Abstract
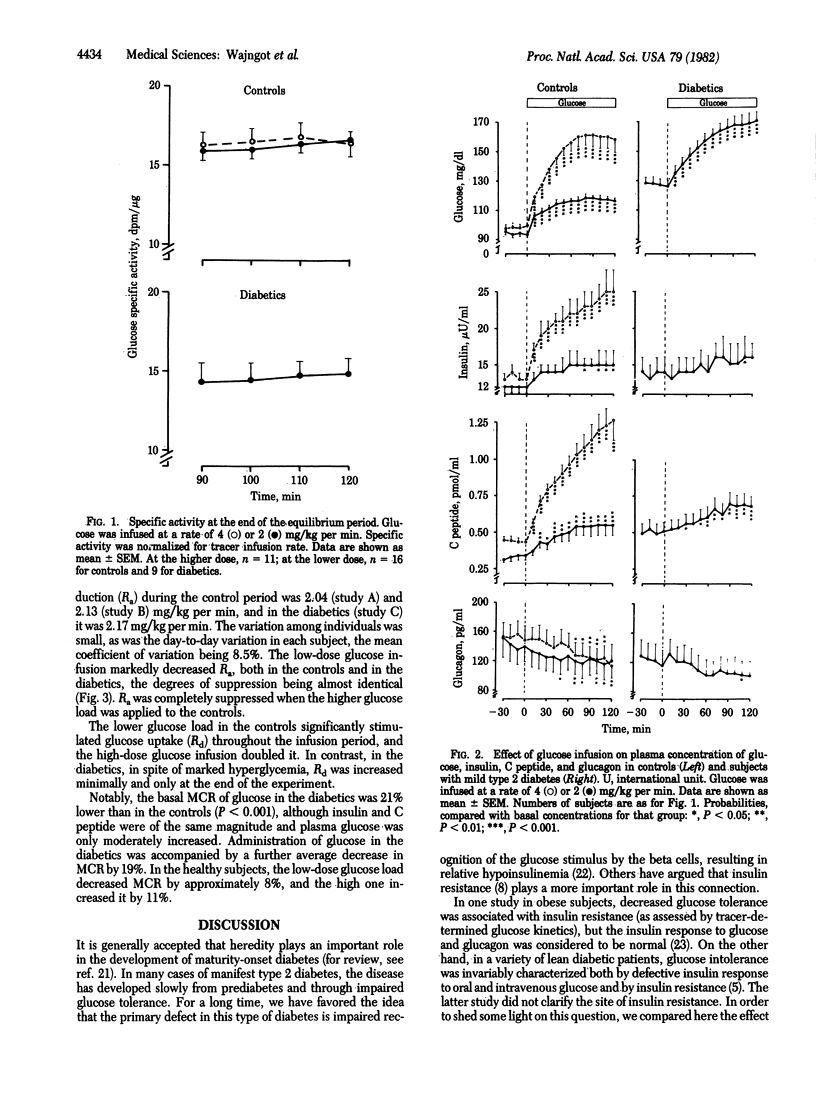
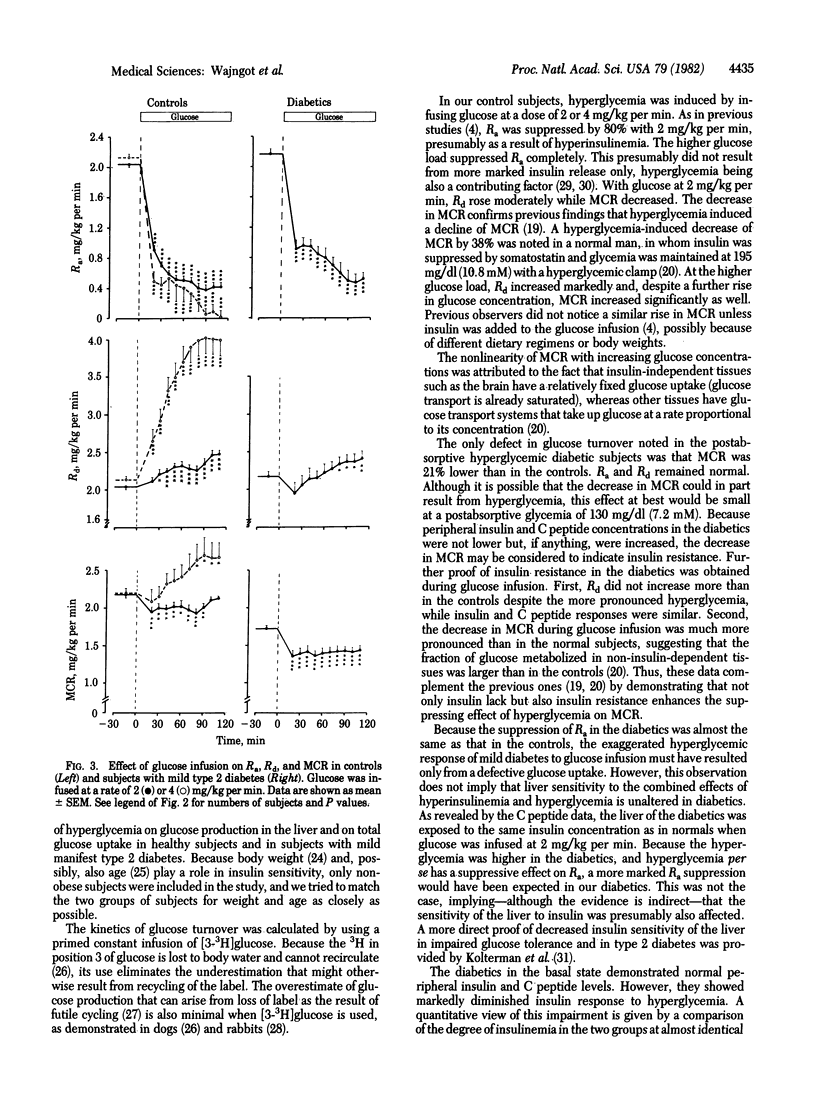
In an attempt to determine the mechanism of decreased glucose tolerance in lean type 2 diabetics, glucose turnover in such subjects and controls was studied under basal conditions and during hyperglycemia induced by intravenous administration of glucose. The diabetics had decreased intravenous glucose tolerance and a fasting plasma glucose of 6-8 mM (108-144 mg/dl). Glucose was infused for 2 hr at 2 mg/kg per min in the controls (n = 16) and diabetics (n = 9). Furthermore, 11 healthy subjects were infused also with glucose at 4 mg/kg per min to match the glycemia of the diabetics. Glucose production, utilization, and metabolic clearance were assessed by the primed constant tracer infusion technique. In the basal state, diabetics showed normal plasma insulin, C peptide, and glucagon concentrations. Their increased basal plasma glucose levels were associated with normal rates of glucose production and utilization, but the metabolic glucose clearance was 21% lower than in the controls (P < 0.001), indicating decreased sensitivity to insulin. During infusion of glucose at 2 mg/kg per min, the hyperglycemia attained in the diabetics (170 mg/dl) was higher than that in controls (115 mg/dl) but comparable to that of the controls exposed to the higher glucose load. With the lower glucose load, metabolic clearance rate decreased more markedly in diabetics, again suggesting insulin resistance. This was further substantiated by the fact that, at the same insulin levels, glucose utilization did not increase more in the diabetics than in the controls, although the glycemia reached was considerably higher in the diabetics. With the lower glucose load, glucose production was suppressed to the same degree in the controls and diabetics, although the attained glycemia was much more marked in the latter. Because both insulin and hyperglycemia can suppress glucose production, some defect in the regulation of glucose production of the diabetics is also indicated. The insulin and C peptide levels were much higher in the controls than in the diabetics at the same levels of glycemia, demonstrating the inadequacy of insulin response to glycemia of the diabetics. Glucagon concentration was equally suppressed in all groups. In conclusion, impaired glucose tolerance of mild type 2 diabetics resulted both from inadequate insulin response and from decreased sensitivity to insulin. The insulin resistance could mainly be ascribed to inadequate glucose uptake, but a defect in glucose-induced suppression of glucose production may also have contributed.
Keywords: early diabetes, pathogenesis
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Bergman R. N. Integrated control of hepatic glucose metabolism. Fed Proc. 1977 Feb;36(2):265–270. [PubMed] [Google Scholar]
- Best J. D., Taborsky G. J., Jr, Halter J. B., Porte D., Jr Glucose disposal is not proportional to plasma glucose level in man. Diabetes. 1981 Oct;30(10):847–850. doi: 10.2337/diab.30.10.847. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Wahren J., Luft R., Felig P., Hendler R. The regulation of splanchnic glucose production in subjects with low insulin response--a compensatory mechanism in prediabetes? Eur J Clin Invest. 1973 May;3(3):193–200. doi: 10.1111/j.1365-2362.1973.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Vranic M. Effect of arginine on glucose turnover and plasma free fatty acids in normal dogs. Diabetes. 1973 Jul;22(7):537–543. doi: 10.2337/diab.22.7.537. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Harris M. S. Relationship between the plasma glucose level and glucose uptake in the conscious dog. Metabolism. 1978 Jul;27(7):787–791. doi: 10.1016/0026-0495(78)90213-5. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Defronzo R. A. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979 Dec;28(12):1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Efendić S., Cerasi E., Elander I., Thornqvist C., Fick G., Berglund B., Luft R. Studies on low insulin responders. Glucose tolerance and insulin response to intravenous and oral glucose challenges in 226 normal subjects and 25 subjects with mild or chemical diabetes. Acta Endocrinol Suppl (Copenh) 1979;224:5–32. [PubMed] [Google Scholar]
- Efendić S., Wajngot A., Cerasi E., Luft R. Insulin release, insulin sensitivity, and glucose intolerance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7425–7429. doi: 10.1073/pnas.77.12.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbath N., Hetenyi G., Jr Glucose dynamics in normal subjects and diabetic patients before and after a glucose load. Diabetes. 1966 Nov;15(11):778–789. doi: 10.2337/diab.15.11.778. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- IKKOS D., LUFT R. On the intravenous glucose tolerance test. Acta Endocrinol (Copenh) 1957 Jul;25(3):312–334. doi: 10.1530/acta.0.0250312. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenquist J. E., Mueller G. L., Cherrington A. D., Perry J. M., Rabinowitz D. Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab. 1979 Jan;48(1):171–175. doi: 10.1210/jcem-48-1-171. [DOI] [PubMed] [Google Scholar]
- Long C. L., Spencer J. L., Kinney J. M., Geiger J. W. Carbohydrate metabolism in normal man and effect of glucose infusion. J Appl Physiol. 1971 Jul;31(1):102–109. doi: 10.1152/jappl.1971.31.1.102. [DOI] [PubMed] [Google Scholar]
- Luft R., Efendić S. On the pathogenesis of maturity-onset diabetes mellitus. Acta Diabetol Lat. 1978 Jan-Apr;15(1-2):1–15. doi: 10.1007/BF02581002. [DOI] [PubMed] [Google Scholar]
- Nagulesparan M., Savage P. J., Unger R. H., Bennett P. H. A simplified method using somatostatin to assess in vivo insulin resistance over a range of obesity. Diabetes. 1979 Nov;28(11):980–983. doi: 10.2337/diab.28.11.980. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Measurement and validation of nonsteady turnover rates with applications to the inulin and glucose systems. Fed Proc. 1974 Jul;33(7):1855–1864. [PubMed] [Google Scholar]
- Verdonk C. A., Rizza R. A., Gerich J. E. Effects of plasma glucose concentration on glucose utilization and glucose clearance in normal man. Diabetes. 1981 Jun;30(6):535–537. doi: 10.2337/diab.30.6.535. [DOI] [PubMed] [Google Scholar]
- Vranic M., Morita S., Steiner G. Insulin resistance in obesity as analyzed by the response of glucose kinetics to glucagon infusion. Diabetes. 1980 Mar;29(3):169–176. doi: 10.2337/diab.29.3.169. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Allsop J. R., Burke J. F. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979 Mar;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]


