Abstract
We report a strategy for generating novel dual-tapered poly(isoprene-b-isoprene/styrene-b-styrene-b-styrene/methyl methacrylate-b-methyl methacrylate) [P(I-IS-S-SM-M)] triblock copolymers that combines anionic polymerization, atom transfer radical polymerization (ATRP), and Huisgen 1,3-dipolar cycloaddition click chemistry. The tapered interfaces between blocks were synthesized via a semi-batch feed using programmable syringe pumps. This strategy allows us to manipulate the transition region between copolymer blocks in triblock copolymers providing control over the interfacial interactions in our nanoscale phase-separated materials independent of molecular weight and block constituents. Additionally, we show the ability to retain a desirous and complex multiply-continuous network structure (alternating gyroid) in our dual-tapered triblock material.
Block copolymer self-assembly offers an ideal opportunity for designing ordered materials with three-dimensionally (3-D) continuous nanoscale domains.1,2 Such nanoscale network morphologies are particularly useful in applications such as catalysis, solar cells, conducting membranes and nanoporous templates due to their desirable size-scale, and unique transport and mechanical properties.1,3–8 In the recent triblock copolymer literature, numerous network morphologies have been reported at weak and intermediate segregation strengths, including the core-shell gyroid, alternating gyroid, and orthorhombic networks.1,9–21 However, generating stable network structures at higher segregation strength remains a challenge in triblock copolymer systems due to kinetic and thermodynamic restrictions.12 To stabilize these morphologies in higher molecular weight and chemically incompatible polymer systems, here we detail a strategy to manipulate the interfaces between the copolymer blocks by synthesizing tapered triblock copolymers.
For the majority of non-tapered block copolymers, the composition profile changes abruptly at the junction between the two blocks.22,23 However, in tapered block copolymers (TBCs), the composition profile is modified along the polymer backbone by introducing a transition region between two pure blocks that tapers from one component to another.24–26 This differs from gradient copolymers, where the graded composition profile extends along the entire length of the copolymer backbone.
TBCs also provide the opportunity to generate well-ordered structures, and introducing a tapered interface between blocks has been shown to decrease the effective Flory-Huggins interaction parameter (χeff), meaning that segregation strength could be tuned independent of polymer molecular weight. This reduction in χeff leads to lower order-to-disorder transition temperatures relative to the corresponding non-tapered block copolymers.24–26 Thus, TBCs can be used to create high molecular weight materials while retaining access to the network phases found at weak and intermediate segregation strengths.26 Though a well-ordered network structure has been found at high molecular weight following additional post-processing in a few select cases,27 our TBCs permit the facile design of network materials with improved mechanical properties (due to higher molecular weights), combined with lowered interfacial energetics for a wider array of potential applications.
Previous examples of TBCs in literature were synthesized using living anionic polymerization; however, this synthetic approach only can be applied to a limited number of monomers.24–26,28 Acar and Matyjaszewski provided an alternative synthetic method by combining living anionic polymerization and atom transfer radical polymerization (ATRP), which allowed for the synthesis of various triblock copolymers with compositional tapers from acrylate- or methacrylate-based monomers.29 While this approach can be used to create TBCs from a larger variety of monomers, Acar and Matyjaszewski's method is limited to low molecular weight polymers due to the poor mobility of living chain ends in high molecular weight materials. Thus, we report a strategy for the preparation of tapered triblock copolymers by click coupling an anionic polymerization-based tapered diblock copolymer to another tapered copolymer synthesized via ATRP. This synthetic route can be applied to a variety of monomers and provides easy access to a library of tapered triblock copolymers by simply coupling different tapered copolymers.
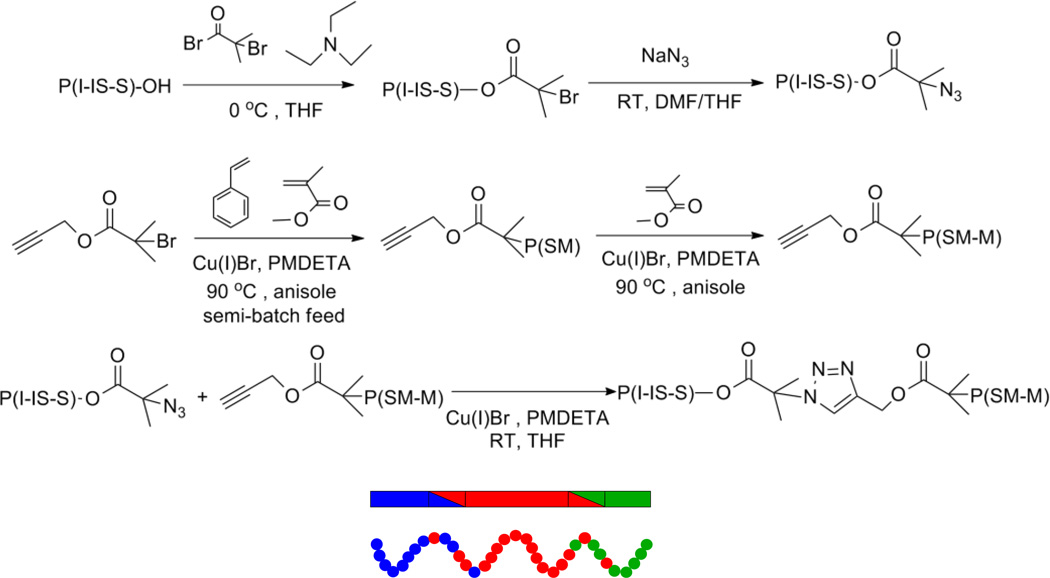
In this report, we used our synthetic strategy to create a P(I-IS-S-SM-M) tapered triblock copolymer by click coupling an azide-terminated P(I-IS-S) tapered diblock copolymer to an alkyne-terminated P(SM-M) tapered copolymer, as shown in Scheme 1. P(I-IS-S)-OH was synthesized using anionic polymerization according to established methods,26 where programmable syringe pumps fed predetermined amounts of the monomers into a semi-batch reactor. The flow rates were varied to adjust the composition of the interfacial segments and to obtain linear composition profiles in each tapered region. To functionalize the P(I-IS-S)-OH with a bromide end group [P(I-IS-S)-Br] the hydroxyl-terminated polymer was reacted with 2-bromoisobutyryl bromide,30 where in our case P(I-IS-S)-OH (0.24 mmol) was first dissolved in 200 mL of dry tetrahydrofuran (THF) containing triethylamine (1.44 mmol). Then, 2-bromoisobutyryl bromide (0.96 mmol) was added dropwise at 0 °C, and the solution was stirred for ≈16 h. The P(I-IS-S)-Br was precipitated into methanol and dried under vacuum. P(I-IS-S)-Br was dissolved in a 3:1 mixture of dimethylformamide (DMF): THF and reacted with sodium azide (0.24 mmol) for 12 h at room temperature, yielding azide-terminated P(I-IS-S)-N3.
Scheme 1.
Schematic illustration of the P(I-IS-S-SM-M) synthesis using a combination of anionic polymerization, ATRP, and Huisgen 1, 3-dipolar cycloaddition click chemistry. The bottom image shows the density profile along a polymer chain and the composition profile along the TBC backbone.
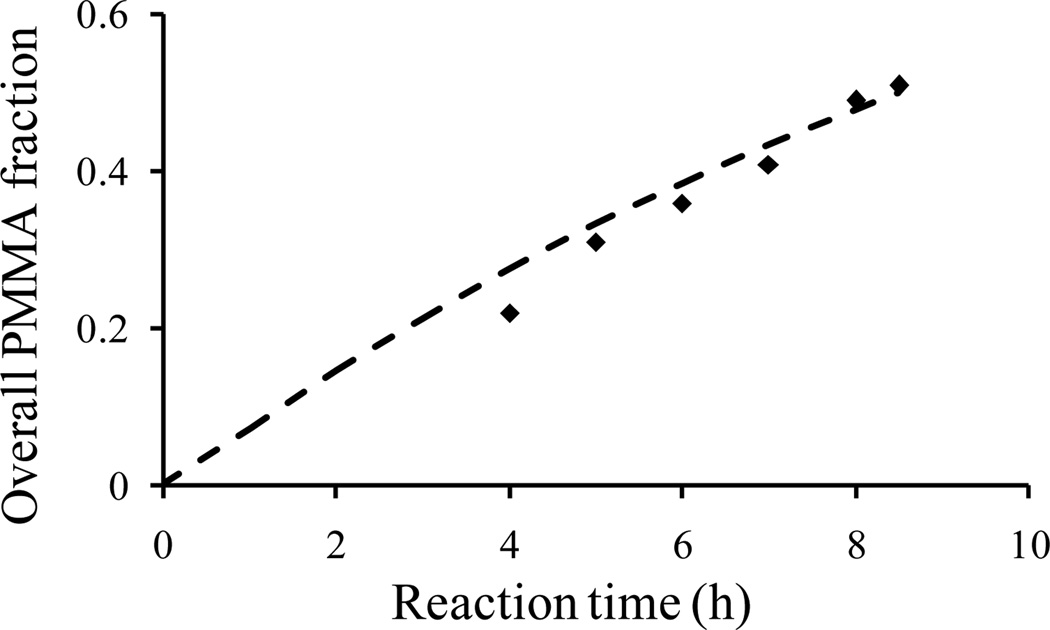
An alkyne-terminated P(SM-M) tapered copolymer was prepared using ATRP. The methyl methacrylate (99%) monomers were purified with sodium hydroxide solution and dried from sodium sulfate and calcium hydride. The initiator (Propargyl 2-bromoisobutyrate, PgBiB, 4.4 mmol), catalyst (copper bromide, Cu(I)Br, 2.2 mmol), ligand (N,N,N',N''N'''-pentamethyldiethylenetriamine, PMDETA, 4.4 mmol), and solvent (anisole, 40 mL) were added to a reactor under argon, and the reactor was immersed in a silicone oil bath at 90 °C. To create the SM tapered segments, styrene and methyl methacrylate monomers were added to the reactor vessel at predetermined flow rates using automated syringe pumps. At the end of the reaction, the polymer was passed through a neutral alumina column to remove the catalyst. Then, the polymer was precipitated from methanol. Aliquots were taken during the polymerization of the SM tapered segment, and analyzed by proton nuclear magnetic resonance (1H NMR) to determine the tapered composition profile as a function of time. As seen in Figure 1, the mole fraction of PMMA increases with reaction time, and the experimental composition (data points) is in a good agreement with the theoretical composition (dash line),28 supporting that the tapered composition profile is linear. Furthermore, comparing the reactivity ratios of styrene (M1) and methyl methacrylate (M2) monomers: r1= 1.16 and r2 = 1.09 (see Figure S1),31 we found that both r1 and r2 are close to unity, indicating that styrene and methyl methacrylate have similar reactivity, and thus we can infer the formation of tapered composition profile in this reaction. We note that the ATRP reaction to form the SM taper was designed to reach the desired composition and molecular weight at relatively low monomer conversions (≈27%) to maximize bromine end-group fidelity. Next, the SM tapered polymer was used as a macroinitiator for the synthesis of the PMMA block by ATRP. The reaction was stirred for 2 h at 90 °C under argon, and the reaction was terminated by exposure of the solution to air. The alkyne-terminated polymer was purified by passage through a neutral alumina column followed by successive THF washes/methanol precipitations to remove the catalyst and unreacted SM tapered material. The solvent was removed under vacuum to obtain the final alkyne-terminated P(SM-M).
Figure 1.
Theoretical prediction (dash line) and experimental data (solid dot) for the overall PMMA mole fraction changes versus reaction time in the ATRP of SM tapered segment.
The P(I-IS-S-SM-M) tapered triblock copolymer was created via a Huisgen 1,3-dipolar cycloaddition click chemistry of azide-terminated P(I-IS-S) (0.036 mmol) and alkyne-terminated P(SM-M) (0.044 mmol) with Cu(I)Br (0.18 mmol) and PMDETA (0.18 mmol) in THF (5 mL) under argon.32–36 The reaction was stirred for 24 h at room temperature, and the polymer was purified by passage through a neutral alumina column followed by successive THF washes/methanol precipitations to remove the catalyst and excess P(SM-M) tapered copolymer. The yield for the coupling reaction based on the initial weight of P(I-IS-S)-N3 was 96%. The chemical characterization and morphology of the resulting tapered material is reported in Table 1.
Table 1.
Characterization data for tapered precursors and final tapered triblock copolymer
| polymer | Mn (g/mol)a | PDI | fI | fS | fM | Phase |
|---|---|---|---|---|---|---|
| P(I-IS-S)-N3 | 34,000 | 1.08 | 0.35 | 0.65 | - | Q230 |
| P(SM-M) | 12,000 | 1.30 | - | 0.39 | 0.61 | Disordered |
| P(I-IS-S-SM-M)b | 46,000 | 1.29 | 0.26 | 0.59 | 0.15 | Q214 |
Molecular weights are determined by a combination of GPC and 1H NMR analysis.
IS tapered vol % = 23 and SM tapered vol % = 17.
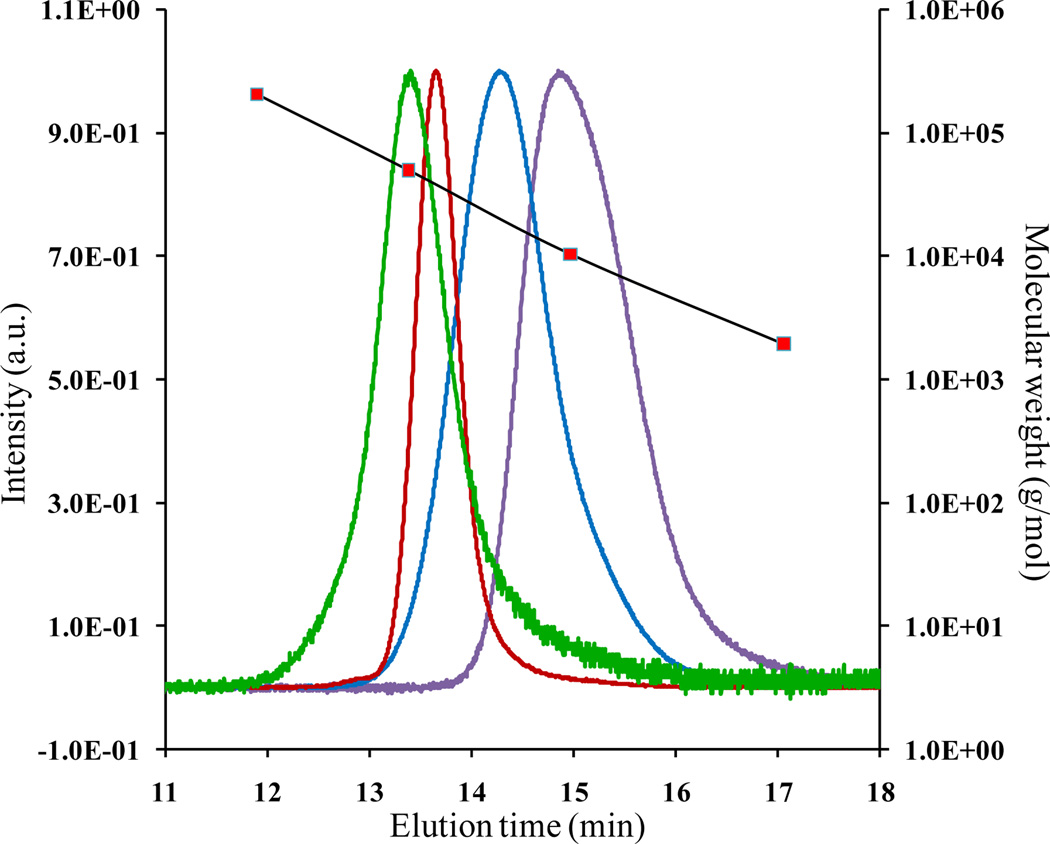
In this work, gel permeation chromatography (GPC) analysis, as shown in Figure 2, confirmed the shifts in molecular weight distributions, indicating the formation of well-defined copolymers at each step. The polymer compositions were determined by 1H NMR peak intensities, and the corresponding volume fractions were calculated using the homopolymer densities at 140 °C (ρPI = 0.83 g/mL, ρPS = 0.97 g/mL, ρPMMA = 1.13 g/mL).37 Notably, the broad peak from the PMMA methylgroup (see Figure S2) was due to the stereo isomerism, which resulted from the mixing of PS and PMMA in the SM tapered segment.38
Figure 2.
Representative Gel Permeation Chromatography data for the P(SM) tapered segment (purple), P(SM-M) tapered copolymer (blue), P(I-IS-S) tapered diblock copolymer (red), and P(I-IS-S-SM-M) tapered triblock copolymer (green). The number average molecular weights and polydispersity indices of TBCs were determined using a Viscotek 270Max instrument fitted with Waters Styragel HR1 and HR4 columns in series, operated with THF as the mobile phase, and calibrated using polystyrene standards.
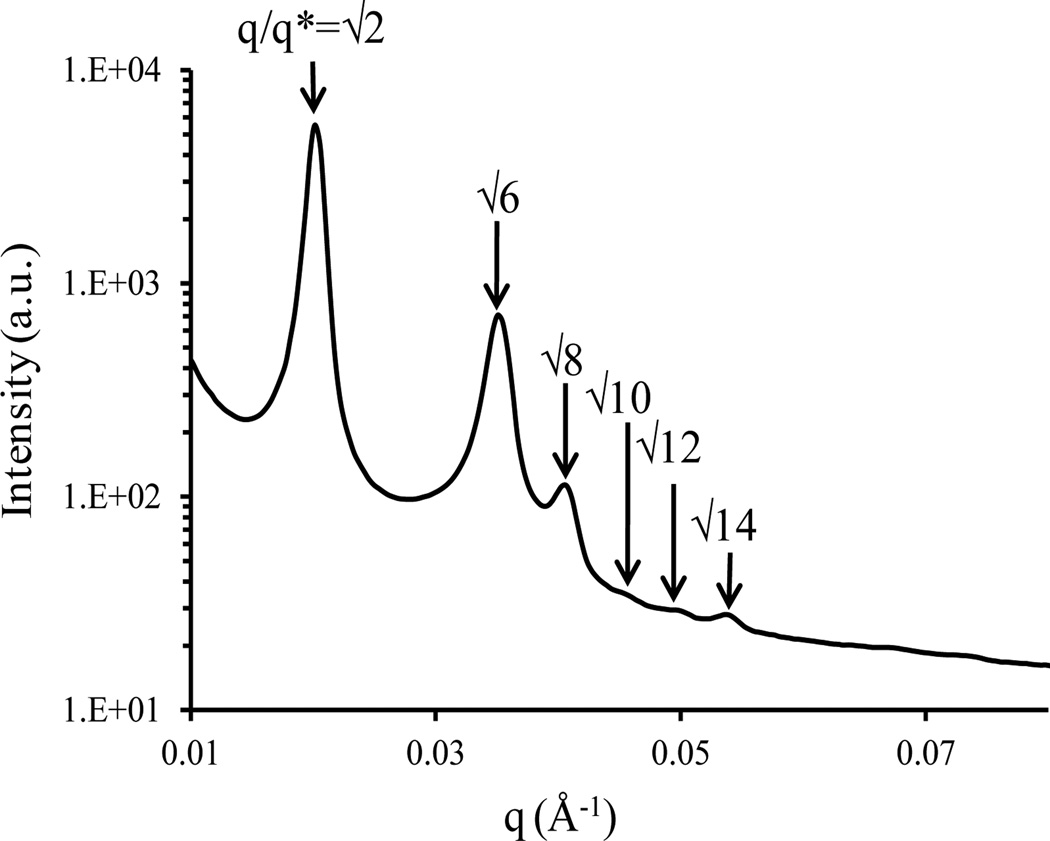
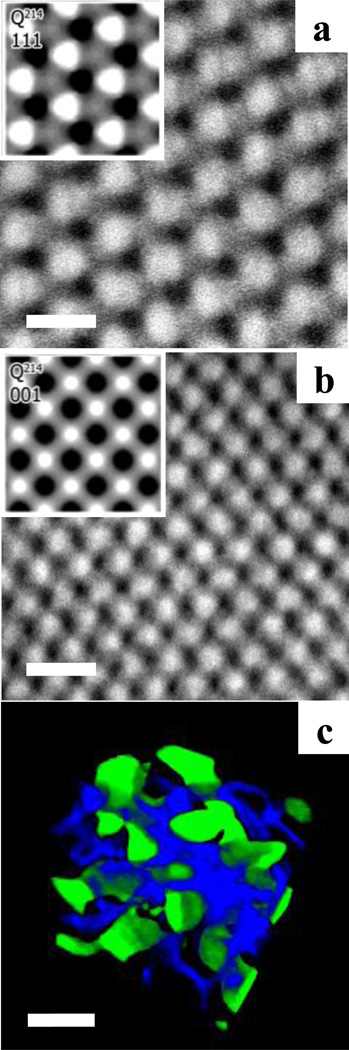
The space group symmetry of the copolymer morphologies was determined from the relative peak positions in the small-angle X-ray scattering (SAXS) patterns. The thermal annealing conditions were 230 °C (6 h), 170 °C (24 h), and 30 °C. The room temperature SAXS profile for the P(I-IS-S-SM-M) is presented in Figure 3. The Bragg reflection peaks for the P(I-IS-S-SM-M) were located at q/q* = √2, √6, √8, √10, √12, and √14 (the primary peak corresponds to q*√2, where q*=q001). The absence of √4 peak was indicative of a I4132 space group symmetry, suggesting that the P(I-IS-S-SM-M) self-assembled into an alternating gyroid (Q214) morphology. The corresponding transmission electron microscopy (TEM) micrographs and transmission electron microtomography (TEMT) image are shown in Figure 4. TEM specimens were cut into ≈70 nm slices at −75 °C on a cryo-microtome from the thermally-annealed SAXS sample. The PI domain was preferentially stained with OsO4 vapors to enhance the TEM contrast. Comparison of our experimental micrographs with simulated TEM images reported for Q214 structures of [111] and [001] projections supports our Q214 assignment in the tapered triblock copolymer.9–11 A reconstructed 3-D image (Figure 4c) was obtained using TEMT experiments; the PS domain was made transparent for visual clarity. PI and PMMA domains formed distinct networks, showing the Q214 symmetry.
Figure 3.
SAXS profile at 30 °C for P(I-IS-S-SM-M). From the primary peak position, the domain spacing was 44 nm, and the peaks were indexed according to I4132 symmetry, characteristic of the alternating gyroid (Q214) morphology.
Figure 4.
TEM micrographs and TEMT image for P(I-IS-S-SM-M). (a) and (b) Experimental TEM images, representing the reflection planes [111] and [001], respectively. The inset images are simulated TEM micrographs from Epps et al.11 Dark domains correspond to OsO4-stained PI. Scale bars represent 50 nm. (c) 3-D reconstructed image. Only PI (blue) and PMMA (green) domains are shown for clarity. Scale bar represents 30 nm.
The morphology of our tapered triblock copolymer was compared to the experimental phase diagram for non-tapered P(I-S-M) triblock copolymers.10 Based on polymer composition, our P(I-IS-S-SM-M) was expected to self-assemble into hexagonally-packed cylinder structures. However, our tapered triblock material was found to form an alternating gyroid (Q214) network. Because the phase diagram is significantly affected by the differences in segregation strength (χN),39,40 this difference in morphology could be due to the interfacial modification of our tapered triblock copolymer (χIS and χSM were decreased by introducing the tapered interfaces between blocks), or the difference in total molecular weight between our copolymer and the triblocks reported by Tureau et al.10 Extensive studies on the effects of interfacial modification on copolymer morphologies are currently under investigation.
In summary, we report for the first time the synthesis of interfacially-manipulated ABC triblock copolymers using a combination of anionic polymerization, ATRP, and Huisgen 1,3-dipolar cycloaddition click chemistry. A network-forming P(I-IS-S-SM-M) tapered triblock copolymer was generated. Our results suggest that it is possible to modify the interfacial composition profile between the pure blocks in linear triblock copolymers, while still retaining the complex co-continuous nanostructures that are a hallmark of triblock copolymers. Though the approach described in this work was applied to ABC triblock copolymers, a similar methodology could be employed to generate tapered tetrablock and pentablock copolymers, as well as ABA triblock copolymers with different tapered profiles at each A–B junction. Finally, the protocols highlighted herein will allow for the design and synthesis of new nanostructured materials with a wide variety of components, providing a significant step towards fabricating 3-D nanoscale devices for nanotechnology applications.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge AFOSR-PECASE (FA9550-09-1-0706), NSF-CAREER (DMR-0645586), and ACS (PRF-46864-67) for financial support. R. Roy acknowledges funding from NIH NCRR COBRE (NIH-2P20RR017716-06A1). Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract DE-AC02-98CH10886. 1H NMR spectra were collected on instrumentation supported by NSF CRIF: MU CEH 0840401. We also thank the W. M. Keck Electron Microscopy Facility at the University of Delaware for use of their TEM and TEMT facilities. The authors acknowledge Dr. T. Smart for assistance with TEMT and Dr. N. Singh for helpful discussion.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Fineman-Ross plot for the evaluation of reactivity ratios and NMR spectra. This information is available free of charge via the Internet at http://pubs.acs.org/journal/amlccd.
REFERENCES
- 1.Meuler AJ, Hillmyer MA, Bates FS. Macromolecules. 2009;42:7221–7250. [Google Scholar]
- 2.Wohlgemuth M, Yufa N, Hoffman J, Thomas EL. Macromolecules. 2001;34:6083–6089. [Google Scholar]
- 3.Crossland EJW, Kamperman M, Nedelcu M, Ducati C, Wiesner U, Smilgies DM, Toombes GES, Hillmyer MA, Ludwigs S, Steiner U, Snaith HJ. Nano Letters. 2008;9:2807–2812. doi: 10.1021/nl803174p. [DOI] [PubMed] [Google Scholar]
- 4.Crossland EJW, Nedelcu M, Ducati C, Ludwigs S, Hillmyer MA, Steiner U, Snaith HJ. Nano Letters. 2008;9:2813–2819. doi: 10.1021/nl800942c. [DOI] [PubMed] [Google Scholar]
- 5.Guo F, Schulte L, Zhang W, Vigild ME, Ndoni S, Chen J. Polymer Chemistry. 2011;2:553–555. [Google Scholar]
- 6.Shefelbine TA, Vigild ME, Matsen MW, Hajduk DA, Hillmyer MA, Cussler EL, Bates FS. Journal of the American Chemical Society. 1999;121:8457–8465. [Google Scholar]
- 7.du Sart GG, Vukovic I, Vukovic Z, Polushkin E, Hiekkataipale P, Ruokolainen J, Loos K, ten Brinke G. Macromolecular Rapid Communications. 2011;32:366–370. doi: 10.1002/marc.201000674. [DOI] [PubMed] [Google Scholar]
- 8.Dair BJ, Honeker CC, Alward DB, Avgeropoulos A, Hadjichristidis N, Fetters LJ, Capel M, Thomas EL. Macromolecules. 1999;32:8145–8152. [Google Scholar]
- 9.Tureau MS, Epps TH., III Macromolecular Rapid Communications. 2009;30:1751–1755. doi: 10.1002/marc.200900298. [DOI] [PubMed] [Google Scholar]
- 10.Tureau MS, Rong L, Hsiao BS, Epps TH., III Macromolecules. 2010;43:9039–9048. [Google Scholar]
- 11.Epps TH, III, Cochran EW, Bailey TS, Waletzko RS, Hardy CM, Bates FS. Macromolecules. 2004;37:8325–8341. [Google Scholar]
- 12.Epps TH, III, Bates FS. Macromolecules. 2006;39:2676–2682. [Google Scholar]
- 13.Suzuki J, Seki M, Matsushita Y. The Journal of Chemical Physics. 2000;112:4862–4868. [Google Scholar]
- 14.Sugiyama M, Shefelbine TA, Vigild ME, Bates FS. The Journal of Physical Chemistry B. 2001;105:12448–12460. [Google Scholar]
- 15.Epps TH, III, Cochran EW, Hardy CM, Bailey TS, Waletzko RS, Bates FS. Macromolecules. 2004;37:7085–7088. [Google Scholar]
- 16.Epps TH, III, Chatterjee J, Bates FS. Macromolecules. 2005;38:8775–8784. [Google Scholar]
- 17.Bailey TS, Hardy CM, Epps TH, III, Bates FS. Macromolecules. 2002;35:7007–7017. [Google Scholar]
- 18.Stadler R, Auschra C, Beckmann J, Krappe U, Voight-Martin I, Leibler L. Macromolecules. 1995;28:3080–3097. [Google Scholar]
- 19.Goldacker T, Abetz V. Macromolecules. 1999;32:5165–5167. [Google Scholar]
- 20.Stefik M, Mahajan S, Sai H, Epps TH, III, Bates FS, Gruner SM, DiSalvo FJ, Wiesner U. Chemistry of Materials. 2009;21:5466–5473. doi: 10.1021/cm902626z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meuler AJ, Ellison CJ, Hillmyer MA, Bates FS. Macromolecules. 2008;41:6272–6275. [Google Scholar]
- 22.Bates FS. Science. 1991;251:898–905. doi: 10.1126/science.251.4996.898. [DOI] [PubMed] [Google Scholar]
- 23.Bates FS, Fredrickson GH. Physics Today. 1999;52:32–38. [Google Scholar]
- 24.Hodrokoukes P, Floudas G, Pispas S, Hadjichristidis N. Macromolecules. 2001;34:650–657. [Google Scholar]
- 25.Singh N, Tureau MS, Epps TH., III Soft Matter. 2009;5:4757–4762. [Google Scholar]
- 26.Roy R, Park JK, Young W-S, Mastroianni SE, Tureau MS, Epps TH., III Macromolecules. 2011;44:3910–3915. doi: 10.1021/ma1025847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbas AM, Maldovan M, DeRege P, Thomas EL. Advanced Materials. 2002;14:1850–1853. [Google Scholar]
- 28.Young RJ, Lovell PA. INTRODUCTION TO POLYMERS Third ed. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- 29.Acar MH, Matyjaszewski K. Macromolecular Chemistry and Physics. 1999;200:1094–1100. [Google Scholar]
- 30.Mahajan S, Cho B-K, Allgaier J, Fetters LJ, Coates GW, Wiesner U. Macromolecular Rapid Communications. 2004;25:1889–1894. [Google Scholar]
- 31.Fineman M, Ross SD. Journal of Polymer Science. 1950;5:259–262. [Google Scholar]
- 32.Hasneen A, Kim S, Paik H-j. Macromolecular Research. 2007;15:541–546. [Google Scholar]
- 33.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angewandte Chemie International Edition. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Huisgen R. Angewandte Chemie International Edition in English. 1963;2:565–598. [Google Scholar]
- 35.Sumerlin BS, Vogt AP. Macromolecules. 2010;43:1–13. [Google Scholar]
- 36.Iha RK, Wooley KL, Nyström AM, Burke DJ, Kade MJ, Hawker CJ. Chemical Reviews. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fetters LJ, Lohse DJ, Richter D, Witten TA, Zirkel A. Macromolecules. 1994;27:4639–4647. [Google Scholar]
- 38.Kotani Y, Kamigaito M, Sawamoto M. Macromolecules. 1998;31:5582–5587. doi: 10.1021/ma980320r. [DOI] [PubMed] [Google Scholar]
- 39.Tyler CA, Qin J, Bates FS, Morse DC. Macromolecules. 2007;40:4654–4668. [Google Scholar]
- 40.Tang P, Qiu F, Zhang H, Yang Y. Physical Review E. 2004;69 doi: 10.1103/PhysRevE.69.031803. 031803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.