Abstract
A major question concerning the learning and memory deficits characteristic of epilepsy is the relative importance of the initial insult that leads to recurrent, unprovoked seizures versus the seizures themselves. A related issue is whether seizure-induced cognitive decline is permanent or reversible when convulsions cease. To address these problems, adult rats were extensively trained in the “spatial accuracy task”, a dry-land analog of the Morris water maze. This task allows the rat’s estimate of the location of a hidden goal zone to be repeatedly measured within each behavioral session. One aim was to measure, in well-trained animals, the time course of any cognitive impairment caused by a daily flurothyl-induced generalized seizure over 11 days. A second aim was to look for possible recovery during 9 subsequent days with no seizures. We saw a cumulative degradation in spatial performance during the seizure days and reversal of the deficit after seizures were stopped such that performance returned to baseline. Interestingly, the rate of learning to an asymptote, the rate of performance decline during one-per-day seizures and the rate of relearning during the recovery period were all similar. Given that the hippocampus plays an important role in spatial memory and that it is the brain structure most vulnerable to abnormal excitation the implication is that the hippocampus remains essential for precise spatial navigation even after prolonged training in locating a fixed goal zone. Clinically, this finding questions the assumption that patients who experience seizures should return to a baseline cognitive level within hours.
Keywords: epilepsy, spatial navigation, spatial memory, learning, hippocampus
Introduction
Epilepsy is a central nervous system disorder that frequently develops after severe brain insults and is characterized by recurrent, unprovoked seizures. Impairments of learning and memory are among the most disabling neuropsychological consequences of epilepsy (Hermann et al., 1997; Helmstaedter, 2002; Jokeit and Ebner, 2002). The mechanisms responsible for this high incidence of memory impairment in individuals with epilepsy are not clear. The initial damage responsible for the subsequent seizures is one possible cause of the memory disturbances (Armstrong, 1993). The brain, particularly mesial temporal lobe structures, are highly susceptible to seizure-induced cell loss, and patients with epilepsy are at high risk for memory deficits (Jokeit and Ebner, 1999, 2002; Jokeit et al., 2001). There is also evidence that in some patients, recurrence of seizures can lead to changes in cognitive function, most notably memory (Jokeit and Ebner, 1999, 2002; Hermann et al., 2006; Cheung et al., 2006). In humans with intractable temporal lobe epilepsy, the decline of ipsilateral hippocampal volume correlates with the lifetime seizure number (Kalviainen and Salmenpera, 2002; Fuerst et al., 2003) supporting the concept that recurrent seizures can have long-term consequences.
Thus, the extent to which deficits in patients with seizures are secondary to the cause of the epilepsy or to the recurrent seizures themselves remains unsettled. In addition, patients with epilepsy faced a variety of problems immediately after a seizure that include confusion and amnesia (Biton et al., 1990; Helmstaedter et al., 1994; Aldenkamp, 1997; Leutmezer and Baumgartner, 2002). It is also unclear how these acute seizure-induced changes contribute to cognitive impairment. Furthermore, the degree to which seizure effects are cumulative and the related issue of the persistence of the changes are not known.
The aim of the present experiment is to ask if recurrent seizures in neurologically intact adult rats produce cumulative performance deficits in a highly practiced spatial task, and if so whether the deficiencies are permanent or reversible. Since rat hippocampal lesions cause specific spatial memory deficits (Morris et al., 1986b; Packard and McGaugh, 1996) and hippocampal place cell activity changes in parallel with changes in map-based navigation (Lenck-Santini et al., 2002; Fenton et al., 2000a; Kubie et al., 2007), we used the “spatial accuracy” task (Kubie et al., 2007) to assess the temporal dynamics of possible performance impairments. This hidden goal task is conceptually similar to the Morris water maze (Morris et al., 1982a) and to the “place preference” task (Rossier et al., 2000) from which it is directly derived. In the spatial accuracy task, the rat must learn to pause inside a small, circular unmarked goal zone to release a single food pellet. It is similar to the water maze in that it is a hidden goal problem; it is different than the place preference task in that the animal must actually pause with good spatial precision to get a reward; simply walking around in the goal zone is not sufficient. The importance of the hippocampus for the selected task is shown by the discovery of secondary firing fields for hippocampal place cells at the goal location (Hok et al., 2007).
Because it is performed in a dry environment, the spatial accuracy task simplifies electrical recordings compared to the water maze, but it has other, crucial advantages for determining the ability of rats to navigate efficiently. Specifically, since it is appetitive in nature, rats are willing to repeat the cycle of navigate-pause-release-forage-navigate many times during an experimental session. Moreover, since the rat is trained to pause, a specific location can be ascribed to each stop of sufficient duration. Because of these properties it is possible to use statistical measures on the individual pauses to characterize spatial ability. In one method, we count the number of pauses made within the experimenter-defined goal zone. In another, we measure the spatial dispersion of pauses by computing the root mean square distance of each pause away from the centroid of pauses.
Using the spatial accuracy task, we looked for cumulative effects of recurrent seizures in adult rats that were highly trained before any seizures were induced. We report both clear-cut impairments that gradually worsened over 11 days of once per day flurothyl-induced seizures and clear-cut recovery over a similar time course once the seizures were stopped.
Materials and Methods
Subjects
Adult male Long-Evans rats were used in all experiments and were treated in accordance with NIH guidelines for the humane treatment of animals. Rat had free access to water but food was restricted to bring the weight to 85% of the ad libitum level; five grams of weight gain were allowed per week. The rats were individually housed in plastic cages under diurnal lighting conditions with 12 hours on and 12 hours off. Twelve rats were used, six in the seizure group and six in the control group.
Apparatus
The experimental chamber was a 90 cm diameter, 70 cm height gray cylinder that was enclosed by a black curtain. The floor of the cylinder was a sheet of speckled yellow formica that was wiped with wet paper after each session to minimize possible odor cues. On the cylinder wall were two cue cards that occupied the upper half of the wall and 45° of the cylinder arc; one card was white, the other black. The card centers were separated by 135° such that the white card center was clockwise relative to the black card center. In the view of the overhead TV camera used for rat tracking, the midpoint between the cards was at 3:00 o’clock; this was also 0° in the angular coordinate whose value increased in the counter-clockwise direction.
Tracking the rat’s position
To prepare the hooded rats for training and tracking, the dark portion of the fur from the neck down was bleached with hydrogen peroxide, leaving only the head its original dark color. Tracking was done via contrast detection between the dark fur and the lighter floor background. Video frames from a FireWire camera were processed at 30Hz. The diameter of the circular tracking region was set at 96cm, ~6% larger than the cylinder diameter; pixels outside this circle were filtered out. A brightness threshold was established to distinguish dark pixels on the rat’s head from other pixels. The rat’s position was taken as the center of gravity of all accepted dark pixels.
Acute seizure induction
Flurothyl (bis-2,2,2-triflurothyl ether), a potent and rapidly acting central nervous system convulsant, was used to induce generalized tonic-clonic seizures (Zhao and Holmes, 2006; Zhou et al., 2007b). Rats that inhale flurothyl go through a series of behaviors consisting of agitation, running, myoclonic, clonic, and tonic activity. Seizure induction was done in a room different from the training room. Seizure group rats were placed in a small rectangle glass tank (40cm × 20cm × 27cm) and liquid flurothyl was delivered at three mL/h using a syringe pump. The flurothyl dripped onto a strip of filter paper just beneath the tank cover from which it evaporated. A rat was exposed to flurothyl until tonic extension of forelimbs and hind limbs was observed at which time the animal fell down. The typical flurothyl volume delivered per seizure induction was ~0.35 mL. The tank was flushed with room air inside a fume hood immediately after the fall. The seizure group rats were given convulsions three hours after session one. The control group rats were placed in the same glass tank but tap water was delivered instead of flurothyl.
Behavioral training
Rats were trained over several weeks to release 25 mg sugar pellets from an overhead feeder by entering a rat-specific, unmarked, circular goal zone and remaining there for ≥ 1.2 sec. After releasing a pellet, the rat had to leave the goal zone for a minimum of three seconds for the next reward to be available. Generally, rats chased down the randomly scattered pellet and stayed out of the goal zone considerably longer than the minimum three seconds.
Each rat was randomly assigned to one of four goal zones. Each zone was initially 35 cm in diameter and centered 30 cm away from the wall at 45°, 135°, 225° and 315°. A modified method of limits procedure was used to change the goal zone diameter according to the following rules: (1) It was reduced by 2.5 cm if the rat obtained ≥ 2 pellets/min for two days. (2) It was restored to the previous value if the rat obtained ≤ 1 pellet for two days. (3) It was unchanged if the number of pellets per minute was > 1 and < 2. The final goal zone diameter was 16 cm for every rat.
Ten-minute training sessions were given twice daily, five days a week. Every other day during the first two training weeks a two cm diameter yellow circular sticker was glued at the goal zone center to visibly mark its location. During the third week, a few pellets were placed in the unmarked goal zone at the start of each session. For the remaining training weeks, the goal zone was not marked in any way. During the last training week, the pellet feeder was turned off for two minutes either at the start or halfway through a session. This extinction training was done to encourage rats to continue to perform even if no pellet was dropped for several minutes.
The experimental protocol
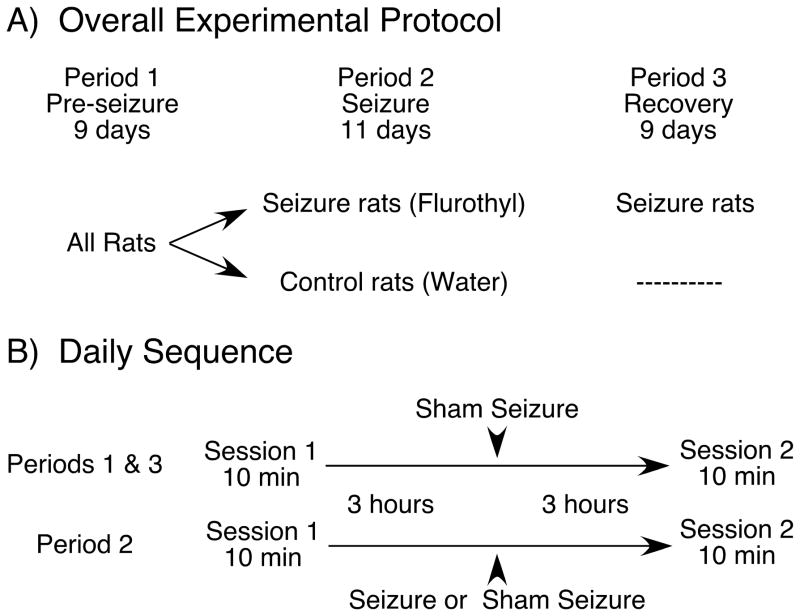
After six to eight weeks of training 12 rats reached the criterion of obtaining > 2 pellets/min in six sessions over three consecutive days. Half the rats were randomly assigned to the seizure group and half to the control group. Data collection began immediately after training and was divided into a pre-seizure period of 9 days, a seizure period of 11 days and a recovery period of 9 days for a total of 29 uninterrupted days (Figure 1A).
Figure 1.
Protocol for the spatial accuracy task. A: Protocol for the entire experiment. There were three periods: (1) Pre-seizure, during which training was completed. At the end of the pre-seizure period, rats were divided into two groups of six each. (2) Seizure, during which flurothyl seizures were induced once a day for the seizure group; the control group was given daily sham seizures in the same chamber used for flurothyl exposure. (3) Recovery, during which testing continued for seizure rats with no additional seizures. B: Daily protocol. Rats were tested twice a day for spatial accuracy task performance; sessions 1 and 2 were separated by six hours. In periods one and three, sham seizure-inductions were given at the midpoint of the six-hour interval between sessions. In period two, the same procedure was followed except that seizure group rats were given flurothyl-induced seizures.
Each day consisted of two 10-min sessions separated by six hours. One of every three sessions was randomly chosen to include a two-minute extinction period at its beginning or after five minutes. During the seizure period, rats were confined to the glass tank three hours after the first daily session and either flurothyl (seizure group) or water (control group) was admitted into the tank (Figure 1B). During the recovery period, testing was continued only for the seizure group.
Pause detection
A pause was defined as a time interval ≥ 1.2 sec during which the rat’s head position did not change by more than eight cm (radius of the final goal zone). When a pause was detected inside the goal zone a pellet was released. Recorded data included all positional information for the session, the start and end of a pause, an indication that the pause was rewarded or not and the cumulative number of rewards during the session. During an extinction interval, the pause detection algorithm remained the same but no pellets were released.
Data analysis
We estimated performance in two ways. In the first, we counted the number of rewarded pauses during a session. A pause was included as a rewarded pause if it occurred during an extinction interval but otherwise would have trigged a pellet release. Performance was considered to improve if the number of rewards went up.
The second performance estimate measured the spatial dispersion of accepted pauses. Performance was considered to improve if the dispersion went down. To calculate dispersion we first eliminated near-wall pauses in a 14.5 cm diameter annulus that extended from the wall inward into the cylinder. This eliminates pauses caused by the rat consuming pellets near the wall, since many pellets rolled near the wall after dropping. The position of the centroid was calculated as:
where Cx is the x coordinate of the centroid, Cy is the y coordinate, and are respectively the x and y coordinates of the ith pause and n is the number of pauses. Pause dispersion was taken as the root mean square (RMS) distance of all pauses from the centroid in a 45 cm diameter circle with the centroid at its middle.
Locomotor speed
To determine if seizures affected locomotor speed, a key aspect of behavior, we measured the distance moved in 0.2 sec intervals.
Statistics
For statistical analysis, any p-value ≤ 0.05 was considered significant.
Results
When generalized seizures were induced once per day in rats for 11 days, a progressive decline was seen in the spatial accuracy performance. When the seizures were stopped, the decline reversed so that after 9 days of recovery performance was back to the levels seen before any seizures were given. We first graphically show these results for an individual rat and contrast them against the performance of a second, control rat never exposed to seizures. We then numerically characterize the performance of control and seizure group over the time course of the experiment.
Across-day effect – Individual examples of spatial performance by control and seizure rats
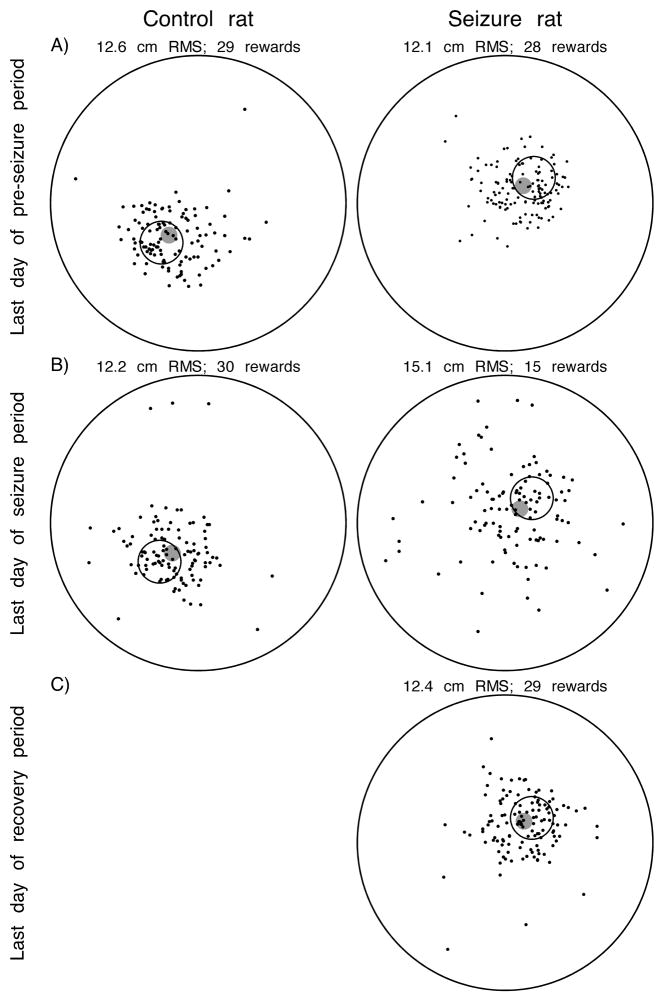
The ability of example control and seizure rats to obtain rewards by entering and remaining in the hidden goal zone was equal during the second daily session at the end of the pre-seizure period. Thus, there were similar, tightly clustered pauses in and around the goal zone for the control rat (Figure 2A left) and the seizure rat (Figure 2A right). The dispersion of pauses as measured by RMS distance from the centroid and the number of rewarded pauses were nearly equal for the two rats.
Figure 2.
Examples of performance at the end of each experimental period for a control rat (left) and a seizure rat (right). The large circles show the extent of the cylinder. The smaller black circles show the location of the goal zone. Each dot shows the location of a pause long enough to have released a pellet had it been made inside the goal zone; pauses made very close to the cylinder wall are excluded (see Methods). The filled grey circles indicate the location of the pause centroid. Summaries of performance are given above each cylinder picture. A: On the last pre-seizure day, pauses are emitted in and near the goal zone for both rats. B: By the last seizure day, performance by the control rat has hardly changed but the much larger dispersion of pauses by the seizure rats is evident. This reduced precision is reflected by the increase of RMS distance and the lower number of rewards. C: On the last recovery day, performance by the seizure rat has returned to pre-seizure baseline according to inspection of the pause distributions, the decreased RMS distance and the increased number of rewards.
After 11 additional days of practice and exposure to sham seizures, the distribution of pauses and the number of rewarded pauses in the second daily session hardly changed for the control rat (Figure 2B left). In contrast, at the end of the 11-day seizure period the RMS distance dispersion has increased by ~28% and the number of rewards has decreased by ~50% in the second daily session for the seizure rat (Figure 2B right). The increased RMS dispersion is visible by comparing the left and right sides of Figure 2B.
No additional sessions were run for control rats but seizure rats were followed for a subsequent 9-day recovery period. At the end of this period (Figure 2C right) the number of rewarded pauses (29) and the RMS distance (12.4 cm) were nearly the same as at the end of the pre-seizure period.
A separate feature of the pause distributions may be noted: in the illustrated cases and in virtually all other sessions (not shown) the pause density in and around the goal zone was greater towards the cylinder center than towards the wall. Apparently, the ability to use the rat’s distance from the wall to serve as a cue for locating the goal zone decreases away from the wall so that the probability of erroneous pauses is asymmetric.
Time course of seizure effects on spatial accuracy performance
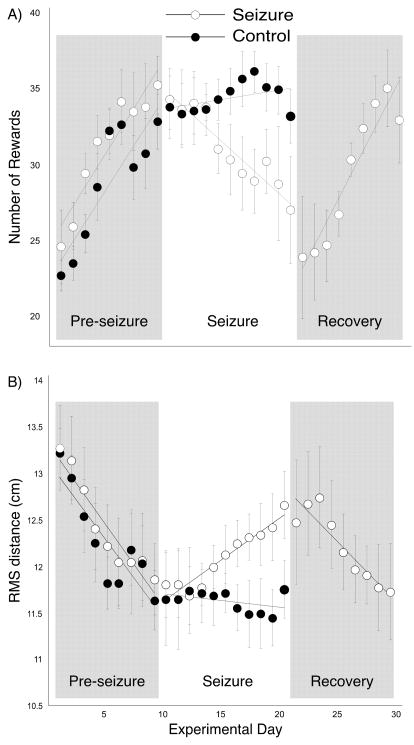
The ability of seizure and control rats to find the hidden goal zone over successive experimental days is shown in Figure 3. For the number of rewarded pauses (Figure 3A) each daily point was obtained in the following way. We first measured and then averaged the number of rewards for the two sessions in a day. Next we averaged daily values within a group. Finally, we took a two-day running average for each group. The calculations were done separately for the pre-seizure, seizure and recovery periods. Points for the RMS distance (Figure 3B) were computed in the same way.
Figure 3.
Grouped daily performance during the three experimental periods. A: Number of rewards. There is continuing improvement over the 9-day pre-seizure period for both groups although the improvement is slowing toward the end of the period. During the seizure period, performance by the control group stays nearly constant whereas performance by the seizure group gets considerably worse. Only the seizure group was tested during the recovery period during which their performance once again improved so that at the end of the 9-day recovery period it was indistinguishable from performance before seizures. In each period the data points are two-day running averages except for the first point which is for a single day. Lines are least square fits to data points in the relevant period. B: RMS distance. Except for the opposite relationship between rewards number and performance, the patterns for both groups reproduce changes seen according to the rewards measure.
In the pre-seizure period, performance by both seizure and control rats improved but appeared to be reaching asymptotes of 33 rewards and ~11.8 cm RMS around days eight and nine. Two-way ANOVAs with repeated measures showed no group effect (Reward: F(1,8), p = 0.31; RMS: F(1,8), p = 0.64) but clear time trends (Reward: F(8,80), p < 0.0001; RMS: F(8,80), p < 0.0001). As expected from the parallel progress, there was no group by time interaction (Reward: F(8,80), p = 0.65; RMS: F(8,80), p = 0.37). The inference that performance was nearly asymptotic during the pre-seizure period is strengthened by the lack of major improvements during the seizure period by the control rats according to either measure.
In the seizure period, the nearly constant performance by control rats was in strong contrast to the progressive decay of performance by seizure rats that showed both decreases of rewarded pauses and increases of RMS distance. Note that the convulsion effects seem to be cumulative; the separation between the two groups grew from the start to the end of the seizure period. For the reward measure, a two-way ANOVA with repeated measures revealed neither a significant group difference (F(1,10) = 1.77; p = 0.21) nor a significant effect of time (F(10,100) = 1.72; p = 0.086) but a large group by time interaction (F(10,100) = 3.45; p = 0.0006). The same overall pattern was seen for the RMS distance measure where there was neither a group (F(1,10) = 1.43; p = 0.26) nor a time (F(10,100) = 1.06; p = 0.38) effect but a clear trend towards a significant group by time interaction (F(10,100) = 1.86; p = 0.059). Thus, recurrent seizures caused performance to get worse as a function of time. To find the day on which performance was first reliably reduced we computed the z-score for each day by subtracting the daily mean for seizure rats from the mean of the final pre-seizure day and normalizing by the standard deviation of the final pre-seizure day. According to the reward measure, the deficit became significant at day five (z = −4.54, p < 0.0001) whereas according to RMS distance (z = 2.25, p = 0.02) performance became reliably worse on day eight.
At the end of the seizure period, the seizure group was run for a 9-day recovery period with no additional convulsions. At the start of this period, performance was poor according to both measures but improved with succeeding days so that by day nine the seizure rats did as well as before any seizures. This impression is corroborated by doing z-tests for each day in the recovery period against the mean and standard deviation of the last pre-seizure day. According to the reward measure, the performance difference was significant until day six of recovery (z = −1.31, p = 0.19) whereas according to the RMS measure performance was back to baseline by day four (z = 1.81, p = 0.07).
It is also interesting that the slopes of performance improvement for seizure rats during the pre-seizure and recovery periods are similar (unpaired t-test for reward: p=0.54; for RMS: p=0.56). It is as if recurrent seizures caused a loss of precision in spatial navigation that was reversible at the original learning rate once the seizures were stopped.
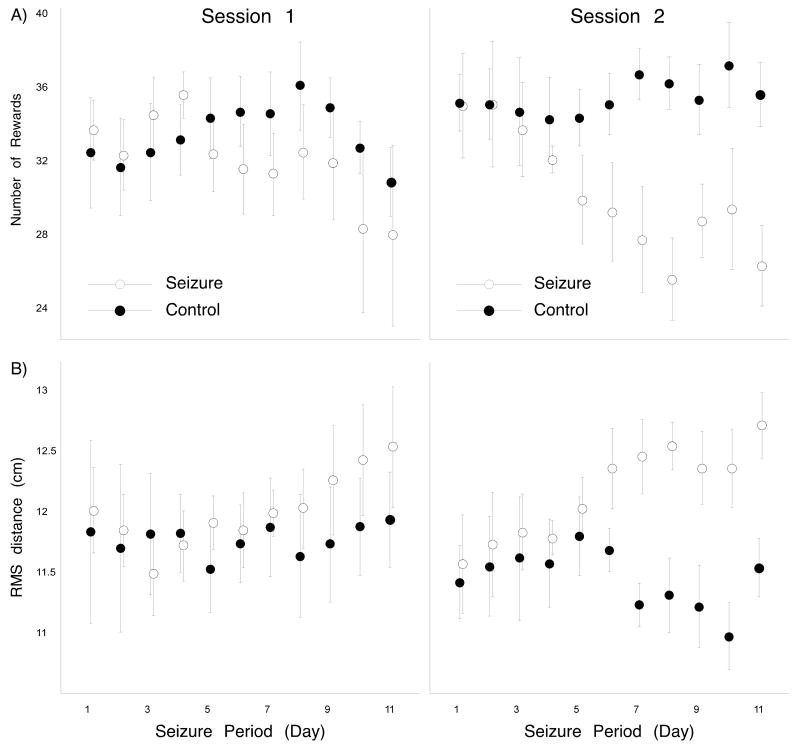
Changes of performance within a day during the seizure period
Since performance was measured at three hours before and three hours after daily seizures or sham seizures we could compare performance in the two sessions. Two-day running averages are shown in Figure 4 for session 1 (left side) and session 2 (right side); rewarded pause counts are presented in Figure 4A and RMS distance values are presented in Figure 4B. For both measures it is clear that the seizure-induced deficit is greater and occurs earlier for session 2 than session 1. This impression is confirmed by two-way repeated measure ANOVAs for session 2. For the reward measure, both group (F(1,10) = 4.26; p = 0.066) and time (F(10,100) = 1.99; p = 0.043) factors are near the 0.05 level of significance. As suggested by the pattern of data points, the interaction effect is very clear (F(10,100) = 3.41; p = 0.0007). For RMS distance, the group factor (F(1,10) = 4.56; p = 0.059) is close to the significance threshold but there is no overall effect of time (F(10,100) = 1.38; p = 0.20). There is a clear group by time interaction, based mainly on the increased RMS distance for the seizure group (F(10,100) = 2.73; p = 0.005).
Figure 4.
The effects of daily seizures appear sooner and are larger during the second session compared to the first session. A: In session 1, the number of rewards is comparable for seizure and control rats up to day six of the seizure period at which time seizure rat performance is significantly worse (see text). A similar separation takes place around day eight according to the RMS distance. B: In session 2, the separation between seizure and control rats takes place around day four of the seizure period according to number of rewards and around day six according to RMS distance.
In contrast to daily session 2, there were no reliable performance differences in session 1; according to a two-way ANOVA with repeated measures there were no group or time effects nor a group by time interaction (Reward: F10,100 = 1.03, p = 0.42; RMS: F10,100 = 0.75; p = 0.68). It therefore seems that overnight rest allowed full recovery for the seizure rats. Nevertheless, additional analysis, however, revealed cumulative effects in session 1 alone. By inspection, performance in session 1 by seizure rats compared to control rats seemed to worsen towards the end of the seizure period. When the last 6 days of the seizure period were pooled, seizure rats got fewer rewards than control rats (unpaired t70 = 2.17, p = 0.03). There was also a trend for poorer performance during the second half of the seizure period according to the RMS distance measure (unpaired t70 = 1.74, p = 0.086).
We also separated the two daily sessions in the recovery period and saw that performance returned towards pre-seizure levels at essentially the same rate for both sessions; for this analysis, daily values in each session were compared to the corresponding session on the last day of the pre-seizure period. For rewards, the session 1 difference underwent a transition between significant on day four (z = −8.33, p < 0.0001) and not significant on day five of the recovery period (z = −1.32, p = 0.19); the transition for session 2 also went from significant on day four (z = −2.25, p = 0.02) to not significant on day five (z = −1.62, p = 0.11). For RMS distance, the session 1 difference went from significant on day three (z = 2.61, p = 0.009) to not significant on day four (z = 0.71, p = 0.48) whereas the session 2 difference went from significant on day two (z = 2.56, p = 0.01) to not significant on day three (z = 1.66, p = 0.10).
Changes of performance during a session
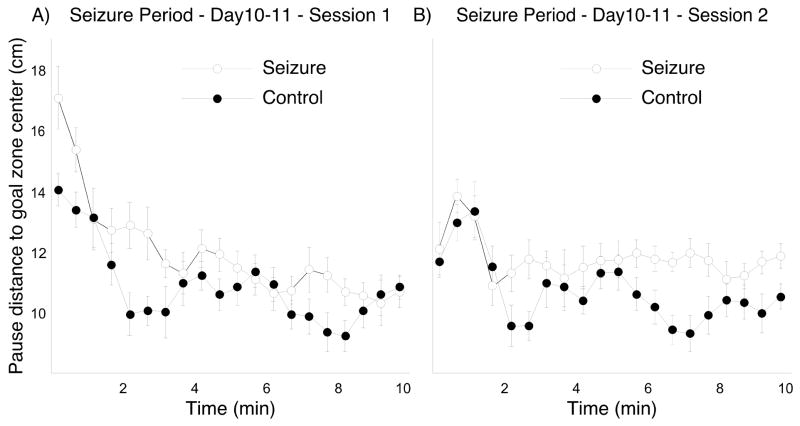
In addition to analyzing across-day and between session seizure effects, we asked if there were systematic changes in the distance of pauses from the goal zone center during both the first and second daily sessions. We were interested, in other words, in whether rats could use information from early successful pellet releases to increase the precision of subsequent pause attempts. To this end, we computed the mean distance between pauses and the goal center at 30-second interval and then the running average of two intervals. Since the performance separation between the two groups was greatest near the end of the seizure period we considered only the last two seizure days. The mean pause to goal center distance for days 10 and 11 combined is shown as a function of time for session 1 in Figure 5A and for session 2 in Figure 5B.
Figure 5.
Pause-distance to the goal center as a function of time during a session; the data are averages of the last two days of the seizure period. A: During session 1, the clearest differences between seizure and control groups occur in the first minute of the session. Although the seizure group performance comes close to control toward the end, there is a clear trend toward group separation. B: During session 2, performance was similar at the session start but persistently worse for the seizure group from the second minute forward.
A two way ANOVA with repeated measures of distance between pauses and the goal center for session 1 revealed that the group effect was very close to the 0.05 level of probability (F(1,10) = 4.70; p = 0.055). The time effect was substantial (F(19,190) = 16.17; p < 0.0001) and there was a just significant interaction (F(19,190) = 1.65; p = 0.048). Paired t-tests showed that the average pause-to-goal distance was greater in the first two 30-sec intervals compared to the rest of the session for both groups, implying overnight forgetting and rapid refreshing of the goal zone location (Control: t5 = 3.25, p = 0.028; Seizure: t5 = 3.56, p = 0.016). In addition, since the pause-to-goal distance was significantly greater for the seizure rats during the first two 30-sec intervals, it appears that the overnight forgetting was greater for the seizure group.
A similar ANOVA for pause-to-goal distance for session 2 revealed a significant group effect (F(1,10) = 7.11; p = 0.024) and a significant time effect (F(19,190) = 4.82; p < 0.0001) but no interaction (F(19,190) = 1.36; p = 0.15). The distance decreases for both groups between the first three 30-sec intervals combined relative to the rest of the session but there is no group difference at the outset of session 2. The performance deficit for the seizure group started to appear after two minutes and persisted all the way to the end of the session.
Behavioral activity during the seizure period
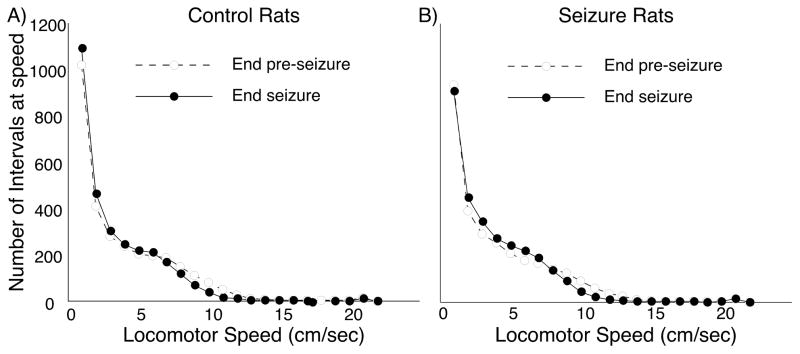
To address the possibility that deficits in spatial accuracy performance are secondary to seizure-induced changes in behavior we made two additional analyses. In the first we measured locomotor speed every 0.2 second for the last day of the pre-seizure and seizure periods. The distribution of time spent at speed is plotted for the control group in Figure 6A and for the seizure group in Figure 6B. The impression that speed distribution is invariant between the last pre-seizure and last seizure days for both groups is borne out by Wilcoxon rank-sum tests (Control: p = 0.67; Seizure: p = 0.55). Wilcoxon rank-sum tests also reveal no differences between the two groups on Day 9 of the pre-seizure period (p = 0.83) or Day 11 of the seizure period (p = 0.86) implying that the reduced number of pellets released by the seizure group is not due to changes in walking speed.
Figure 6.
Time spent by rats walking at different locomotor speeds. The bin size is one cm/sec. Open circle/dashed lines show the last day of the pre-seizure period; solid circle/solid lines show the last day of the seizure period. By inspection, it is clear that neither the passage of time (control group), nor time plus convulsions (seizure group) altered the distribution of time that rats spent walking at different speeds. Superimposing the pre-seizure lines and the seizure period lines for the two groups shows there were no differences in locomotor behavior at either time point.
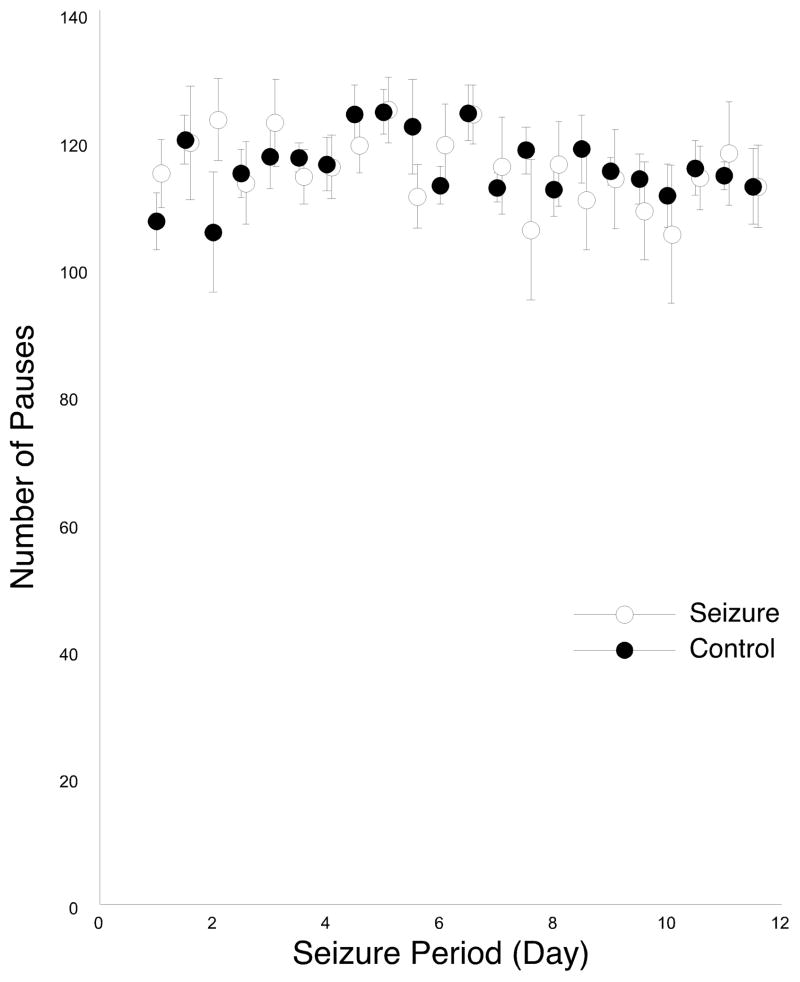
We also counted the total number of pause attempts detected in the 45 cm diameter zone around the pause centroid during the seizure period for both sessions 1 and 2. The outcome of this analysis for both groups is shown in Figure 7. No trend for changes in the number of emitted pauses was seen, suggesting that the smaller number of rewards for seizure rats is not due to an overall reduction in the number of pause attempts to release a pellet. These impressions are confirmed by two-way repeated measure ANOVAs for the separate sessions. For session 1, there is no effect of main factor group (F(1,10) = 0.47, p = 0.51), nor any effect of main factor time (F(10, 100) = 1.48, p = 0.16). And as expected, there is no group by time interaction (F(10,100) = 0.72, p = 0.70). Analysis for session 2 also show no effect of group (F(1,10) = 0.64, p = 0.44) or time (F(10,100) = 1.57, p = 0.13), as well as any interaction effect (F(10,100) = 0.46, p = 0.91).
Figure 7.
Number of pauses made by seizure and control rats during the seizure period; sessions 1 and 2 are plotted on the same graph. The lack of a trend for either session and the lack of difference between the groups are statistically confirmed (see text). This analysis suggests that changes in motivation and memory for the need to emit pauses are unaffected by seizures.
For completeness, we note that less formal behavioral measures indicate some group differences. Although the gait of seizure rats appeared normal, they spent less time pacing in the home cage. In addition, seizure rats responded less vigorously to taps on their home cages and they assumed a sleeping posture more often. Thus, there seem to be changes in alertness or emotionality that can last for hours after a seizure even though there was no evidence that such changes alter performance in the spatial accuracy task. It is also possible that increased number of flurothyl-induced seizures could cause a kindling effect (Holmes et al., 1993). Nevertheless, we saw no spontaneous seizures while the rats were performing the task or when they were observed in their home cages. Moreover, place cell and hippocampal EEG recordings made while subjecting rats to one per day repetitive flurothyl seizures showed gradual slowing of the theta peak frequency but revealed no paroxysmal electrical activity after eight seizures (Lin et al., 2008), a total nearly equal to the number given in the present study.
DISCUSSION
There were four main findings from this study: (1) Recurrent seizures caused a progressive decline of performance in the spatial accuracy task for highly trained adult rats. (2) During the seizure period, the performance decline appeared on earlier days in the second daily session (3 hours post-seizure) than in the first daily session (21 hours post-seizure); the degree of decline was also greater for the second session. (3) Performance deficits persisted for most part of a 10-minute session. (4) Once seizures were terminated, performance returned to normal over a time course of several days.
Cumulative effect of recurrent seizures
The effect of recurrent seizures on the spatial accuracy task was cumulative; there was little if any performance change at the start of the seizure period but by its end rats given convulsions obtained fewer rewards and scattered their pauses over a considerably larger region than control rats. This initial performance decline appeared to take place over a linear time course and not with the delay characteristic of a sigmoid function. In this view, improved measures might reveal a performance drop after even a single seizure. Also, given that performance would eventually asymptote after more seizures, we suggest that the linear time course is the beginning of a first order exponential decline that was not allowed to go to completion. The implication is that each seizure interferes with a constant fraction of the remaining ability of rats to find the hidden goal zone; this is in contrast to the idea that performance begins to degrade detectably only when the system loses a critical amount of its computational power.
The overall performance pattern reported here agrees with the conclusion that flurothyl-induced seizures degrade spatial memory. In a previous study it was found that a similar number of flurothyl seizures delivered twice per day for five days slows acquisition of a standard water maze task (Zhou et al., 2007b). Here we saw a loss of precision in spatial navigation in extensively trained rats rather than learning problems during the course of seizure induction. The extended one-a-day, 11 day seizure protocol in conjunction with two tests per day allowed us to characterize the temporal dynamics of impairment in a detailed manner.
Is sleep neuro-restorative?
The markedly delayed and smaller performance deficits in sessions before compared to sessions after the daily seizure implies that significant recuperation occurs during the 21 hour seizure-test interval. It is unclear if the improvement would occur simply as a function of time or if intervening sleep episodes are important. We hypothesize that it is the increased number of sleep episodes that are likely to serve a neuro-restorative role since seizure rats are informally observed to be in sleeping positions more often as well as reduced alertness to external noise. In this regard, it is noteworthy that recent work shows that sleep favors net synaptic depression whereas wakefulness favors net synaptic potentiation (Tononi and Cirelli, 2006; Vyazovskiy et al., 2008). If seizures are a mechanism of inducing a pathological form of long-term potentiation it follows that sleep could play an important role in recovery by depotentiation or by long-term depression, as well as many other aspects of homeostatic restoration (Mignot, 2008).
Recovery after the 21-hour interval is, however, incomplete as the number of seizures increases. Thus, we saw that the average pause-distance to the goal zone center for seizure rats is greater than for control rats during the first minute of session 1 on the last two days of the seizure period. Apparently, the seizure rats remember the general area they must search for the goal zone after the overnight interval but their recall of the exact goal location to make pause attempts is less precise. Once informed of the goal zone location by a successful drop, however, they appear to come close in their performance to that of control toward the end of the session. Nonetheless, there is a strong trend toward group separation (Figure 5A). Recovery is also known to be incomplete simply from the cumulative effects of seizures on session 2 and eventually session 1 performance.
Relatively persistent but reversible cognitive impairment
Performance deficits for the seizure group improved only gradually after seizures were stopped; full recovery for both sessions 1 and 2 was not seen until day six according to the reward measure. The deficits may be due to a form of “erasure” of the learned skill in locating the goal zone with good precision and recovery may reflect normal learning from a lower baseline. Alternatively, the deficits and their persistence may be due to seizure-induced long-term modifications of the hippocampal neuronal network or changes in receptor subunit composition (Bo et al., 2004; Ni et al., 2004). In this case, recovery may occur by tapping a “reserve” capacity of the network that was not needed for the initial learning. This raises the possibility that there may be a critical amount of damage after which full recovery cannot happen. We speculate, in other words, that there are combinations of seizure severity and frequency that make render cognitive impairment permanent.
The role of hippocampus in processing of long-term memory
Our results suggest that multiple daily seizures impair the ability of highly trained rats to precisely locate the goal zone but leave essentially intact the knowledge that pausing is required for food to be released and that the pauses must be made in a certain location. Thus, there is no trend for the number of pauses to decrease during the seizure period compared to the pre-seizure or recovery periods and the emitted pauses are still biased towards the vicinity of the goal zone (Figure 2B, right).
This pattern of effects can be accounted for on two assumptions: (1) Control over task performance is partitioned into a mainly spatial component whose accuracy depends on the state of the hippocampus and related areas and a procedural component in which the know-how has come to depend on other brain areas with the striatum likely an important player. (2) The relatively weak, short duration seizures predominantly affect the hippocampus, which has the lowest threshold for abnormal excitability of any brain structure.
In this view, the hippocampus remains essential for precise navigation even after extensive practice in going to a fixed goal location. The residual bias for the animal to make pauses in the vicinity of the goal zone may be due to remaining capacity of the hippocampus or to a cruder navigational capability centered elsewhere in the brain. On the other hand, after nearly two months of combined training and pre-seizure experience, the general performance rules are encoded in other brain areas even if the hippocampus was originally in control of the entire task. Based on earlier results (McDonald and White, 1993; Packard and McGaugh, 1996) we imagine that the extra-hippocampal contribution may be by the dorsal striatum (see White and McDonald, 2002).
Clinical Implications
While extrapolation of rodent studies to humans must be done with caution, our results indicate that recurrent seizures can result in cumulative, but reversible difficulties with memory. Despite relatively brief seizures, post-ictal impairment was quite long and progressive. Humans having flurries of seizures may be at risk for memory disturbances that extend well after the last seizure. Our study would question the assumption that the patient who has a seizure should be back to their baseline cognitive level within a few hours of the seizure. Fortunately, our work also shows that full recovery, at least with a limited number of seizures, is possible.
Acknowledgments
We thank Mr. Matthew Holtzer for developing the rat tracking system used in the spatial accuracy task. The work was supported by NIH grant NS20686 and funds from the Craigmyle Foundation, Glen Head, NY 11545.
Grant sponsor: The Craigmyle Foundation; SUNY Research Account 1009342
References
- Aldenkamp AP. Effect of seizures and epileptiform discharges on cognitive function. Epilepsia. 1997;38(Suppl 1):S52–55. doi: 10.1111/j.1528-1157.1997.tb04520.x. [DOI] [PubMed] [Google Scholar]
- Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol. 1993;52(5):433–443. doi: 10.1097/00005072-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Biton V, Gates JR, dePadua Sussman L. Prolonged postictal encephalopathy. Neurology. 1990;40(6):963–966. doi: 10.1212/wnl.40.6.963. [DOI] [PubMed] [Google Scholar]
- Bo T, Jiang Y, Cao H, Wang J, Wu X. Long-term effects of seizures in neonatal rats on spatial learning ability and N-methyl-D-aspartate receptor expression in the brain. Brain Res Dev Brain Res. 2004;152(2):137–142. doi: 10.1016/j.devbrainres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Cheung MC, Chan AS, Chan YL, Lam JM, Lam W. Effects of illness duration on memory processing of patients with temporal lobe epilepsy. Epilepsia. 2006;47(8):1320–1328. doi: 10.1111/j.1528-1167.2006.00556.x. [DOI] [PubMed] [Google Scholar]
- Fenton AA, Csizmadia G, Muller RU. Conjoint control of hippocampal place cell firing by two visual stimuli. I. The effects of moving the stimuli on firing field positions. J Gen Physiol. 2000;116(2):191–209. doi: 10.1085/jgp.116.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol. 2003;53(3):413–416. doi: 10.1002/ana.10509. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C. Effects of chronic epilepsy on declarative memory systems. Prog Brain Res. 2002;135:439–453. doi: 10.1016/S0079-6123(02)35041-6. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Elger CE, Lendt M. Postictal courses of cognitive deficits in focal epilepsies. Epilepsia. 1994;35(5):1073–1078. doi: 10.1111/j.1528-1157.1994.tb02557.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, Bell B. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60(1):80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54(4):369–376. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- Hok V, Lenck-Santini PP, Roux S, Save E, Muller RU, Poucet B. Goal-related activity in hippocampal place cells. J Neurosci. 2007;27(3):472–482. doi: 10.1523/JNEUROSCI.2864-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, Chronopoulos A, Stafstrom CE, Mikati MA, Thurber SJ, Hyde PA, Thompson JL. Effects of kindling on subsequent learning, memory, behavior, and seizure susceptibility. Brain Res Dev Brain Res. 1993;73(1):71–77. doi: 10.1016/0165-3806(93)90047-e. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Daamen M, Zang H, Janszky J, Ebner A. Seizures accelerate forgetting in patients with left-sided temporal lobe epilepsy. Neurology. 2001;57(1):125–126. doi: 10.1212/wnl.57.1.125. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: a cross sectional study. J Neurol Neurosurg Psychiatry. 1999;67(1):44–50. doi: 10.1136/jnnp.67.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokeit H, Ebner A. Effects of chronic epilepsy on intellectual functions. Prog Brain Res. 2002;135:455–463. doi: 10.1016/S0079-6123(02)35042-8. [DOI] [PubMed] [Google Scholar]
- Kalviainen R, Salmenpera T. Do recurrent seizures cause neuronal damage? A series of studies with MRI volumetry in adults with partial epilepsy. Prog Brain Res. 2002;135:279–295. doi: 10.1016/S0079-6123(02)35026-X. [DOI] [PubMed] [Google Scholar]
- Kubie JL, Fenton A, Novikov N, Touretzky D, Muller RU. Changes in goal selection induced by cue conflicts are in register with predictions from changes in place cell field locations. Behav Neurosci. 2007;121(4):751–763. doi: 10.1037/0735-7044.121.4.751. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini PP, Muller RU, Save E, Poucet B. Relationships between place cell firing fields and navigational decisions by rats. J Neurosci. 2002;22(20):9035–9047. doi: 10.1523/JNEUROSCI.22-20-09035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutmezer F, Baumgartner C. Postictal signs of lateralizing and localizing significance. Epileptic Disord. 2002;4(1):43–48. [PubMed] [Google Scholar]
- Lin H, Barry JM, Muller RU. Effects of recurrent seizures on hippocampal neuronal activity. Society Neuroscience Abstracts (645.8) 2008 [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107(1):3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Mignot E. Why we sleep: the temporal organization of recovery. PLoS Biol. 2008;6(4):e106. doi: 10.1371/journal.pbio.0060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris RG, Hagan JJ, Rawlins JN. Allocentric spatial learning by hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q J Exp Psychol B. 1986;38(4):365–395. [PubMed] [Google Scholar]
- Ni H, Jiang YW, Bo T, Wang JM, Pan H, Wu XR. Long-term effects of neonatal seizures on subsequent N-methyl-D-aspartate receptor-1 and gamma-aminobutyric acid receptor A-alpha 1 receptor expression in hippocampus of the Wistar rat. Neurosci Lett. 2004;368(3):254–257. doi: 10.1016/j.neulet.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Rossier J, Kaminsky Y, Schenk F, Bures J. The place preference task: a new tool for studying the relation between behavior and place cell activity in rats. Behav Neurosci. 2000;114(2):273–284. [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11(2):200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple Parallel Memory Systems in the Brain of the Rat. Neurobiology of Learning and Memory. 2002;77(2):125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Holmes GL. Repetitive seizures in the immature brain. In: Pitkänen A, Schwartzkroin PA, Moshé S, editors. Models of Seizures and Epilepsy. Elsevier Academic Press; 2006. pp. 341–350. [Google Scholar]
- Zhou JL, Shatskikh TN, Liu X, Holmes GL. Impaired single cell firing and long-term potentiation parallels memory impairment following recurrent seizures. Eur J Neurosci. 2007;25(12):3667–3677. doi: 10.1111/j.1460-9568.2007.05598.x. [DOI] [PubMed] [Google Scholar]