differences between the regression lines for boys and girls (data not shown) and the data were combined. There were no differences based on race/ethnicity (data not shown). Using the reference range defined here, NTproCNP SDS were calculated in order to quantify levels relative to others of the same age. There were no significant correlations between NTproCNP SDS and height SDS, weight SDS, or BMI SDS (p>0.2 for all). Secondary analysis identified a modest, but significant correlation between NTproCNP SDS and height velocity SDS (r=0.174, p<0.05), as well as a negative correlation between NTproCNP SDS and upper-to-lower segment ratio SDS (r=−0.181, p<0.05)(Table 2).
Table 2.
Correlations with NTproCNP levels
| Comparison | r | p |
|---|---|---|
| NTproCNP vs. CNP | 0.566 | <0.0005 |
| CNP vs. height velocity | 0.314 | <0.0005 |
| NTproCNP vs. height velocity | 0.711 | <0.0005 |
| NTproCNP SDS vs. height SDS | 0.076 | 0.6 |
| NTproCNP SDS vs. height velocity SDS | 0.174 | 0.04 |
| NTproCNP SDS vs. US:LS SDS | −0.181 | 0.004 |
Figure 3.
Correlation between height velocity and NTproCNP levels. Plasma for NTproCNP was taken at the first visit. Annualized height velocity was determined using the height at the first visit and a follow up height taken between 2 and 12 months later. Line, least mean squares linear regression line. The correlation is significant (n=139, r=0.711, p<0.0005).
Comparison of assays
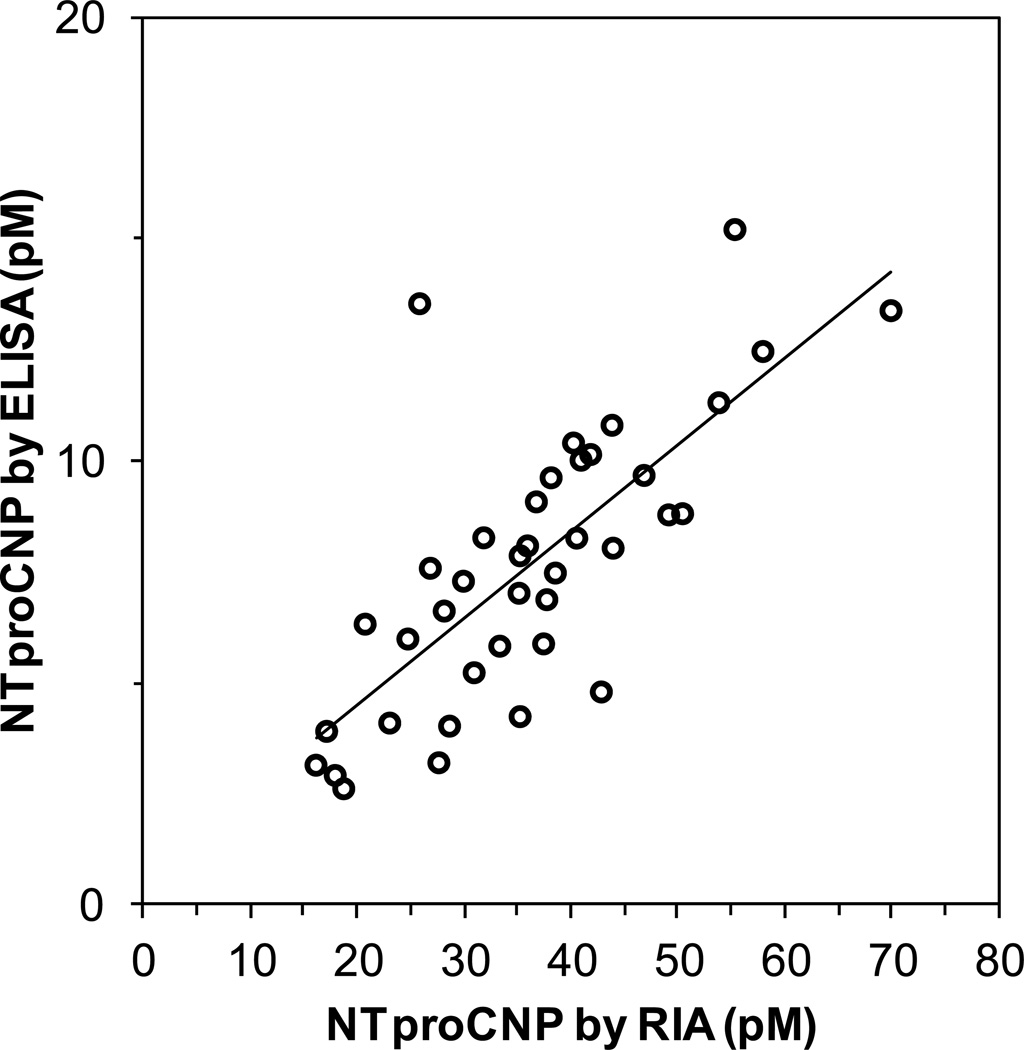
A commercial ELISA kit is available for NTproCNP and a comparison was made with our in house RIA. Plasma samples from forty subjects of widely varying ages (1.4 to 19.8 years; 21 boys, 19 girls) were evaluated by measuring NTproCNP in both assays. Results from one sample were eliminated as a clear statistical outlier. The correlation between the assays was significant (n=39, r=0.748, p<0.0005)(Figure 4). However, the commercial ELISA gave values that were an average of 21% of the RIA values (range 11 – 52%). Running the reference standard provided with the ELISA kit using the RIA demonstrated a disagreement of 15% (stated concentration of 40 pM, measured concentration of 46 pM). A dilution curve was parallel to the RIA standard curve.
Figure 4.
Correlation between the NTproCNP measured by RIA and by ELISA. Plasma samples from 40 healthy children were assayed for NTproCNP both by the in house RIA and the commercially available ELISA. Line, least mean squares linear regression line. A single data point was discarded as a statistical outlier. The correlation is significant (n=39, r=0.748, p<0.0005).
Discussion
This study recruited healthy children in order to study the relationship between plasma NTproCNP levels and height velocity across all phases of linear growth, as well as to define the reference range of CNP and NTproCNP as measured by RIA. The subject population accurately reflected the racial/ethnic distribution of Northeast Florida, including a low representation of people of Asian descent. Since we found no differences between racial/ethnic groups, this distribution should be comparable to other populations, with the exception of Asians, for whom we did not have enough data. Compared to the US population in general, our subjects were somewhat taller and heavier. However, within our population, we found no differences in CNP or NTproCNP levels based on height, weight or BMI, again suggesting the results should be comparable to the general population.
The results for both CNP and NTproCNP show a clear variation based on age. Levels from birth to 2 months of age have been previously reported 21 and showed very high levels in newborns that rise during the first week of life, then trend downward after six weeks of age. We show here that levels fall rapidly in the first year of life, leveling off in prepubertal children. At these ages, there is no difference between boys and girls. During the pubertal years, levels rise again. This occurs at an earlier age in girls than boys. Later in puberty, the levels drop again, eventually reaching the low adult levels. In adults, levels slowly decline during the third decade of life (manuscript in preparation). This pattern parallels that of height velocity, with the exception that, unlike height velocity, CNP and NTproCNP levels do not drop to zero at the completion of puberty. Notably, the age of the median peak NTproCNP (14.1 years for boys, 11.9 years for girls) is almost identical to the age of peak height velocity [13.6±1.1 (mean±SD) in boys and 11.5±1.2 in girls] as reported by Berkey, et al. 22, for children in the US. These strong temporal links are consistent with a dominant contribution to circulating levels of NTproCNP from tissues participating in linear growth.
During puberty, CNP and NTproCNP levels similarly varied by genitalia Tanner stage, peaking in boys at Tanner stage III and IV and in girls at Tanner stage II and III. This agrees with the data by Tanner and Davies 17 for the pubertal stage at the time of peak height velocity in North American children.
A direct comparison of NTproCNP and height velocity demonstrated a strong, positive linear relationship. We had previously identified this relationship in a smaller, heterogeneous population (n=23, r=0.571, p=0.005)10. In this larger study population studied prospectively, the correlation is even stronger (r=0.711). Based on this result, we conclude that height velocity explains 51% of the variability of plasma NTproCNP levels in healthy children.
We have previously reported that NTproCNP levels increase in children starting growth-promoting therapy, either rhGH in children with growth hormone deficiency or idiopathic short stature, or testosterone in boys with constitutional delay of growth and puberty 11. Using pre- and post-treatment height velocities, we identified a similar significant correlation with NTproCNP levels.
Secondary analysis identified a correlation between NTproCNP and height velocity SDS (i.e. at any given age, children growing faster than the population had higher NTproCNP levels), but no relationship between NTproCNP SDS and height SDS (i.e. children taller than the population did not have higher NTproCNP levels). This confirms the clinical dogma that tall children are not necessarily fast growing children at a given point in time. We also identified a negative relationship between NTproCNP SDS and upper-to-lower segment ratio SDS. Information from the genetically altered mice (CNP and NPR-B knockout mice and CNP transgeneic mice) as well as from clinical genetic studies (acromesomelic dysplasia, Maroteaux type, 2q37 translocations, and NPR-B activating mutations) demonstrate that CNP plays a larger role in the regulation of growth of the appendicular skeleton than the axial skeleton. Primary CNP growth disorders result in skeletal disproportion; disruption results in shortened limbs, with the distal elements being most affected, while CNP overexpression results in disproportionately long limbs, again with the distal elements being most affected. Our data suggest a similar effect is operational in healthy children. Those children with relatively high CNP production (as demonstrated by higher NTproCNP levels) have relatively longer limbs and a lower upper-to-lower segment ratio. This finding would require a prospective study for confirmation.
The results reported here for NTproCNP are from an in-house RIA. An ELISA kit is commercially available. When the two assays are compared, the RIA gave values roughly five times those of the ELISA. The values have a significant degree of correlation, however. Cross-platform analysis showed the standards used in both assays are comparable and hence not the source of the difference. High performance liquid chromatography analysis of plasma shows that the NTproCNP peak is broad and contains degraded, lower molecular weight forms 13. We also have evidence that the carboxy-terminal amino acid of the predicted peptide is not present in circulating NTproCNP, suggesting that carboxy-terminal degradation occurs in vivo. In addition, the first two residues from the amino-terminus of circulating NTproCNP are removed by endogenous dipeptidyl peptidase-4 activity. The ELISA utilizes antibodies raised against amino acids 1–19 and 30–50. Any amount of terminal degradation would likely reduce the amount of product being measured by the ELISA. The RIA utilizes a polyclonal antiserum raised to the synthetic peptide proCNP1–15 13 which is the amino-terminus of the propeptide and is therefore not affected by carboxy-terminal degradation. The epitope of the antiserum used by the RIA requires residues 3–15 of proCNP; loss of the two aminoterminal amino acids would not affect binding. We believe this is the source of the difference between the assays. Given the differences in the two platforms, it would not be appropriate to use the reference range we present here for results obtained with the commercial ELISA.
Many peptides have been identified as potential biomarkers correlating with linear growth, most of them products of bone metabolism. They include markers of bone synthesis 23,24 and bone resorption 25, all of which increase during puberty 25,26. Among these, bone specific alkaline phosphatase (BsALP)23, urinary deoxypyridinoline cross-links (DPD)27 and the cartilage specific matrix protein (COMP)28 appeared to show the best correlation with linear growth when assessed during periods characterized by rapid growth. However longitudinal studies show little or no relation to height velocity in healthy children 29,30. In contrast to products of bone metabolism, CNP is synthesized by chondrocytes and acts to promote expansion within all zones of the growth plate 31. While it is rapidly degraded at its source, the bio-inactive metabolite of the prohormone (NTproCNP) enters the circulation and is therefore a logical candidate as a marker of skeletal growth. However, like classical bone turnover markers, CNP synthesis is not limited to the skeleton; it is also expressed in a range of mammalian tissues including osteoblasts 32, brain, reproductive tissues and vascular endothelium 33. Contributions made by such tissues to circulating levels are difficult to assess although in the adult, where plasma levels of NTproCNP are relatively invariant 10, it is likely that constitutive production from the vasculature, bone, heart and liver 34 all contribute to some degree. The much higher levels in children, the evidence of an arterio–venous CNP gradient across bone dense tissues in growing lambs 35 and the marked and concordant changes in NTproCNP accompanying changes in height velocity 10,11,21,36 indicate that the growing skeleton is a dominant source in children. This view is further supported by our finding in the present study showing that in the context of normal growth throughout childhood, 51% of the variance in NTproCNP concentration is determined by variation in height velocity.
To our knowledge, NTproCNP is the first peptide described whose blood levels correlate with height velocity in healthy children of all ages. As such, there is potential clinical utility in assessing NTproCNP levels. In the evaluation of children with short stature, NTproCNP levels might differentiate those with short stature, but normal height velocity from those with poor current growth. Levels before and during growth-promoting therapy may provide information about the efficacy of the therapy. And finally, levels may be useful in identifying when growth is completed. The first step in evaluating these potential uses is the development of a reference range for NTproCNP, as presented here. Future work should establish the impact of renal function or other potentially confounding effects of drugs or illness on plasma concentrations of NTproCNP, and their interpretation in children with growth disorders.
In summary, CNP is expressed and acts in the epiphyseal growth plate, plays a key regulatory role of the growth plate and hence in linear growth. NTproCNP, a marker of CNP biosynthesis, strongly correlates with height velocity in healthy children and in children receiving growth promoting therapies. This is strong evidence for NTproCNP being a biomarker for linear growth.
Acknowledgment
This project was supported by grants from Quest Diagnostics, Inc., and The Nemours Research Programs. The authors would like to thank Dr. Nelly Mauras for providing data and samples on the previously reported subjects, Tina Ewen for administrative assistance, and Shawn Sweeten and Karl Mann for technical assistance with this study.
Footnotes
T.C.R.P. and E.A.E. have a patent pending for NTproCNP peptides and uses thereof.
References
- 1.Chusho H, Tamura N, Ogawa Y, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl.Acad Sci U.S.A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamura N, Doolittle LK, Hammer RE, et al. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl.Acad Sci U.S.A. 2004;101:17300–17305. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasoda A, Komatsu Y, Chusho H, et al. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004;10:80–86. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- 4.Bartels CF, Bukulmez H, Padayatti P, et al. Mutations in the Transmembrane Natriuretic Peptide Receptor NPR-B Impair Skeletal Growth and Cause Acromesomelic Dysplasia, Type Maroteaux. Am J Hum Genet. 2004;75:27–34. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocciardi R, Giorda R, Buttgereit J, et al. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum Mutat. 2007;28:724–731. doi: 10.1002/humu.20511. [DOI] [PubMed] [Google Scholar]
- 6.Moncla A, Missirian C, Cacciagli P, et al. A cluster of translocation breakpoints in 2q37 is associated with overexpression of NPPC in patients with a similar overgrowth phenotype. Hum Mutat. 2007;28:1183–1188. doi: 10.1002/humu.20611. [DOI] [PubMed] [Google Scholar]
- 7.Miura K, Namba N, Fujiwara M, et al. A gain-of-function type mutation of the natriuretic peptide receptor B causes acceleration of skeletal growth and osteoporotic change in humans and mice (abstract) Osteoporos.Int. 2011;22(suppl. 4):S536. [Google Scholar]
- 8.Suda M, Tanaka K, Yasoda A, et al. C-type natriuretic peptide/guanylate cyclase B system in ATDC5 cells, a chondrogenic cell line. J Bone Miner Metab. 2002;20:136–141. doi: 10.1007/s007740200019. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Wu F, Pan J, et al. Furin-mediated processing of Pro-C-type natriuretic peptide. J Biol Chem. 2003;278:25847–25852. doi: 10.1074/jbc.M301223200. [DOI] [PubMed] [Google Scholar]
- 10.Prickett TC, Lynn AM, Barrell GK, et al. Amino-terminal proCNP: a putative marker of cartilage activity in postnatal growth. Pediatr Res. 2005;58:334–340. doi: 10.1203/01.PDR.0000169964.66260.4B. [DOI] [PubMed] [Google Scholar]
- 11.Olney RC, Prickett TC, Yandle TG, et al. Amino-terminal propeptide of C-type natriuretic peptide and linear growth in children: effects of puberty, testosterone, and growth hormone. J Clin Endocrinol Metab. 2007;92:4294–4298. doi: 10.1210/jc.2007-0567. [DOI] [PubMed] [Google Scholar]
- 12.Yandle TG, Fisher S, Charles C, et al. The ovine hypothalamus and pituitary have markedly different distribution of C-type natriuretic peptide forms. Peptides. 1993;14:713–716. doi: 10.1016/0196-9781(93)90102-m. [DOI] [PubMed] [Google Scholar]
- 13.Prickett TC, Yandle TG, Nicholls MG, et al. Identification of amino-terminal pro-C-type natriuretic peptide in human plasma. Biochem Biophys Res Commun. 2001;286:513–517. doi: 10.1006/bbrc.2001.5419. [DOI] [PubMed] [Google Scholar]
- 14.Prickett TC, Kaaja RJ, Nicholls MG, et al. N-terminal pro-C-type natriuretic peptide, but not C-type natriuretic peptide, is greatly elevated in the fetal circulation. Clin Sci (Lond) 2004;106:535–540. doi: 10.1042/CS20030307. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. 2000 CDC Growth Charts for the United States: Methods and Development. Vital and Health Statistics. 2002;11:1–203. [PubMed] [Google Scholar]
- 16.Dept. of Nutrition for Health and Development, W.H.O. WHO Child Growth Standards: Growth velocity based on weight, length and head circumference: Methods and development. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 17.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–329. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- 18.McKusick VA. Heritable disorders of connective tissue. Mosby; 1972. [Google Scholar]
- 19.Hall JG, Allanson JE, Gripp KW, Slavotinek AM. Handbook of Physical Measurements. Oxford: Oxford University Press; 2007. [Google Scholar]
- 20.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 21.Prickett TC, Dixon B, Frampton C, et al. Plasma amino-terminal pro C-type natriuretic Peptide in the neonate: relation to gestational age and postnatal linear growth. J Clin Endocrinol Metab. 2008;93:225–232. doi: 10.1210/jc.2007-1815. [DOI] [PubMed] [Google Scholar]
- 22.Berkey CS, Dockery DW, Wang X, et al. Longitudinal height velocity standards for U.S. adolescents. Stat.Med. 1993;12:403–414. doi: 10.1002/sim.4780120321. [DOI] [PubMed] [Google Scholar]
- 23.Tobiume H, Kanzaki S, Hida S, et al. Serum bone alkaline phosphatase isoenzyme levels in normal children and children with growth hormone (GH) deficiency: a potential marker for bone formation and response to GH therapy. J Clin Endocrinol Metab. 1997;82:2056–2061. doi: 10.1210/jcem.82.7.4081. [DOI] [PubMed] [Google Scholar]
- 24.Kanbur NO, Derman O, Kinik E. The relationships between pubertal development, IGF-1 axis, and bone formation in healthy adolescents. J Bone Miner Metab. 2005;23:76–83. doi: 10.1007/s00774-004-0544-9. [DOI] [PubMed] [Google Scholar]
- 25.Crofton PM, Wade JC, Taylor MR, et al. Serum concentrations of carboxyl-terminal propeptide of type I procollagen, amino-terminal propeptide of type III procollagen, cross-linked carboxyl-terminal telopeptide of type I collagen, and their interrelationships in schoolchildren. Clin Chem. 1997;43:1577–1581. [PubMed] [Google Scholar]
- 26.Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007;92:443–449. doi: 10.1210/jc.2006-1706. [DOI] [PubMed] [Google Scholar]
- 27.Rauch F, Georg M, Stabrey A, et al. Collagen markers deoxypyridinoline and hydroxylysine glycosides: pediatric reference data and use for growth prediction in growth hormone-deficient children. Clin Chem. 2002;48:315–322. [PubMed] [Google Scholar]
- 28.Posey KL, Hecht JT. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr Drug Targets. 2008;9:869–877. doi: 10.2174/138945008785909293. [DOI] [PubMed] [Google Scholar]
- 29.Bjarnason R, Andersson B, Kim HS, et al. Cartilage oligomeric matrix protein increases in serum after the start of growth hormone treatment in prepubertal children. J Clin Endocrinol Metab. 2004;89:5156–5160. doi: 10.1210/jc.2004-0587. [DOI] [PubMed] [Google Scholar]
- 30.Federico G, Baroncelli GI, Vanacore T, et al. Pubertal changes in biochemical markers of growth. Horm.Res. 2003;60:46–51. doi: 10.1159/000071225. [DOI] [PubMed] [Google Scholar]
- 31.Yasoda A, Kitamura H, Fujii T, et al. Systemic administration of C-type natriuretic peptide as a novel therapeutic strategy for skeletal dysplasias. Endocrinology. 2009;150:3138–3144. doi: 10.1210/en.2008-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suda M, Komatsu Y, Tanaka K, et al. C-Type natriuretic peptide/guanylate cyclase B system in rat osteogenic ROB-C26 cells and its down-regulation by dexamethazone. Calcif.Tissue Int. 1999;65:472–478. doi: 10.1007/s002239900735. [DOI] [PubMed] [Google Scholar]
- 33.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine dependent signaling functions. Endocr.Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 34.Palmer SC, Prickett TC, Espiner EA, et al. Regional release and clearance of C-type natriuretic peptides in the human circulation and relation to cardiac function. Hypertension. 2009;54:612–618. doi: 10.1161/HYPERTENSIONAHA.109.135608. [DOI] [PubMed] [Google Scholar]
- 35.Prickett TC, Charles CJ, Yandle TG, et al. Skeletal contributions to plasma CNP forms: evidence from regional sampling in growing lambs. Peptides. 2011;30:2343–2347. doi: 10.1016/j.peptides.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Reh CS, Olney R, Azen C, et al. Plasma CNP Forms and Thyroid Status in Prepubertal Children with Acquired Thyroid Disease. Clin Endocrinol (Oxf) 2011 doi: 10.1111/j.1365-2265.2011.04187.x. Aug 4 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]